Fabrication of Beta-Barium Borate Sensing Head for Non-Invasive Measurement of Fluidic Concentration Variations
Abstract
:1. Introduction
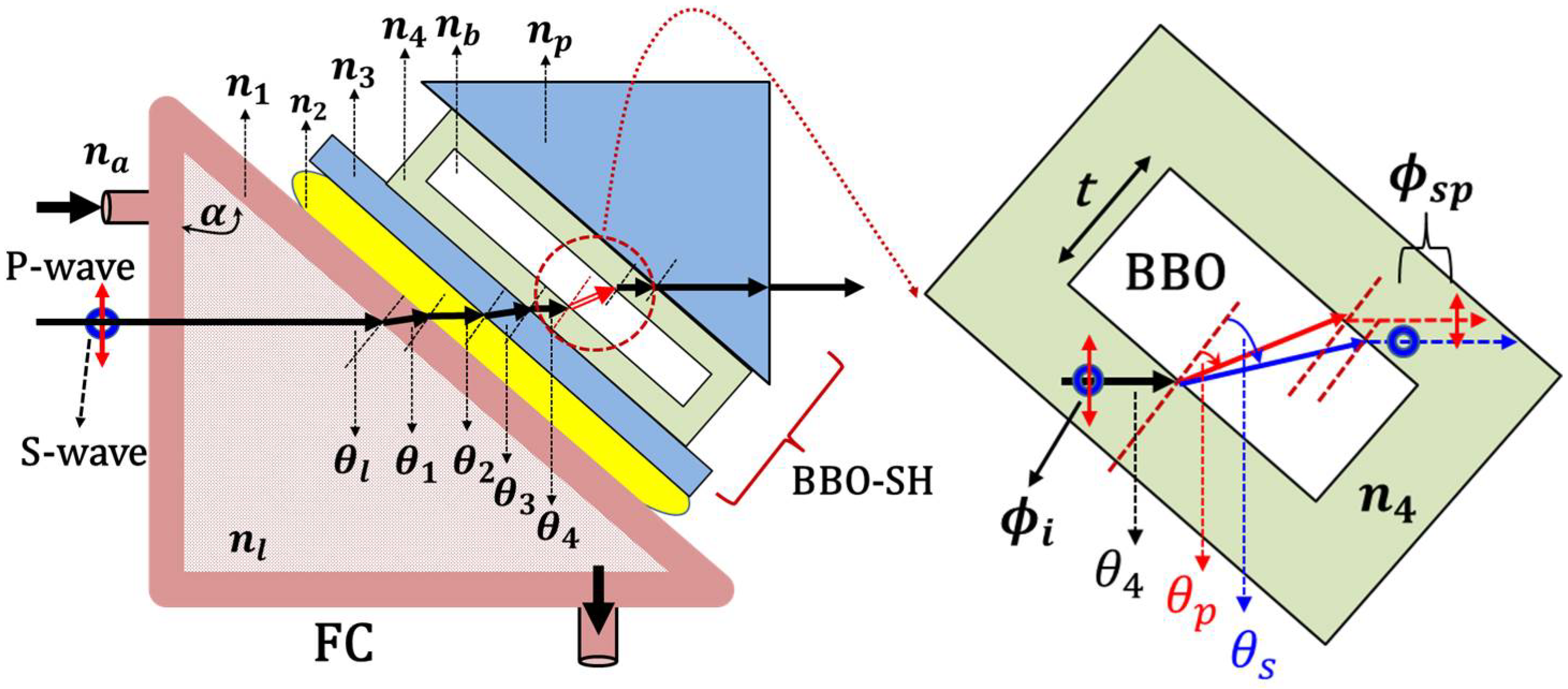
2. Measurement Principles
3. Non-Invasive Measurement System
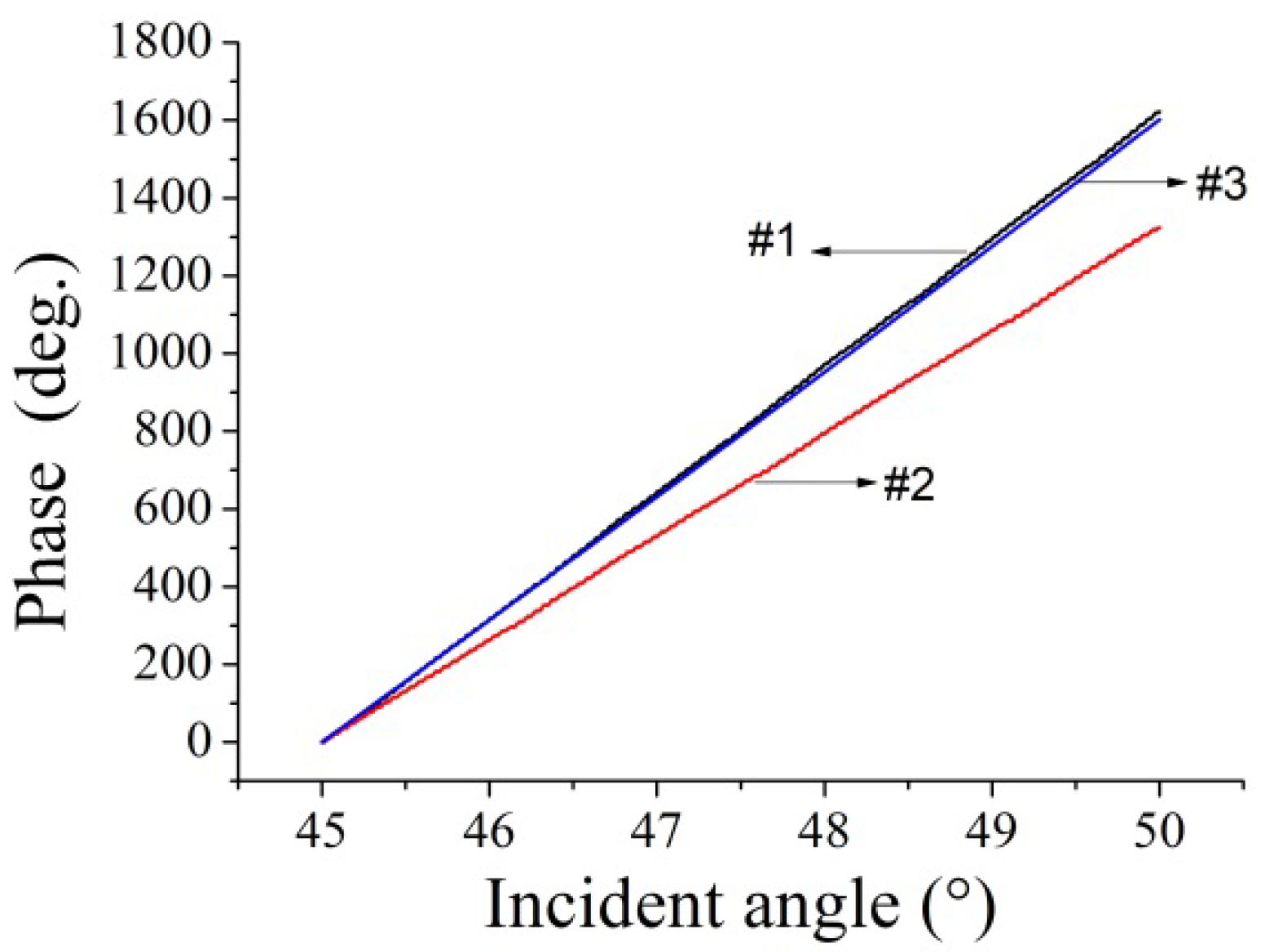
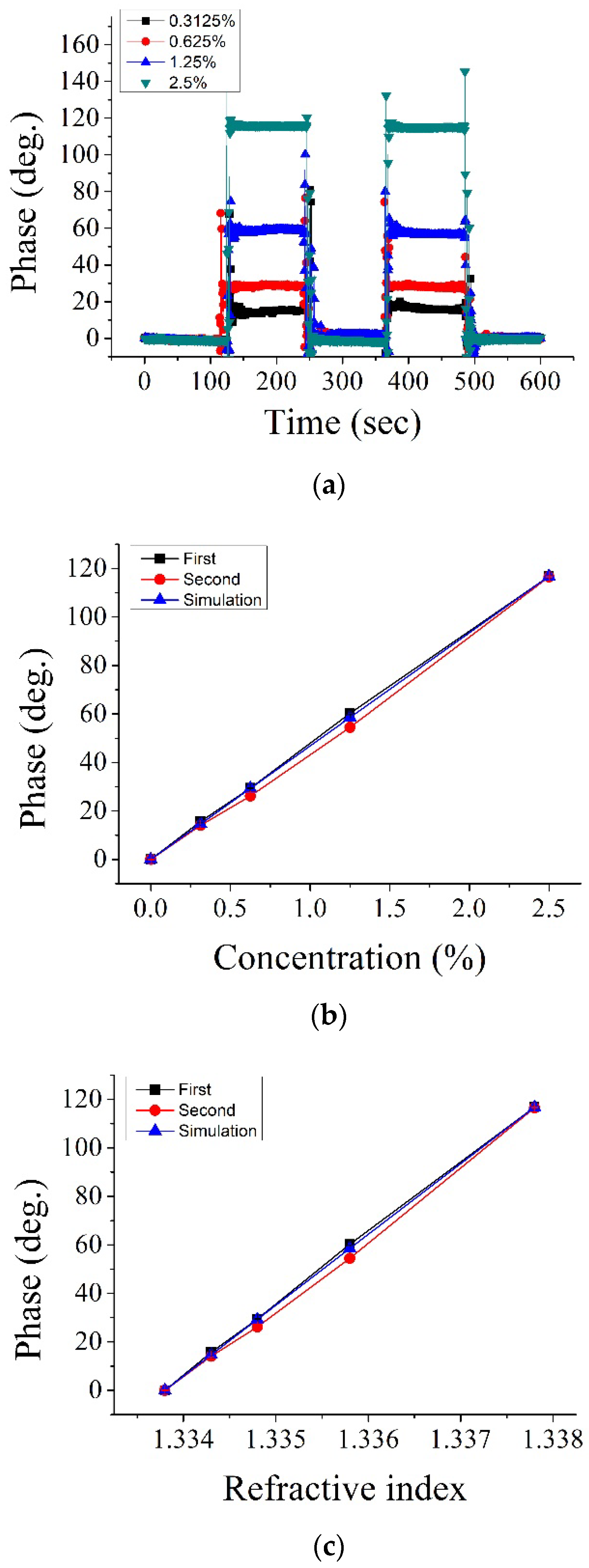
4. Results and Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laskar, J.M.; Kumar, P.S.; Herminghaus, S.; Daniels, K.E.; Schröter, M. High refractive index immersion liquid for superresolution 3D imaging using sapphire-based aplanatic numerical aperture increasing lens optics. Appl. Opt. 2016, 55, 3165–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Muntzeck, M.; Arcos, T.; Grundmeier, G.; Wilhelm, R.; Wagner, T. Determination of the refractive indices of ionic liquids by ellipsometry, and their application as immersion liquids. Appl. Opt. 2018, 57, 9215–9222. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Y.; Li, X.; Zhou, H.; Hong, X.; Geng, Y. Fiber-optic urine specific gravity sensor based on surface plasmon resonance. Sens. Actuators B Chem. 2016, 226, 412–418. [Google Scholar] [CrossRef]
- Burton, C.; Shi, H.; Ma, Y. Normalization of urinary pteridines by urine specific gravity for early cancer detection. Clin. Chim. Acta 2014, 435, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Electron Machine Corporation. Available online: https://electronmachine.com/ (accessed on 28 October 2022).
- Ameta, R.K.; Singh, M.; Kale, R.K. Comparative study of density, sound velocity and refractive index for (water+alkali metal) phosphates aqueous systems at T = (298.15,303.15, and 308.15) K. J. Chem. Thermodyn. 2013, 60, 159–168. [Google Scholar] [CrossRef]
- Sparks, D.; Smith, R.; Straayer, M.; Cripe, J.; Schneider, R.; Chimbayo, A.; Anasari, S.; Najafi, N. Measurement of density and chemical concentration using a microfluidic chip. Lab Chip 2003, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wu, Y.; Rohani, S. Simultaneous measurement of solution concentration and slurry density by Raman spectroscopy with artificial neural network. Cryst. Growth Des. 2020, 20, 1752–1759. [Google Scholar] [CrossRef]
- Toledo, J.; Ruiz-Díez, V.; Velasco, J.; Hernando-García, J.; Sánchez-Rojas, J.L. 3D-Printed Liquid Cell Resonator with Piezoelectric Actuation for In-Line Density-Viscosity Measurements. Sensors 2021, 21, 7654. [Google Scholar] [CrossRef]
- Yasuda, K.; Bando, Y.; Yamaguchi, S.; Nakamura, M.; Oda, A.; Kawase, Y. Analysis of concentration characteristics in ultrasonic atomization by droplet diameter distribution. Ultrason. Sonochemistry 2005, 12, 37–41. [Google Scholar] [CrossRef]
- Fan, M.; Zhou, R.; Jiang, H.; Zhou, G. Effect of liquid conductivity on optical and electric performances of the electrowetting display system with a thick dielectric layer. Results Phys. 2020, 16, 102904. [Google Scholar] [CrossRef]
- Smith, K.C.; Garza, A. Using Conductivity Measurements To Determine the Identities and Concentrations of Unknown Acids: An Inquiry Laboratory Experiment. J. Chem. Educ. 2015, 92, 1373–1377. [Google Scholar] [CrossRef]
- Kumar, P.K.; Kabanda, M.M.; Bahadur, I.; Venkatesu, P.; Ebenso, E.E. Influence of temperature and concentration on the molecular interactions of pyrrolidinium-based ionic liquid with water and alcohols: An experimental and DFT studies. J. Mol. Liq. 2022, 360, 119554. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Tovar-Lopez, F.J.; Scott, J.; Ghorbani, K. Differential Microwave Sensor for Characterization of Glycerol-Water Solutions. Sens. Actuators B Chem. 2020, 321, 128561. [Google Scholar] [CrossRef]
- Baghelani, M.; Hosseini, N.; Daneshmand, M. Non-contact real-time water and brine concentration monitoring in crude oil based on multi-variable analysis of microwave resonators. Measurement 2021, 177, 109286. [Google Scholar] [CrossRef]
- Sharma, P.; Lao, L.; Falcone, G. A microwave cavity resonator sensor for water-in-oil measurements. Sens. Actuators B Chem. 2018, 262, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.K.; Chamuah, N.; Sarkar, D.; Bezboruah, T. Non-Intrusive Technique for Measuring Refractive Index of Clear and Transparent Liquids. IEEE Sens. J. 2014, 14, 313–314. [Google Scholar] [CrossRef]
- Vilitis, O.; Shipkovs, P.; Merkulov, D. Determining the refractive index of liquids using a cylindrical cuvette. Meas. Sci. Technol. 2009, 20, 117001. [Google Scholar] [CrossRef]
- Jiménez-Márquez, F.; Vázquez, J.; Úbeda, J.; Sánchez-Rojas, J.L. Temperature dependence of grape must refractive index and its application to winemaking monitoring. Sens. Actuators B Chem. 2016, 225, 121–127. [Google Scholar] [CrossRef]
- Huang, L.; Glebov, A.L.; Aoki, S.; Yokouchi, K. A Novel Method to Measure Refractive Index of Liquid and Curable Liquid Substances. IEEE Photonics Technol. Lett. 2004, 16, 1137–1139. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, H.; Zhong, L.; Li, J.; Li, B.; Tian, J.; Lu, X. Dynamic refractive index distribution measurement of dynamic process by combining dual-channel simultaneous phase-shifting interferometry and total internal reflection. Sci. Rep. 2018, 8, 15231. [Google Scholar] [CrossRef]
- Yuan, B.; Tong, S.; Zhang, X.; Li, L.; Wang, C. Automatic monitoring refractive index variations of transient solution during electrochemical reactions. Measurement 2017, 98, 10–16. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Ishikura, H.; Radchenko, A.; Arreguin-Campos, R.; Rogosic, R.; Heidt, B.; Monroy, K.J.; Peeters, M.; Diliën, H.; Eersels, K.; et al. Rapid Colorimetric Screening of Elevated Phosphate in Urine: A Charge-Transfer Interaction. ACS Omega 2020, 5, 21054–21066. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Chin, L.K.; Ser, W.; Chen, H.F.; Hsieh, C.M.; Lee, C.H.; Sung, K.B.; Ayi, T.C.; Yap, P.H.; Liedberg, B.; et al. Cell refractive index for cell biology and disease diagnosis: Past, present and future. Lab Chip 2016, 16, 634–644. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, K.; Wang, C.; Zhao, Y.; Zhao, J.; Zhang, L. Temperature-calibrated high-precision refractometer using a tilted fiber Bragg grating. Opt. Express 2017, 25, 25910. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Semenova, Y.; Wang, P.; Farrell, G. High sensitivity SMS fiber structure based refractometer--analysis and experiment. Opt. Express 2011, 19, 7937–7944. [Google Scholar] [CrossRef]
- Sudas, D.P.; Zakharov, L.Y.; Jitov, V.A.; Golant, K.M. Silicon Oxynitride Thin Film Coating to Lossy Mode Resonance Fiber-Optic Refractometer. Sensors 2022, 22, 3665. [Google Scholar] [CrossRef]
- Torii, T.; Chiba, H.; Tanabe, T.; Oyama, Y. Measurements of glucose concentration in aqueous solutions using reflected THz radiation for applications to a novel sub-THz radiation non-invasive blood sugar measurement method. Digit. Heath 2017, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, H.; Tang, M.; Huang, J.; Liu, W.; Done, J.; Chen, X.; Fu, W.; Zhang, Y. The medical application of terahertz technology in non-invasive detection of cells and tissues: Opportunities and challenges. RSC Adv. 2019, 9, 9354–9363. [Google Scholar]
- Villar, A.; Lohrmann, A.; Ling, A. Experimental entangled photon pair generation using crystals with parallel optical axes. Opt. Express 2018, 26, 12396–12402. [Google Scholar] [CrossRef]
- Fu, Q.; Hanrahan, N.; Xu, L.; Lane, S.; Lin, D.; Jung, Y.; Mahajan, S.; Richardson, D.J. High-power, high-efficiency, all-fiberized-laser-pumped, 260-nm, deep-UV laser for bacterial deactivation. Opt. Express 2021, 29, 42485–42494. [Google Scholar] [CrossRef]
- Dabu, R. Femtosecond Laser Pulses Amplification in Crystals. Crystals 2019, 9, 347. [Google Scholar] [CrossRef]
- Nikolaev, N.A.; Andreev, Y.M.; Antsygin, V.D.; Bekker, T.B.; Ezhov, D.M.; Kokh, A.E.; Kokh, K.A.; Lanskii, G.V.; Mamrashev, A.A.; Svetlichnyi, V.A. Optical properties of β-BBO and potential for THz applications. J. Phys. Conf. Ser. 2018, 951, 012003. [Google Scholar] [CrossRef]
- Li, C.; Shen, X.; Zeng, R. Optical electric-field sensor based on angular optical bias using singleβ-BaB2O4 crystal. Appl. Opt. 2013, 52, 7580–7585. [Google Scholar] [CrossRef]
- Goldberg, L.; Cole, B.; Mcintoshi, C.; King, V.; Hays, A.D.; Chinn, S.R. Narrow-band 1 W source at 257 nm using frequency quadrupled passively Q-switched Yb:YAG laser. Opt. Express 2016, 24, 17397–17405. [Google Scholar] [CrossRef]
- Twu, R.C.; Lin, B.L. Development and investigation of birefringent Beta-Barium borate for optical angle sensor. Opt. Laser Technol. 2022, 155, 108369. [Google Scholar] [CrossRef]
- Goh, S.H.; Sheppard, C.J.R. High aperture focusing through a spherical interface: Application to refractive solid immersion lens (RSIL) for subsurface imaging. Opt. Commun. 2009, 282, 1036–1041. [Google Scholar] [CrossRef]
- Twu, R.C.; Wang, C.S. A Birefringent-Refraction Transducer for Measuring Angular Displacement Based on Heterodyne Interferometry. Appl. Sci. 2016, 6, 208. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twu, R.-C.; Sun, Y.-R. Fabrication of Beta-Barium Borate Sensing Head for Non-Invasive Measurement of Fluidic Concentration Variations. Sensors 2022, 22, 9566. https://doi.org/10.3390/s22249566
Twu R-C, Sun Y-R. Fabrication of Beta-Barium Borate Sensing Head for Non-Invasive Measurement of Fluidic Concentration Variations. Sensors. 2022; 22(24):9566. https://doi.org/10.3390/s22249566
Chicago/Turabian StyleTwu, Ruey-Ching, and Yi-Ren Sun. 2022. "Fabrication of Beta-Barium Borate Sensing Head for Non-Invasive Measurement of Fluidic Concentration Variations" Sensors 22, no. 24: 9566. https://doi.org/10.3390/s22249566
APA StyleTwu, R.-C., & Sun, Y.-R. (2022). Fabrication of Beta-Barium Borate Sensing Head for Non-Invasive Measurement of Fluidic Concentration Variations. Sensors, 22(24), 9566. https://doi.org/10.3390/s22249566





