The Real-Time Validation of the Effectiveness of Third-Generation Hyperbranched Poly(ɛ-lysine) Dendrons-Modified KLVFF Sequences to Bind Amyloid-β1-42 Peptides Using an Optical Waveguide Light-Mode Spectroscopy System
Abstract
:1. Introduction
2. Materials and Methods
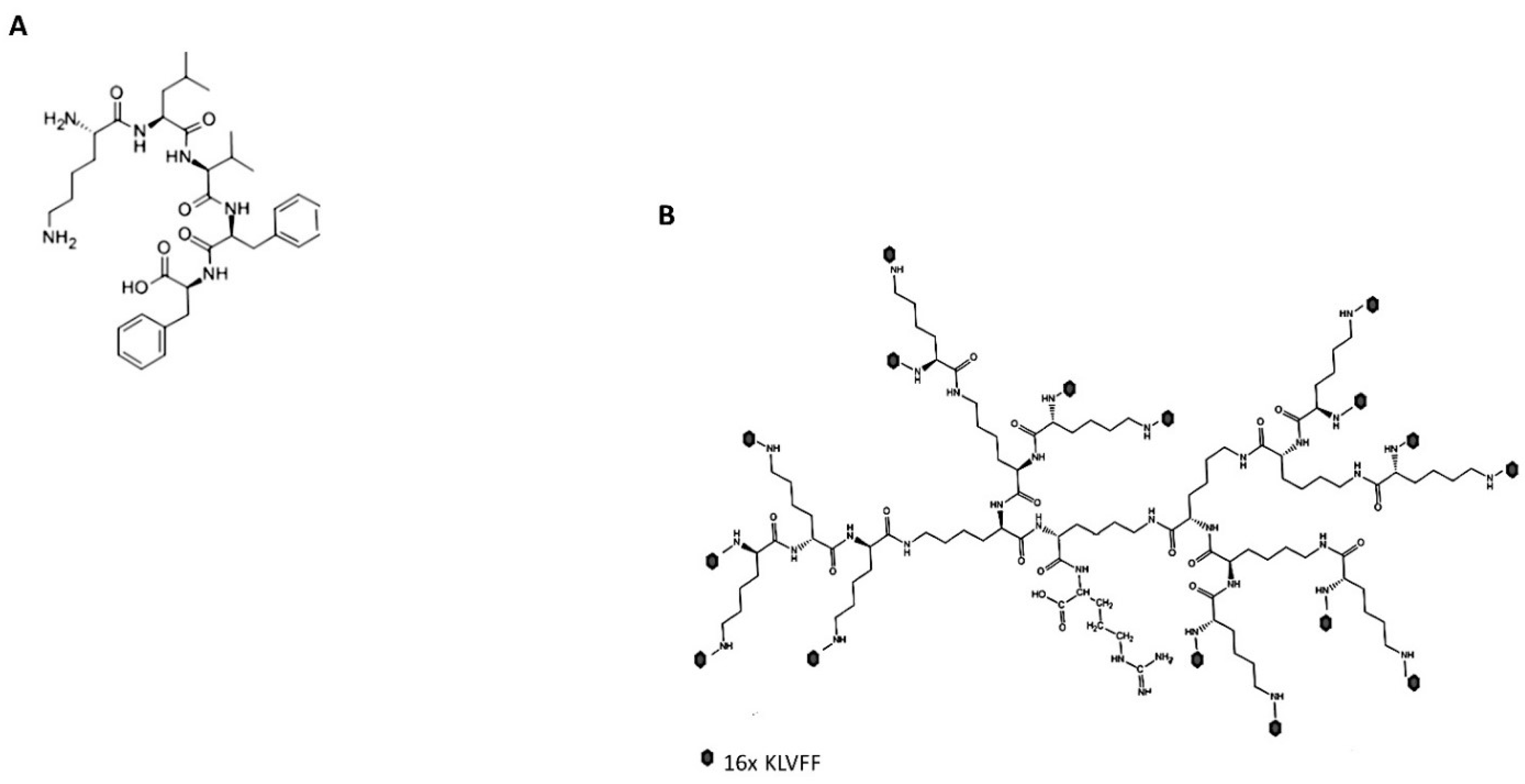
2.1. Hyperbranched KLVFF Design
2.2. Synthesis and Characterisation of Linear and Hyperbranched KLVFF
2.3. Thioflavin T Staining
2.4. Congo Red Assay
2.5. Optical Waveguide Lightmode Spectroscopy Chip Surface Functionalisation
2.6. OWLS Study of Aβ1-42 Binding
(dn/dc)
2.7. Statistical Analysis
3. Results
3.1. Characterisation of Linear KLVFF and Rgen3K(KLVFF)16 Peptides
Physicochemical Characterisation
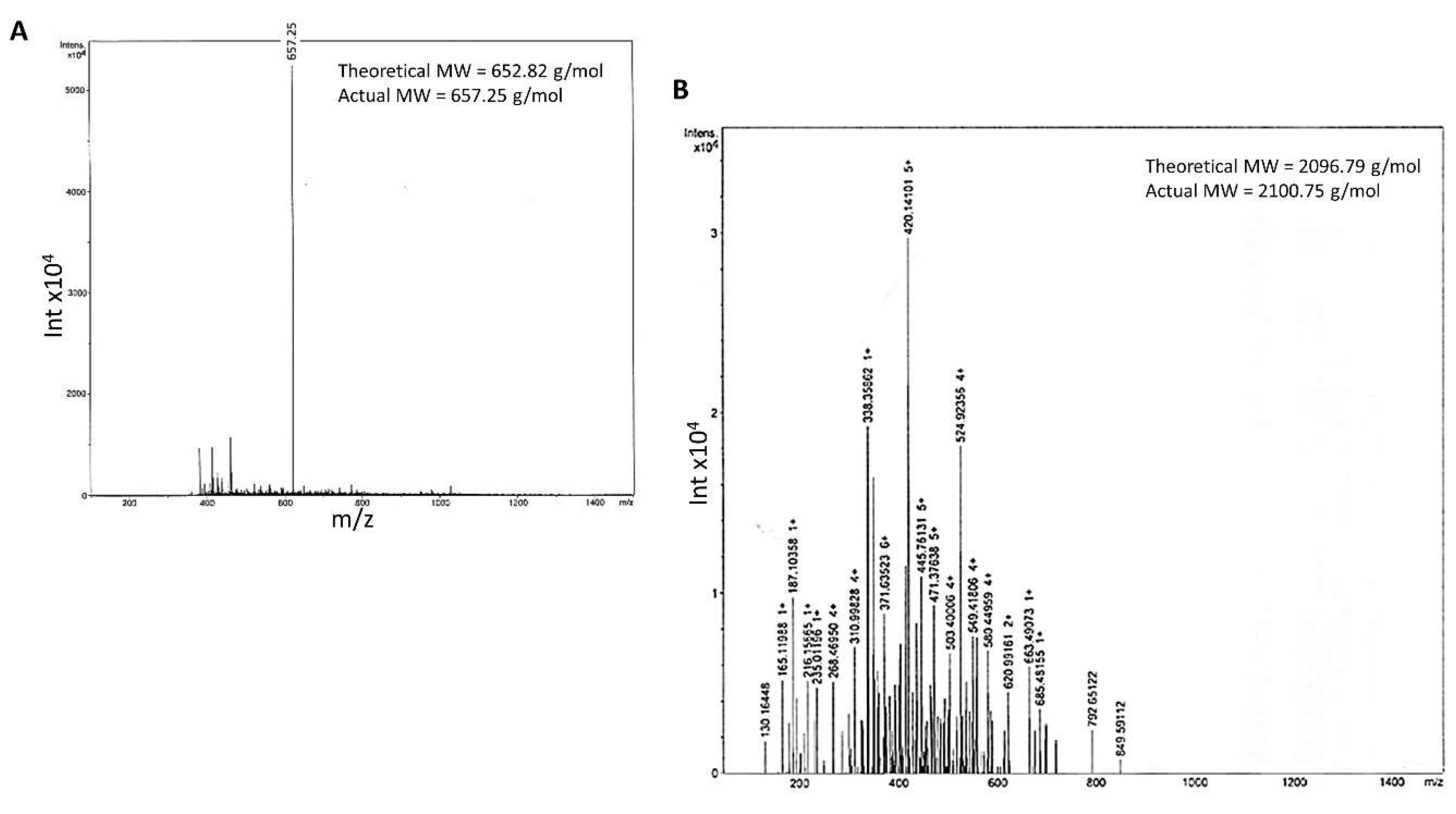
- Both linear and branched KLVFF peptides (Figure 1A,B) were assembled in batches of approximately 80 mg and successfully characterised by mass spectrometry (Figure 2A,B) and HPLC (Figure 3). Their degree of purity was always above 95% with yields of reaction 75 and 81% for KLVFF and Rgen3K(KLVFF)16, respectively.

3.2. Biospecific Binding Tests and OWLS Sensing of Aβ1-42
3.2.1. Linear and Branched KLVFF Peptides Blocking Effect on Aβ1-42 Fibrils Formation
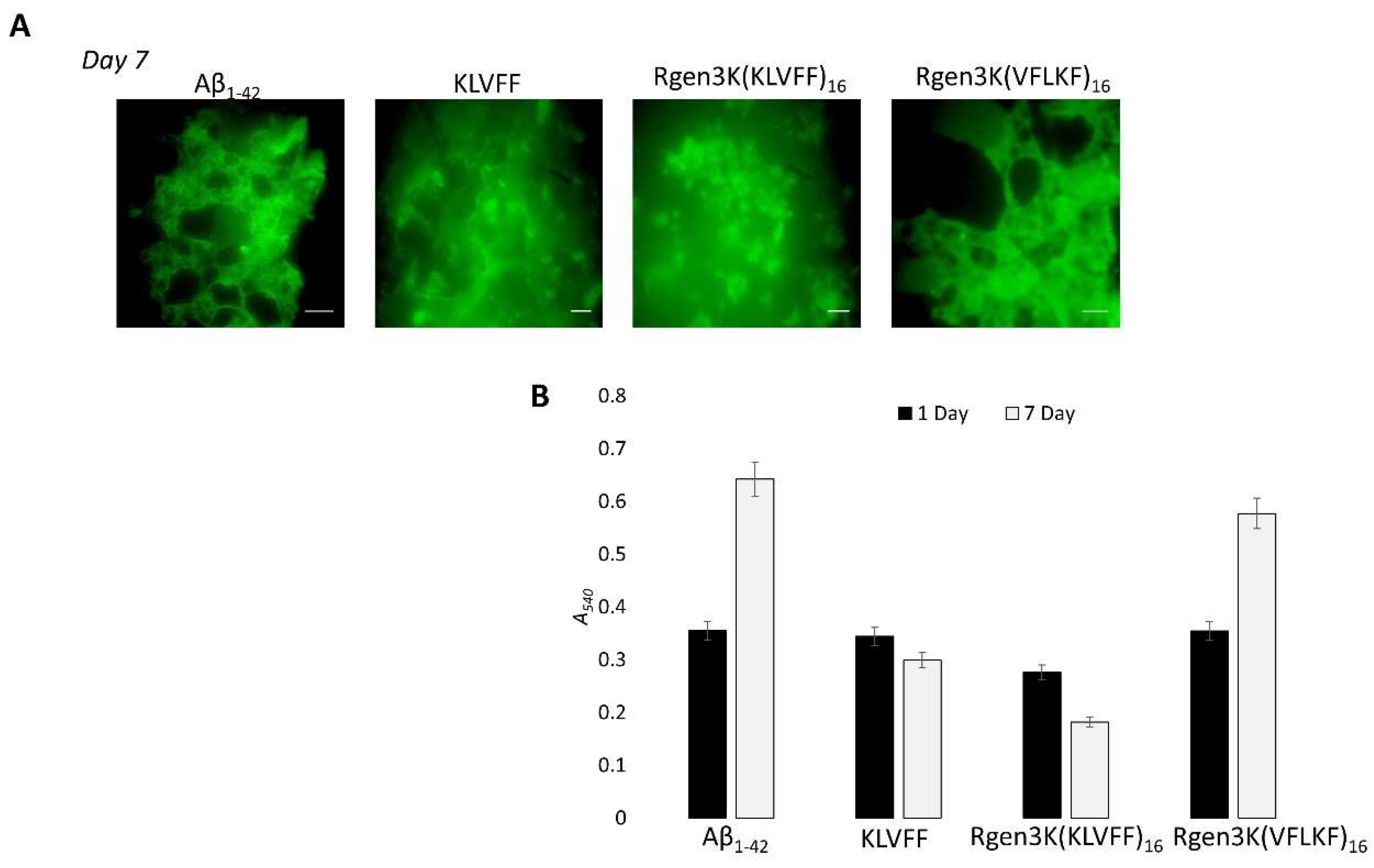
- To monitor the potential of both peptides to inhibit the self-aggregation of Aβ1-42 monomers into fibrils as well as the disruption of formed fibrils, ThT and Congo red analysis were performed and used to quantify the amounts of formed fibrils when Aβ1-42 was incubated with either linear or branched KLVFF peptides for 1 and 7 days (Figure 5A,B). Both fluorescent microscopy of ThT-stained samples (Figure 5A) and spectrophotometric measurement of Congo Red staining (Figure 5B) clearly show the enhanced inhibition of Aβ1-42 peptide aggregation when incubated with hyperbranched Rgen3K(KLVFF)16. The specificity of the binding was also demonstrated when a scramble sequence (i.e., VFLKF) of the peptide was tested showing no disaggregation properties.
3.2.2. OWLS Real-Time Monitoring of Aβ1-42
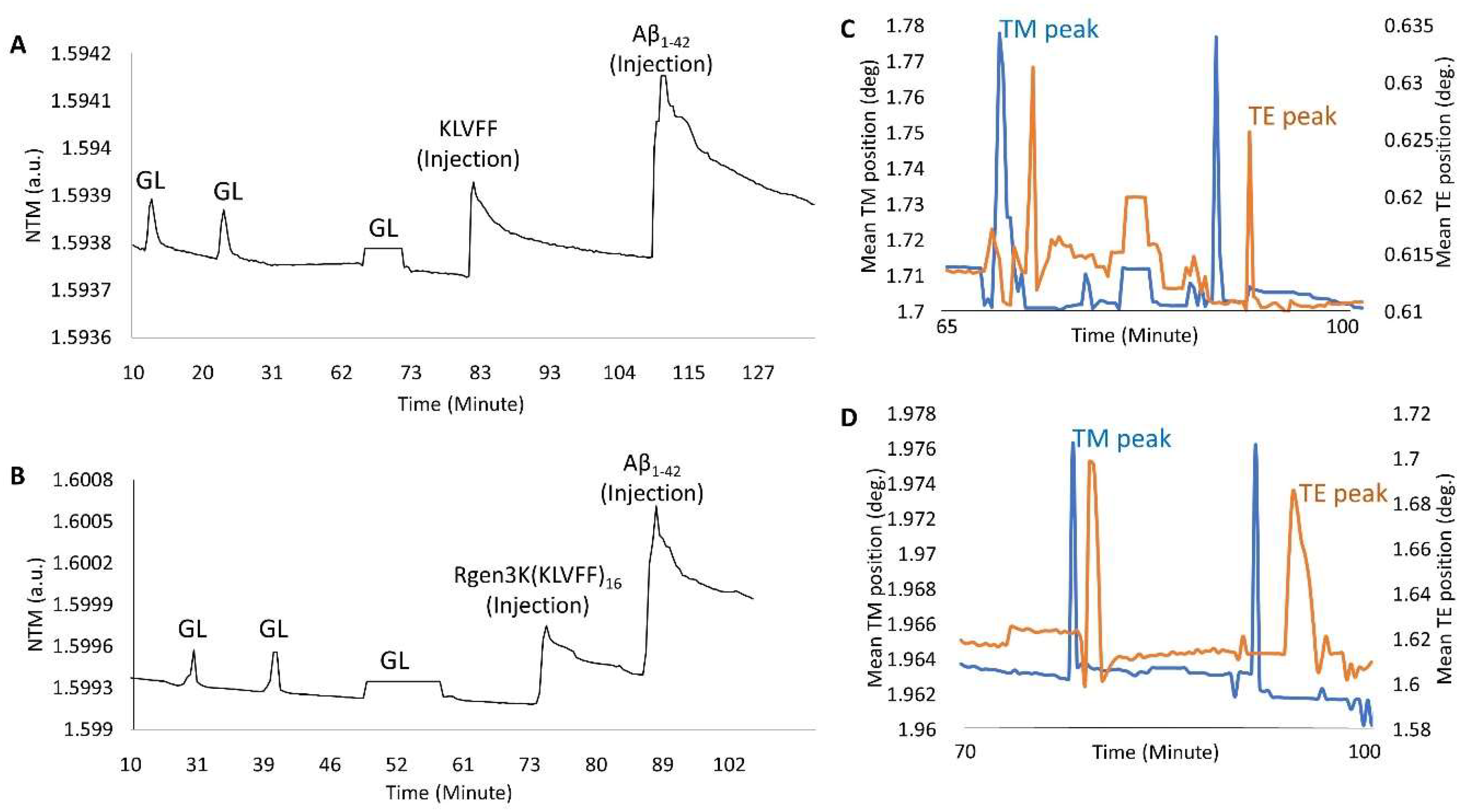
- OWLS studies clearly demonstrated the ability of both KLVFF peptides to form macromolecular complexes with Aβ1-42 when used to functionalise the surface of the waveguide sensor (Figure 6A,B). The binding of the linear and hyperbranched peptides to the metal-oxide thin layer of the sensor was obtained by three injections of glutaraldehyde up to allege saturation of the whole surface (Figure 6A,B,G,L); this surface derivatisation step allowed each peptide to be effectively grafted onto the sensor chip surface. However, a slightly weaker immobilisation was recorded in the case of the linear KLVFF peptides (NTM = 1.5939 v NTM = 1.5999). The data were also confirmed by their respective InTM (absorption phase, KLVFF = 1.78°; Rgen3K(KLVFF)16 = 1.97°) and InTE (desorption phase, KLVFF = 0.63°; Rgen3K(KLVFF)16 = 1.7°) peaks (Figure 6C,D) whereby the introduction of a water solution and indeed the stabilisation of a baseline was reached after 32 min by KLVFF rather than 15 min as observed for Rgen3K(KLVFF)16. This in turn directly raised the thicknesses of the coating layer from 0.01 to 2.51 [KLVFF] and 3.41 nm [Rgen3K(KLVFF)16] and induced the OWLS signals to significantly change within few minutes after the injections of Aβ1-42 [KLVFF NTM = 1.5941; Rgen3K(KLVFF)16 NTM = 1.6007].
- NTM peaks also showed the successful binding of Aβ1-42 on OWLS surfaces functionalised with both linear and branched KLVFF (Figure 6A,B). In particular, Aβ1-42 binding to the linear KLVFF showed a broader peak with an elution process characterised by a slower and irregular decay of the signals. Consistently, the OWLS-driven transformation of the binding data into mass values (ng/cm2) showed less chip surface functionalisation by the linear KLVFF peptides (Figure 7A) whereby a slower process of molecular adsorption reached a peak of 250 ng/cm2 and a slower wash out of the non-covalently bound excess (Figure 7A, 83–104 min) yielding ca 125 ng/cm2 covalently-bound peptide mass, whilst in the case of the functionalisation of the OWLS chip surface by Rgen3K(KLVFF)16, faster, more efficient covalent binding was observed after 74 min followed by a rapid removal (ca 1 min) of the unbound excess to yield a mass level of bound-branched peptides at level similar to that of the linear molecule (ca 125 ng/cm2) (Figure 7B). The similar level of coupled mass led to significantly different Aβ1-42, binding profiles. Following injection, the AD biomarker molecules were captured by the chip surface functionalised with the linear aptamer at a slower rate and reached lower peaks of mass interaction and the slower removal of unbound excess molecules than those captured by the surface functionalised with Rgen3K(KLVFF)16 (Figure 7A,B). The resulting adsorbed Aβ1-42, bound mass slowly increased from 125 to 387 ng/cm2 in the case of surfaces functionalised with KLVFF. The weakly bound excess mass being slowly removed to yield 301 ng/cm2 of stably bound Aβ1-42. Instead, Rgen3K(KLVFF)16 -modified chip surfaces showed a sharp and fast increase from ca 100 ng/cm2 505 ng/cm2 in less than 2 min, followed by a fast and more efficient washing up of the unbound species in ca 3 min, thereby yielding a reliably measured Aβ1-42-detected mass of 250 ng/cm2. In other words, the dissociation of excessive bound Aβ1-42 observed in the case of chip surfaces functionalised with the linear peptide showed prolonged elution times highlighting less binding specificity and the likelihood of Aβ1-42 aggregate formation rolling and remaining adsorbed on the surface (Figure 7A, 110–130 min curve). Instead, in the case of chips functionalised with Rgen3K(KLVFF)16, the sharper and higher peak was followed by a very rapid decline to stable value (Figure 7B, 90–100 min) indicating a more specific and stable interaction, hence giving higher and more reliable measurements. No significant stable binding was observed when non-functionalised OWLS chip surfaces were used (data not shown).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s disease. Subcell Biochem. 2012, 65, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Ow, S.Y.; Dunstan, D.E. A brief overview of amyloids and Alzheimer’s disease. Protein Sci. 2014, 23, 1315–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Kedia, N.; Illes-Toth, E.; Haralampiev, I.; Prisner, S.; Herrmann, A.; Wanker, E.E.; Bieschke, J. Amyloid-β(1-42) Aggregation Initiates Its Cellular Uptake and Cytotoxicity. J. Biol. Chem. 2016, 291, 19590–19606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verberk, I.M.W.; Thijssen, E.; Koelewijn, J.; Mauroo, K.; Vanbrabant, J.; de Wilde, A.; Zwan, M.D.; Verfaillie, S.C.J.; Ossenkoppele, R.; Barkhof, F.; et al. Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res. Ther. 2020, 12, 118. [Google Scholar] [CrossRef]
- Smach, M.A.; Charfeddine, B.; Othman, L.B.; Lammouchi, T.; Dridi, H.; Nafati, S.; Ltaief, A.; Bennamou, S.; Limem, K. Evaluation of cerebrospinal fluid tau/beta-amyloid(42) ratio as diagnostic markers for Alzheimer disease. Eur. Neurol. 2009, 62, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Neddenriep, B.; Calciano, A.; Conti, D.; Sauve, E.; Paterson, M.; Bruno, E.; Moffet, D.A. Short peptides as inhibitors of amyloid aggregation. Open Biotechnol. J. 2011, 5, 39–46. [Google Scholar] [CrossRef]
- Nie, Q.; Du, X.G.; Geng, M.Y. Small molecule inhibitors of amyloid β peptide aggregation as a potential therapeutic strategy for Alzheimer’s disease. Acta Pharmacol. Sin. 2011, 32, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Yu, X.; Li, L.; Zheng, J. Inhibition of amyloid-β aggregation in Alzheimer’s disease. Curr. Pharm. Des. 2014, 20, 1223–1243. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Rabbani, G.; Choi, I. Use of Peptides for the Management of Alzheimer’s Disease: Diagnosis and Inhibition. Front. Aging Neurosci. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Funke, S.A.; Willbold, D. Peptides for therapy and diagnosis of Alzheimer’s disease. Curr. Pharm. Des. 2012, 18, 755–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perugini, V.; Santin, M. A substrate mimicking the basement membrane drives the organisation of human mesenchymal stromal cells and endothelial cells into perivascular niche-like structures. Front. Cell Dev. Biol. 2021, 9, 701842. [Google Scholar] [CrossRef] [PubMed]
- Meikle, S.T.; Piñeiro, Y.; Bañobre López, M.; Rivas JSantin, M. Surface functionalization superparamagnetic nanoparticles conjugated with thermoresponsive poly(epsilon-lysine) dendrons tethered with carboxybetaine for the mild hyperthermia-controlled delivery of VEGF. Acta Biomater. 2016, 40, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; He, Y.; Feng, X.; Fu, D. ε-Polylysine and next-generation dendrigraft poly-L-lysine: Chemistry, activity, and applications in biopharmaceuticals. J. Biomater. Sci. Polym. Ed. 2015, 26, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Hug, T.S.; Prenosil, J.E.; Morbidelli, M. Optical waveguide lightmode spectroscopy as a new method to study adhesion of anchorage-dependent cells as an indicator of metabolic state. Biosens. Bioelectron. 2001, 16, 865–874. [Google Scholar] [CrossRef]
- Adányi, N.; Majer-Baranyi, K.; Nagy, A.; Németh, G.; Szendrő, I.; Székács, A. Optical waveguide lightmode spectroscopy immunosensor for detection of carp vitellogenin. Sens. Actuators B Chem. 2013, 176, 932–939. [Google Scholar] [CrossRef]
- Cooper, I.; Meikle, S.; Standen, G.; Hanlon, G.; Santin, M. The rapid and specific real-time detection of Legionella pneumophila in water samples using Optical Waveguide Lightmode Spectroscopy. J. Microbiol. Meth. 2009, 78, 40–44. [Google Scholar] [CrossRef]
- Székács, I.; Kaszás, N.; Gróf, P.; Erdélyi, K.; Szendrő, I.; Mihalik, B.; Pataki, A.; Antoni, F.A.; Madarász, E. Optical Waveguide Lightmode Spectroscopic Techniques for Investigating Membrane-Bound Ion Channel Activities. PLoS ONE 2013, 8, e81398. [Google Scholar] [CrossRef] [Green Version]
- Tjernberg, L.O.; Lilliehöök, C.; Callaway, D.J.; Näslund, J.; Hahne, S.; Thyberg, J.; Terenius, L.; Nordstedt, C. Controlling amyloid beta-peptide fibril formation with protease-stable ligands. J. Biol. Chem. 1997, 272, 12601–12605. [Google Scholar] [CrossRef] [Green Version]
- Qu, A.; Huang, F.; Li, A.; Yang, H.; Zhou, H.; Long, J.; Shi, L. The synergistic effect between KLVFF and self-assembly chaperones on both disaggregation of beta-amyloid fibrils and reducing consequent toxicity. Chem. Commun. 2017, 53, 1289–1292. [Google Scholar] [CrossRef]
- Straub, J.E.; Thirumalai, D. Toward a molecular theory of early and late events in monomer to amyloid fibril formation. Annu. Rev. Phys. Chem. 2011, 62, 437–463. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Ayyannan, S.R.; Panda, G. Perspectives on Inhibiting β-Amyloid Aggregation through Structure-Based Drug Design. Chem. Med. Chem. 2015, 10, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Kozin, S.A.; Barykin, E.P.; Mitkevich, V.A.; Makarov, A.A. Anti-amyloid Therapy of Alzheimer’s Disease: Current State and Prospects. Biochemistry 2018, 83, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ma, B.; McElheny, D.; Parthasarathy, S.; Long, F.; Hoshi, M.; Nussinov, R.; Ishii, Y. Aβ(1-42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 2015, 22, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perchiacca, J.M.; Ladiwala, A.R.; Bhattacharya, M.; Tessier, P.M. Structure-based design of conformation- and sequence-specific antibodies against amyloid β. Proc. Natl. Acad. Sci. USA 2012, 109, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Castelletto, V.; Ryumin, P.; Cramer, R.; Hamley, I.W.; Taylor, M.; Allsop, D.; Reza, M.; Ruokolainen, J.; Arnold, T.; Hermida-Merino, D.; et al. Self-Assembly and Anti-Amyloid Cytotoxicity Activity of Amyloid beta Peptide Derivatives. Sci. Rep. 2017, 7, 43637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austen, B.M.; Paleologou, K.E.; Ali, S.A.; Qureshi, M.M.; Allsop, D.; El-Agnaf, O.M. Designing peptide inhibitors for oligomerization and toxicity of Alzheimer’s beta-amyloid peptide. Biochemistry 2008, 47, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Takahashi, T.; Oshima, H.; Matsumura, S.; Mihara, H. Design of peptides that form amyloid-like fibrils capturing amyloid beta1-42 peptides. Chemistry 2007, 13, 7745–7752. [Google Scholar] [CrossRef]
- Chafekar, S.M.; Malda, H.; Merkx, M.; Meijer, E.W.; Viertl, D.; Lashuel, H.A.; Baas, F.; Scheper, W. Branched KLVFF tetramers strongly potentiate inhibition of beta-amyloid aggregation. Chembiochem 2007, 8, 1857–1864. [Google Scholar] [CrossRef] [Green Version]
- Akikusa, S.; Watanabe, K.I.; Horikawa, E.; Nakamura, K.; Kodaka, M.; Okuno, H.; Konakahara, T. Practical assay and molecular mechanism of aggregation inhibitors of beta-amyloid. J. Pept. Res. 2003, 61, 1–6. [Google Scholar] [CrossRef]
- Pallitto, M.M.; Ghanta, J.; Heinzelman, P.; Kiessling, L.L.; Murphy, R.M. Recognition sequence design for peptidyl modulators of beta-amyloid aggregation and toxicity. Biochemistry 1999, 38, 3570–3578. [Google Scholar] [CrossRef]
- Heegaard, P.M.; Boas, U.; Otzen, D.E. Dendrimer effects on peptide and protein fibrillation. Macromol. Biosci. 2007, 7, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Supattapone, S.; Wille, H.; Uyechi, L.; Safar, J.; Tremblay, P.; Szoka, F.C.; Cohen, F.E.; Prusiner, S.B.; Scott, M.R. Branched polyamines cure prion-infected neuroblastoma cells. J. Virol. 2001, 75, 3453–3461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giehm, L.; Christensen, C.; Boas, U.; Heegaard, P.M.; Otzen, D.E. Dendrimers destabilize proteins in a generation-dependent manner involving electrostatic interactions. Biopolymers 2008, 89, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Lichtemberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perugini, V.; Santin, M. The Real-Time Validation of the Effectiveness of Third-Generation Hyperbranched Poly(ɛ-lysine) Dendrons-Modified KLVFF Sequences to Bind Amyloid-β1-42 Peptides Using an Optical Waveguide Light-Mode Spectroscopy System. Sensors 2022, 22, 9561. https://doi.org/10.3390/s22239561
Perugini V, Santin M. The Real-Time Validation of the Effectiveness of Third-Generation Hyperbranched Poly(ɛ-lysine) Dendrons-Modified KLVFF Sequences to Bind Amyloid-β1-42 Peptides Using an Optical Waveguide Light-Mode Spectroscopy System. Sensors. 2022; 22(23):9561. https://doi.org/10.3390/s22239561
Chicago/Turabian StylePerugini, Valeria, and Matteo Santin. 2022. "The Real-Time Validation of the Effectiveness of Third-Generation Hyperbranched Poly(ɛ-lysine) Dendrons-Modified KLVFF Sequences to Bind Amyloid-β1-42 Peptides Using an Optical Waveguide Light-Mode Spectroscopy System" Sensors 22, no. 23: 9561. https://doi.org/10.3390/s22239561
APA StylePerugini, V., & Santin, M. (2022). The Real-Time Validation of the Effectiveness of Third-Generation Hyperbranched Poly(ɛ-lysine) Dendrons-Modified KLVFF Sequences to Bind Amyloid-β1-42 Peptides Using an Optical Waveguide Light-Mode Spectroscopy System. Sensors, 22(23), 9561. https://doi.org/10.3390/s22239561







