Two-Photon Imaging for Non-Invasive Corneal Examination
Abstract
1. Introduction
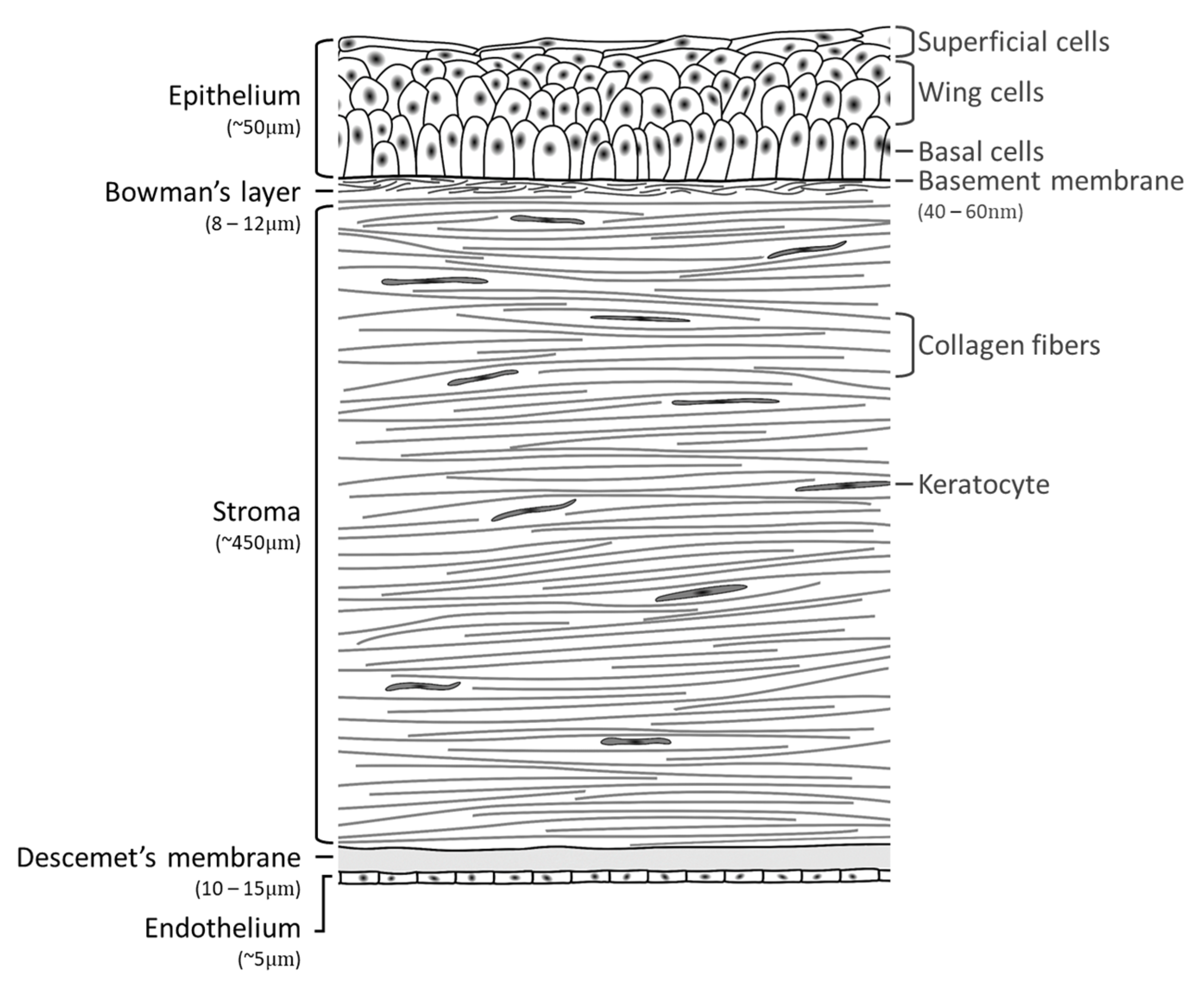
2. Cornea Anatomy and Morphology
2.1. Epithelium
2.2. Bowman’s Layer
2.3. Corneal Stroma
2.4. Descemet’s Membrane
2.5. Endothelium
2.6. Corneal Nerves
3. Principles of Two-Photon Imaging
3.1. Two-Photon Excited Fluorescence
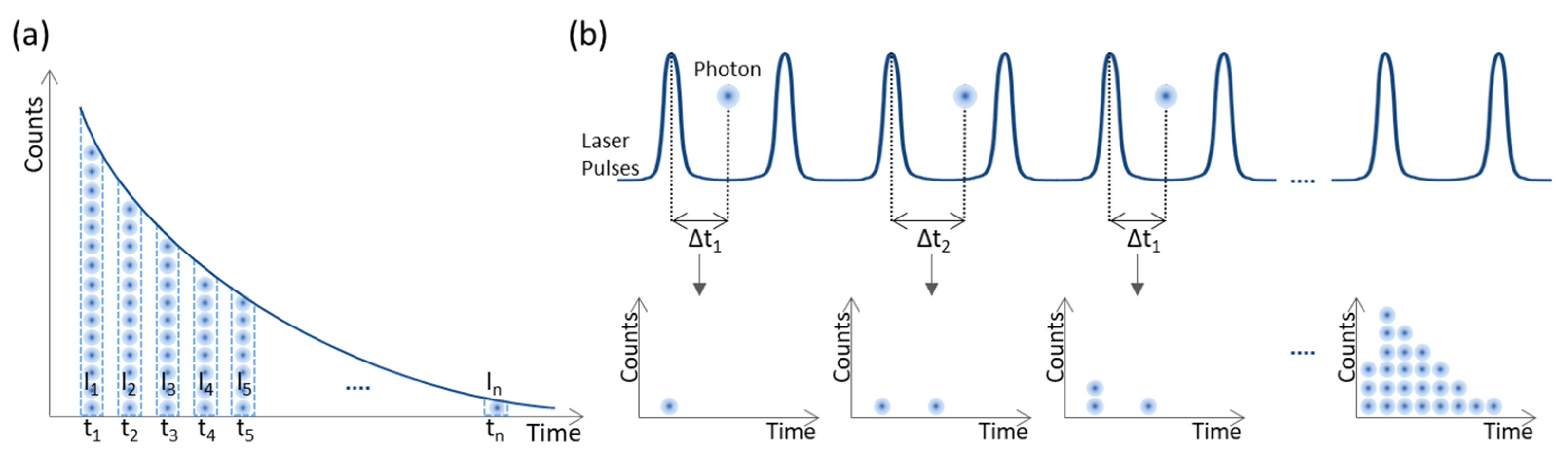
3.1.1. Fluorescence Lifetime Imaging
3.1.2. Corneal Endogenous Sources of Contrast
| Endogenous Fluorophore | NAD(PH) | Flavins | Collagen | ||
|---|---|---|---|---|---|
| Two-photon excitation maximum (nm) | 690–730 | 700–730 900 | 500–1700 | ||
| Emission max (nm) | Free | Bound | 525 | 380–520 | |
| 460 | 445 | ||||
| Two-photon ) | 0.02 | 0.08 | - | ||
| Fluorescence ) | Free | Bound | Free | Bound | 0.3 2.0–2.5 |
| 0.4 | 1.0–6.5 * | 0.1 | 2.0–3.0 | ||
| Reference Examples | [35,53,57,59,60] | [35,53] | [35,63,64,65,66] | ||
3.2. Second-Harmonic Generation
3.2.1. Second-Harmonic Generation in Biological Tissues
3.2.2. Quantification of Tissue Organization
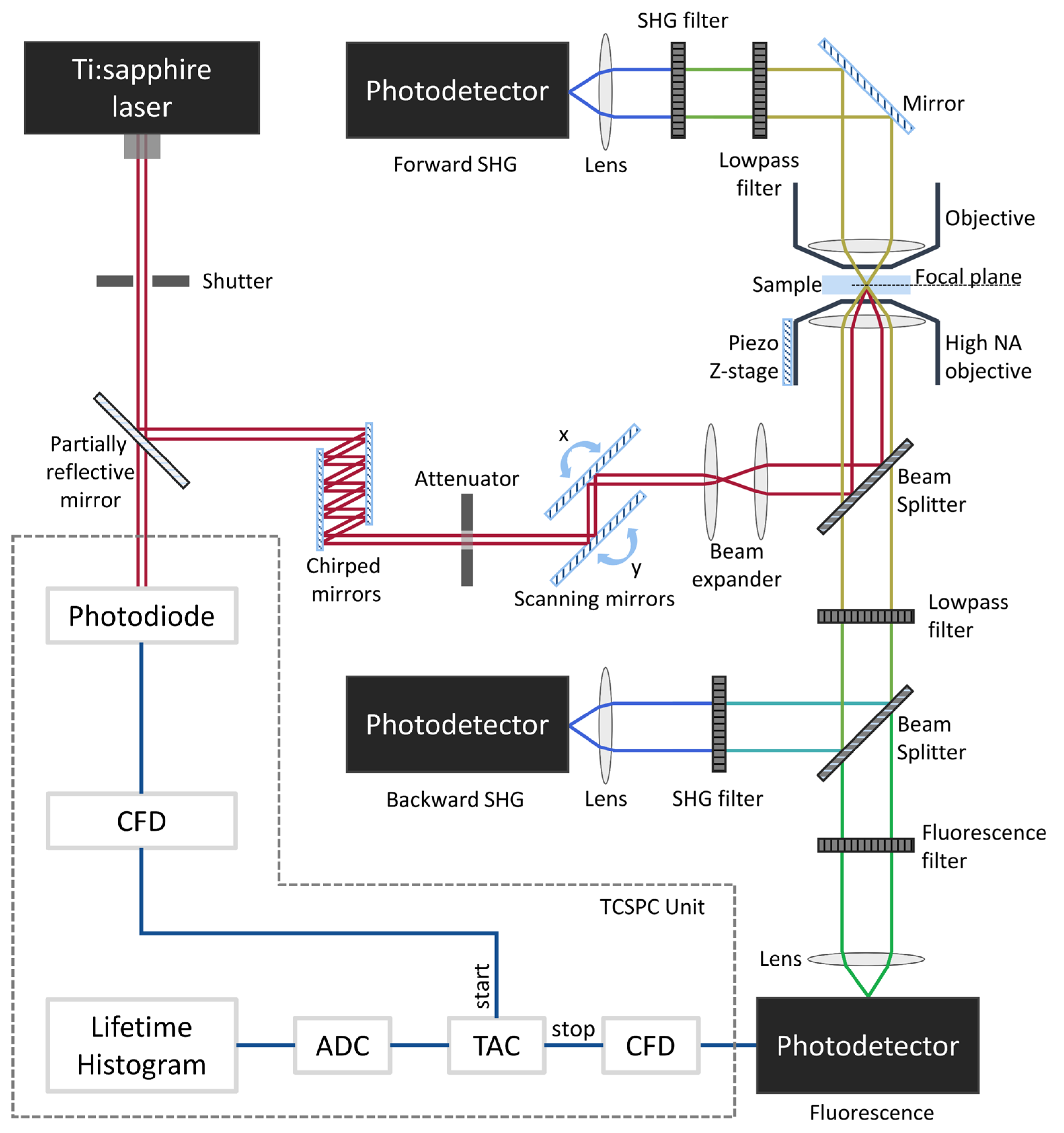
4. Two-Photon Imaging Microscope Setup/Instrumentation
Safety Considerations for In Vivo Two-Photon Imaging of the Cornea
5. Advances in Two-Photon Imaging
5.1. Excitation Sources
5.2. Photodetectors
5.3. Analysis Methods
6. Two-Photon Imaging of the Cornea
6.1. Two-Photon Imaging for Non-Invasive Corneal Evaluation
6.2. Clinical Applications of Two-Photon Imaging
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shapiro, B.L.; Cortés, D.E.; Chin, E.K.; Li, J.Y.; Werner, J.S.; Redenbo, E.; Mannis, M.J. High-Resolution Spectral Domain Anterior Segment Optical Coherence Tomography in Type 1 Boston Keratoprosthesis. Cornea 2013, 32, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jhanji, V.; Dorairaj, S.; Liu, A.; Lam, D.S.; Leung, C.K. Anterior Segment Optical Coherence Tomography and its Clinical Applications in Glaucoma. Curr. J. Glaucoma Pract. with DVD 2012, 6, 68–74. [Google Scholar] [CrossRef]
- Remington, L.A. Clinical Anatomy of the Visual System, 2nd ed.; Elsevier: St. Louis, MO, USA, 2005; ISBN 9780750674904. [Google Scholar]
- Lang, G.K.; Amann, J. Ophthalmology: A Short Textbook, 1st ed.; Thieme: Stuttgart, Germany, 2000; ISBN 9783131261618. [Google Scholar]
- Nishida, T.; Saika, S. Cornea and Sclera: Anatomy and Physiology. In Cornea—Fundamentals, Diagnosis and Management (Volume 1); Krachmer, J.H., Mannis, M.J., Holland, E.J., Eds.; Mosby/Elsevier: Maryland Heights, MO, USA, 2011; ISBN 9780323063876. [Google Scholar]
- Bye, L.; Modi, N.; Stanford, M. Basic Sciences for Ophthalmology, 1st ed.; Oxford University Press: Oxford, UK, 2013; ISBN 0199584990. [Google Scholar]
- Batista, A. Two-Photon Imaging of the Cornea Using Femtoseconf Laser Microscopes and Tomographs. Ph.D. Thesis, Saarland University, Saarbrücken, Germany, 2018. [Google Scholar]
- Duke-Elder, S. System of Ophthalmology; Mosby: St. Louis, MI, USA, 2011. [Google Scholar]
- Kabosova, A.; Azar, D.T.; Bannikov, G.A.; Campbell, K.P.; Durbeej, M.; Ghohestani, R.F.; Jones, J.C.R.; Kenney, M.C.; Koch, M.; Ninomiya, Y.; et al. Compositional differences between infant and adult human corneal basement membranes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4989–4999. [Google Scholar] [CrossRef]
- Marshall, J.; Trokel, S.L.; Rothery, S.; Krueger, R.R. Long-term Healing of the Central Cornea after Photorefractive Keratectomy Using an Excimer Laser. Ophthalmology 1988, 95, 1411–1421. [Google Scholar] [CrossRef]
- Goodman, G.L.; Trokel, S.L.; Stark, W.J.; Munnerlyn, C.R.; Green, W.R. Corneal healing following laser refractive keratectomy. Arch.Ophthalmol. 1989, 107, 1799–1803. [Google Scholar] [CrossRef]
- Amano, S.; Shimizu, K.; Tsubota, K. Corneal Epithelial Changes After Excimer Laser Photorefractive Keratectomy. Am. J. Ophthalmol. 1993, 115, 441–443. [Google Scholar] [CrossRef]
- Meek, K.M.; Boote, C. The organization of collagen in the corneal stroma. Exp. Eye Res. 2004, 78, 503–512. [Google Scholar] [CrossRef]
- Yue, B.Y.J.T.; Sugar, J.; Schrode, K. Collagen staining in corneal tissues. Curr. Eye Res. 2009, 5, 559–564. [Google Scholar] [CrossRef]
- Knupp, C.; Pinali, C.; Lewis, P.N.; Parfitt, G.J.; Young, R.D.; Meek, K.M.; Quantock, A.J. The Architecture of the Cornea and Structural Basis of Its Transparency. Adv. Protein Chem. Struct. Biol. 2009, 78, 25–49. [Google Scholar] [CrossRef]
- Giraud, J.P.; Pouliquen, Y.; Offret, G.; Payrau, P. Statistical morphometric studies in normal human and rabbit corneal stroma. Exp. Eye Res. 1975, 21, 221–229. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alkanaan, A.; Barsotti, R.; Kirat, O.; Khan, A.; Almubrad, T.; Akhtar, S. Collagen fibrils and proteoglycans of peripheral and central stroma of the keratoconus cornea—Ultrastructure and 3D transmission electron tomography. Sci. Rep. 2019, 9, 19963. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.J.; Pels, E.; Vrensen, G.F. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br. J. Ophthalmol. 2001, 85, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M. The structure and transparency of the cornea. J. Physiol. 1957, 136, 263–286. [Google Scholar] [CrossRef]
- Meek, K.M.; Leonard, D.W. Ultrastructure of the corneal stroma: A comparative study. Biophys. J. 1993, 64, 273–280. [Google Scholar] [CrossRef]
- Murphy, C.; Alvarado, J.; Juster, R. Prenatal and postnatal growth of the human Descemet’s membrane. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1402–1415. [Google Scholar]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef]
- Waring III, G.O.; Bourne, W.M.; Edelhauser, H.F.; Kenyon, K.R. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 1982, 89, 531–590. [Google Scholar]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef]
- He, J.; Bazan, N.G.; Bazan, H.E.P. Mapping the entire human corneal nerve architecture. Exp. Eye Res. 2010, 91, 513–523. [Google Scholar] [CrossRef]
- Oliveira-Soto, L.; Efron, N. Morphology of corneal nerves using confocal microscopy. Cornea 2001, 20, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Guthoff, R.F.; Baudouin, C.; Stave, J. Confocal Laser Scanning In Vivo Microscopy. In Atlas of Confocal Laser Scanning In-Vivo Microscopy in Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 31–148. [Google Scholar]
- Patel, D.V.; McGhee, C.N.J. Mapping of the normal human corneal sub-basal nerve plexus by in vivo laser scanning confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4485–4488. [Google Scholar] [CrossRef]
- Müller, L.J.; Pels, L.; De Wolf, A.; Kliffen, M.; Vrensen, G.F.J.M. Ultrastructural analysis of human corneal nerves. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2506. [Google Scholar]
- Petropoulos, I.N.; Ponirakis, G.; Ferdousi, M.; Azmi, S.; Kalteniece, A.; Khan, A.; Gad, H.; Bashir, B.; Marshall, A.; Boulton, A.J.M.; et al. Corneal Confocal Microscopy: A Biomarker for Diabetic Peripheral Neuropathy. Clin. Ther. 2021, 43, 1457–1475. [Google Scholar] [CrossRef] [PubMed]
- Göppert-Mayer, M. Uber Elementarakte mit zwei Quantensprüngen. Ann. Phys. 1931, 401, 273–294. [Google Scholar] [CrossRef]
- Kaiser, W.; Garrett, C. Two-Photon Excitation in CaF2:Eu2+. Phys. Rev. Lett. 1961, 7, 229–231. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.; Webb, W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2007; ISBN 9780387463124. [Google Scholar]
- Anthony, N.; Guo, P.; Berland, K. Principles of Fluorescence for Quantitative Fluorescence Microscopy. In FLIM Microscopy in Biology and Medicine; Periasamy, A., Clegg, R., Eds.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- So, P.T.C.C.; Dong, C.Y.; Masters, B.R.; Berland, K.M. Two-photon Excitation Fluorescence Microscopy. Biomed. Photonics Handb. 2003, 2, 399–429. [Google Scholar] [CrossRef]
- König, K.; Uchugonova, A. Multiphoton Fluorescence Lifetime Imaging at the Dawn of Clinical Application. In FLIM Microscopy in Biology and Medicine; Periasamy, A., Clegg, R., Eds.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Diaspro, A.; Sheppard, C.J.R. Two-Photon Microscopy: Basic Principles and Architectures. In Confocal and Two-Photon Microscopy: Foundations, Applications, and Advances; Diaspro, A., Ed.; Wiley-Liss: Hoboken, NJ, USA, 2001; p. 567. ISBN 9780471409205. [Google Scholar]
- Herman, P.; Lin, H.-J.; Lakowicz, J.R. Lifetime-Based Imaging. In Biomedical Photonics Handbook; Vo-Dinh, T., Ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0-8493-1116-0. [Google Scholar]
- Clegg, R. Fluorescence Lifetime-Resolved Imaging—What, Why, How—A Prologue. In FLIM Microscopy in Biology and Medicine; Periasamy, A., Clegg, R., Eds.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420078909. [Google Scholar]
- Datta, R.; Heaster, T.M.; Sharick, J.T.; Gillette, A.A.; Skala, M.C. Fluorescence lifetime imaging microscopy: Fundamentals and advances in instrumentation, analysis, and applications. J. Biomed. Opt. 2020, 25, 1. [Google Scholar] [CrossRef]
- Spring, B.Q.; Clegg, R. Frequency-Domain FLIM. In FLIM Microscopy in Biology and Medicine; Periasamy, A., Clegg, R., Eds.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420078909. [Google Scholar]
- Tkachenko, N. V Optical Spectroscopy: Methods and Instrumentations, 1st ed.; Elsevier Science: London, UK, 2006; ISBN 9780080461724. [Google Scholar]
- Becker, W. Introduction to Multi-dimensional TCSPC. In Advanced Time-Correlated Single Photon Counting Applications; Becker, W., Ed.; Springer Series in Chemical Physics; Springer International Publishing: New York, NY, USA, 2015; ISBN 978-3-319-14928-8. [Google Scholar]
- Becker, W.; Bergmann, A.; Schweitzer, D.; Hammer, M. Time- and Wavelength-Resolved Autofluorescence Detection by Multi-Dimensional TCSPC. In Proceedings of the Diagnostic Optical Spectroscopy in Biomedicine III; Mycek, A., Ed.; Optical Society of America: Washington, DC, USA, 2005; p. ThE2. [Google Scholar]
- Becker, W.; Bergmann, A.; Hink, M.A.; König, K.; Benndorf, K.; Biskup, C. Fluorescence lifetime imaging by time-correlated single-photon counting. Microsc. Res. Tech. 2004, 63, 58–66. [Google Scholar] [CrossRef]
- Schweitzer, D.; Hammer, M. Fluorescence Lifetime Imaging in Ophthalmology. In Advanced Time-Correlated Single Photon Counting Applications; Becker, W., Ed.; Springer Series in Chemical Physics; Springer International Publishing: New York, NY, USA, 2015; ISBN 978-3-319-14928-8. [Google Scholar]
- Becker, W.; Bergmann, A. Lifetime-Resolved Imaging in Nonlinear Microscopy. In Handbook of Biomedical Nonlinear Optical Microscopy; Masters, B.R., So, P., Eds.; Oxford University Press: Oxford, UK, 2008; ISBN 9780198036821. [Google Scholar]
- Digman, M.A.; Caiolfa, V.R.; Zamai, M.; Gratton, E. The phasor approach to fluorescence lifetime imaging analysis. Biophys. J. 2008, 94, L14–L16. [Google Scholar] [CrossRef]
- Stringari, C.; Cinquin, A.; Cinquin, O.; Digman, M.A.; Donovan, P.J.; Gratton, E. Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 13582–13587. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, L.; Ranjit, S.; Jameson, D.M.; Gratton, E. The Phasor Plot: A Universal Circle to Advance Fluorescence Lifetime Analysis and Interpretation. Annu. Rev. Biophys. 2021, 50, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Georgakoudi, I.; Quinn, K.P. Optical imaging using endogenous contrast to assess metabolic state. Annu. Rev. Biomed. Eng. 2012, 14, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Karp, G. Aerobic Respiration and Mitochondrion. In Cell and Molecular Biology: Concepts and Experiments; Karp, G., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 173–205. ISBN 9780470483374. [Google Scholar]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G.; Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Raphael, A.P.; Lin, L.; Grice, J.E.; Soyer, H.P.; Breunig, H.G.; Roberts, M.S.; Prow, T.W. Applications of multiphoton tomographs and femtosecond laser nanoprocessing microscopes in drug delivery research. Adv. Drug Deliv. Rev. 2011, 63, 388–404. [Google Scholar] [CrossRef]
- Palero, J.A.; Bader, A.N.; de Bruijn, H.S.; der Ploeg van den Heuvel, A.; Sterenborg, H.J.; Gerritsen, H.C. In vivo monitoring of protein-bound and free NADH during ischemia by nonlinear spectral imaging microscopy. Biomed. Opt. Express 2011, 2, 1030–1039. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Szmacinski, H.; Nowaczyk, K.; Johnson, M.L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc. Natl. Acad. Sci. USA 1992, 89, 1271–1275. [Google Scholar] [CrossRef]
- Blacker, T.S.; Mann, Z.F.; Gale, J.E.; Ziegler, M.; Bain, A.J.; Szabadkai, G.; Duchen, M.R. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat. Commun. 2014, 5, 3936. [Google Scholar] [CrossRef]
- Vishwasrao, H.D.; Heikal, A.A.; Kasischke, K.A.; Webb, W.W. Conformational dependence of intracellular NADH on metabolic state revealed by associated fluorescence anisotropy. J. Biol. Chem. 2005, 280, 25119–25126. [Google Scholar] [CrossRef]
- Batey, D.W.; Eckhert, C.D. Analysis of flavins in ocular tissues of the rabbit. Invest. Ophthalmol. Vis. Sci. 1991, 32, 1981–1985. [Google Scholar] [PubMed]
- Masters, B.R.; Ghosh, A.K.; Wilson, J.; Matschinsky, F.M. Pyridine nucleotides and phosphorylation potential of rabbit corneal epithelium and endothelium. Invest. Ophthalmol. Vis. Sci. 1989, 30, 861–868. [Google Scholar] [PubMed]
- Sekar, S.K.V.; Bargigia, I.; Mora, A.D.; Taroni, P.; Ruggeri, A.; Tosi, A.; Pifferi, A.; Farina, A. Diffuse optical characterization of collagen absorption from 500 to 1700 nm. J. Biomed. Opt. 2017, 22, 015006. [Google Scholar] [CrossRef]
- Shi, L.; Lu, L.; Harvey, G.; Harvey, T.; Rodríguez-Contreras, A.; Alfano, R.R. Label-Free Fluorescence Spectroscopy for Detecting Key Biomolecules in Brain Tissue from a Mouse Model of Alzheimer’s Disease. Sci. Rep. 2017, 7, 2599. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, Y.; Li, D.; Qu, J.Y. Autofluorescence of epithelial tissue: Single-photon versus two-photon excitation. J. Biomed. Opt. 2008, 13, 54010. [Google Scholar] [CrossRef] [PubMed]
- Lutz, V.; Sattler, M.; Gallinat, S.; Wenck, H.; Poertner, R.; Fischer, F. Characterization of fibrillar collagen types using multi-dimensional multiphoton laser scanning microscopy. Int. J. Cosmet. Sci. 2012, 34, 209–215. [Google Scholar] [CrossRef]
- König, K. Clinical multiphoton tomography. J. Biophotonics 2008, 1, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Shirshin, E.A.; Gurfinkel, Y.I.; Priezzhev, A.V.; Fadeev, V.V.; Lademann, J.; Darvin, M.E. Two-photon autofluorescence lifetime imaging of human skin papillary dermis in vivo: Assessment of blood capillaries and structural proteins localization. Sci. Rep. 2017, 7, 1171. [Google Scholar] [CrossRef]
- Manickavasagam, A.; Hirvonen, L.M.; Melita, L.N.; Chong, E.Z.; Cook, R.J.; Bozec, L.; Festy, F. Multimodal optical characterisation of collagen photodegradation by femtosecond infrared laser ablation. Analyst 2014, 139, 6135–6143. [Google Scholar] [CrossRef]
- Lutz, V.; Sattler, M.; Gallinat, S.; Wenck, H.; Poertner, R.; Fischer, F. Impact of collagen crosslinking on the second harmonic generation signal and the fluorescence lifetime of collagen autofluorescence. Ski. Res. Technol. 2012, 18, 168–179. [Google Scholar] [CrossRef]
- Franken, P.A.; Hill, A.E.; Peters, C.W.; Weinreich, G. Generation of optical harmonics. Phys. Rev. Lett. 1961, 7, 118–119. [Google Scholar] [CrossRef]
- Fine, S.; Hansen, W.P. Optical second harmonic generation in biological systems. Appl. Opt. 1971, 10, 2350–2353. [Google Scholar] [CrossRef] [PubMed]
- Freund, I.; Deutsch, M. Second-harmonic microscopy of biological tissue. Opt. Lett. 1986, 11, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Gauderon, R.; Lukins, P.B.; Sheppard, C.J. Optimization of second-harmonic generation microscopy. Micron 2001, 32, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Kable, E.; Jones, A.; Fraser, I.; Manconi, F.; Gorrell, M.D. 3-Dimensional imaging of collagen using second harmonic generation. J. Struct. Biol. 2003, 141, 53–62. [Google Scholar] [CrossRef] [PubMed]
- New, G. Introduction to Nonlinear Optics, 1st ed.; Cambridge University Press: Cambridge, UK, 2011; ISBN 9780511975851. [Google Scholar]
- Boyd, R. Nonlinear Optics, 3rd ed.; Boyd, R., Ed.; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780123694706. [Google Scholar]
- Sutherland, R.L. Handbook of Nonlinear Optics, 2nd ed.; Sutherland, R.L., Ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780824742430. [Google Scholar]
- Yeh, A.T.; Choi, B.; Nelson, J.S.; Tromberg, B.J. Reversible dissociation of collagen in tissues. J. Invest. Dermatol. 2003, 121, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Theodossiou, T.A.; Thrasivoulou, C.; Ekwobi, C.; Becker, D.L. Second harmonic generation confocal microscopy of collagen type I from rat tendon cryosections. Biophys. J. 2006, 91, 4665–4677. [Google Scholar] [CrossRef]
- Tian, L.; Qu, J.; Guo, Z.; Jin, Y.; Meng, Y.; Deng, X. Microscopic second-harmonic generation emission direction in fibrillous collagen type I by quasi-phase-matching theory. J. Appl. Phys. 2010, 108, 54701–54709. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Dong, C.Y. Second harmonic generation microscopy: Principles and applications to disease diagnosis. Laser Photon. Rev. 2011, 5, 13–26. [Google Scholar] [CrossRef]
- McCance, K.L.; Huether, S.E.; Geneser, F.; Shoulders, M.D.; Raines, R.T.; Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P.; et al. Collagen Structure and Stability. PLoS ONE 2010, 78, 929–958. [Google Scholar] [CrossRef]
- Bianchini, P.; Diaspro, A. Three-dimensional (3D) backward and forward second harmonic generation (SHG) microscopy of biological tissues. J. Biophotonics 2008, 1, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Campagnola, P.J. Second-Harmonic Generation Imaging Microscopy of Structural Protein Arrays in Tissue. In Handbook of Biomedical Nonlinear Optical Microscopy; Masters, B.R., So, P., Eds.; Oxford University Press: Oxford, UK, 2008; ISBN 9780198036821. [Google Scholar]
- Mertz, J.; Moreaux, L. Second-harmonic generation by focused excitation of inhomogeneously distributed scatterers. Opt. Commun. 2001, 196, 325–330. [Google Scholar] [CrossRef]
- LaComb, R.; Nadiarnykh, O.; Townsend, S.S.; Campagnola, P.J. Phase matching considerations in second harmonic generation from tissues: Effects on emission directionality, conversion efficiency and observed morphology. Opt. Commun. 2008, 281, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Giese, G.; Bille, J.F. Second harmonic generation imaging of collagen fibrils in cornea and sclera. Opt. Express 2005, 13, 5791. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nadiarynkh, O.; Plotnikov, S.; Campagnola, P.J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc. 2012, 7, 654–669. [Google Scholar] [CrossRef]
- Williams, R.M.; Zipfel, W.R.; Webb, W.W. Interpreting Second-Harmonic Generation Images of Collagen I Fibrils. Biophys. J. 2005, 88, 1377–1386. [Google Scholar] [CrossRef]
- Mostaço-Guidolin, L.; Rosin, N.L.; Hackett, T.L. Imaging collagen in scar tissue: Developments in second harmonic generation microscopy for biomedical applications. Int. J. Mol. Sci. 2017, 18, 1772. [Google Scholar] [CrossRef]
- Cicchi, R.; Kapsokalyvas, D.; De Giorgi, V.; Maio, V.; Van Wiechen, A.; Massi, D.; Lotti, T.; Pavone, F.S. Scoring of collagen organization in healthy and diseased human dermis by multiphoton microscopy. J. Biophotonics 2010, 3, 34–43. [Google Scholar] [CrossRef]
- Zhuo, S.; Chen, J.; Wu, G.; Xie, S.; Zheng, L.; Jiang, X.; Zhu, X. Quantitatively linking collagen alteration and epithelial tumor progression by second harmonic generation microscopy. Appl. Phys. Lett. 2010, 96, 94–97. [Google Scholar] [CrossRef]
- Latour, G.; Gusachenko, I.; Kowalczuk, L.; Lamarre, I.; Schanne-Klein, M.-C. In vivo structural imaging of the cornea by polarization-resolved second harmonic microscopy. Biomed. Opt. Express 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Breunig, G.; Batista, A.; Uchugonova, A.; König, K.; Breunig, H.G.; Batista, A.; Uchugonova, A.; König, K. Motionless polarization-resolved second harmonic generation imaging of corneal collagen. Proc. SPIE 2015, 9329, 265–270. [Google Scholar]
- Ávila, F.; del Barco, O.; Bueno, J. Polarization response of second-harmonic images for different collagen spatial distributions. J. Biomed. Opt. 2016, 21, 066015. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.M.; Martínez-Ojeda, R.M.; Yago, I.; Ávila, F.J. Collagen Organization, Polarization Sensitivity and Image Quality in Human Corneas using Second Harmonic Generation Microscopy. Photonics 2022, 9, 672. [Google Scholar] [CrossRef]
- Hristu, R.; Stanciu, S.G.; Tranca, D.E.; Stanciu, G.A. Improved quantification of collagen anisotropy with polarization-resolved second harmonic generation microscopy. J. Biophotonics 2017, 10, 1171–1179. [Google Scholar] [CrossRef]
- Raoux, C.; Schmeltz, M.; Bied, M.; Alnawaiseh, M.; Hansen, U.; Latour, G.; Schanne-Klein, M.-C. Quantitative structural imaging of keratoconic corneas using polarization-resolved SHG microscopy. Biomed. Opt. Express 2021, 12, 4163. [Google Scholar] [CrossRef]
- Mostaço-Guidolin, L.B.; Ko, A.C.T.; Wang, F.; Xiang, B.; Hewko, M.; Tian, G.; Major, A.; Shiomi, M.; Sowa, M.G. Collagen morphology and texture analysis: From statistics to classification. Sci. Rep. 2013, 3, 2190. [Google Scholar] [CrossRef]
- Batista, A.; Breunig, H.G.; König, A.; Schindele, A.; Hager, T.; Seitz, B.; Morgado, A.M.; König, K. Assessment of Human Corneas Prior to Transplantation Using High-Resolution Two-Photon Imaging. Investig. Opthalmology Vis. Sci. 2018, 59, 176. [Google Scholar] [CrossRef]
- Ávila, F.; Bueno, J. Analysis and quantification of collagen organization with the structure tensor in second harmonic microscopy images of ocular tissues. Appl. Opt. 2015, 54, 9848–9854. [Google Scholar] [CrossRef]
- Bueno, J.; Ávila, F.; Lorenzo-Martín, E.; Gallego-Muñoz, P.; Carmen Martínez-García, M. Assessment of the corneal collagen organization after chemical burn using second harmonic generation microscopy. Biomed. Opt. Express 2021, 12, 756. [Google Scholar] [CrossRef]
- Bueno, J.; Ávila, F.; Mart, M.C. Quantitative Analysis of the Corneal Collagen Distribution after In Vivo Cross-Linking with Second Harmonic Microscopy. Biomed Res. Int. 2019, 2019, 3860498. [Google Scholar] [CrossRef]
- Ávila, F.J.; Artal, P.; Bueno, J.M. Quantitative Discrimination of Healthy and Diseased Corneas With Second Harmonic Generation Microscopy. Transl. Vis. Sci. Technol. 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.; Chen, W.-L.L.; Hsueh, C.M.; Ghazaryan, A.A.; Chen, S.J.; Ma, D.H.; Dong, C.Y.; Tan, H.Y.; Hui-Kang Ma, D.; Dong, C.Y.; et al. Fast fourier transform-based analysis of second-harmonic generation image in keratoconic cornea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3501–3507. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Lee, J.K.; Chuck, R.S. Second harmonic generation imaging analysis of collagen arrangement in human cornea. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Chang, Y.L.; Lo, W.; Hsueh, C.M.; Chen, W.L.; Ghazaryan, A.A.; Hu, P.S.; Young, T.H.; Chen, S.J.; Dong, C.Y. Characterizing the morphologic changes in collagen crosslinked-treated corneas by Fourier transform-second harmonic generation imaging. J. Cataract Refract. Surg. 2013, 39, 779–788. [Google Scholar] [CrossRef]
- Mega, Y.; Robitaille, M.; Zareian, R.; McLean, J.; Ruberti, J.; DiMarzio, C. Quantification of lamellar orientation in corneal collagen using second harmonic generation images. Opt. Lett. 2012, 37, 3312–3314. [Google Scholar] [CrossRef]
- Mega, Y.; McLean, J.; Zareian, R.; Karasek, S.; Lai, Z.; DiMarzio, C. The arrangement of fibrous collagen in cornea using second harmonic generation (SHG) microscopy. Proc. SPIE 2013, 8588, 269–274. [Google Scholar]
- Matteini, P.; Ratto, F.; Rossi, F.; Cicchi, R.; Stringari, C.; Kapsokalyvas, D.; Pavone, F.S.; Pini, R. Photothermally-induced disordered patterns of corneal collagen revealed by SHG imaging. Opt. Express 2009, 17, 4868–4878. [Google Scholar] [CrossRef]
- Lee, S.-L.; Chen, Y.-F.; Dong, C.-Y. Second harmonic generation imaging of chick corneal development. Ophthalmic Technol. XXIX 2019, 10858, 65. [Google Scholar] [CrossRef]
- Lee, S.-L.; Chen, Y.-F.; Dong, C.-Y. Probing superstructure of chicken corneal stroma by Fourier transform second harmonic generation microscopy. Proc. SPIE 2017, 10045, 162–165. [Google Scholar]
- Batista, A.; Breunig, G.; König, A.; Schindele, A.; Hager, T.; Seitz, B.; König, K. High-resolution, label-free two-photon imaging of diseased human corneas. JBO 2018, 23, 036002. [Google Scholar] [CrossRef]
- James, D.S.; Campagnola, P.J. Recent Advancements in Optical Harmonic Generation Microscopy: Applications and Perspectives. BME Front. 2021, 2021, 3973857. [Google Scholar] [CrossRef]
- Cicchi, R.; Sacconi, L.; Vanzi, F.; Pavone, F.S. How to build and SHG Apparatus. In Second Harmonic Generation Imaging: Series in Cellular and Clinical Imaging; Pavone, F.S., Campagnola, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Campagnola, P.J.; Millard, A.C.; Terasaki, M.; Hoppe, P.E.; Malone, C.J.; Mohler, W.A. Three-Dimensional High-Resolution Second-Harmonic Generation Imaging of Endogenous Structural Proteins in Biological Tissues. Biophys. J. 2002, 82, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Eichler, J.; Da Silva, L.B. Frequency doubling of ultrashort laser pulses in biological tissues. Appl. Opt. 1999, 38, 7145. [Google Scholar] [CrossRef] [PubMed]
- Apolonskiy, A. Femtosecond Ti: Sapphire Laser; Praktikum für fortgeschrittene Physikstudenten; LMU München: München, Germany, 2013. [Google Scholar]
- Fermann, M.E.; Galvanauskas, A.; Sucha, G. Ultrafast Lasers: Technology and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780824743499. [Google Scholar]
- Krueger, A. Ultrafast lasers in biophotonics. In Multiphoton Microscopy and Fluorescence Lifetime Imaging; König, K., Ed.; De Gruyter: Berlin, Germany, 2018; ISBN 9783110429985. [Google Scholar]
- Niemz, M.H. Interaction Mechanisms. Laser-Tissue Interact. 2002, 45–149. [Google Scholar] [CrossRef]
- Ziegelberger, G. Icnirp guidelines on limits of exposure to laser radiation of wavelengths between 180 nm and 1000 μm. Health Phys. 2013, 105, 271–295. [Google Scholar] [CrossRef]
- Ziegelberger, G.; Okuno, T.; Lund, J.; O’Hagan, J.; Schulmeister, K.; Sliney, D.; Stuck, B.; van Rongen, E.; Croft, R.; Feychting, M.; et al. Comments on the 2013 ICNIRP laser guidelines. Health Phys. 2020, 118, 543–548. [Google Scholar] [CrossRef]
- IEC 60825-1:2014; Safety of Laser Products—Part 1: Equipment Classification and Requirements. IEC: Geneva, Switzerland, 2014.
- American National Standard Institute. American National Standard for Safe Use of Lasers (ANSI Z136.1-2014); Laser Institute of America: Orlando, FL, USA, 2014; ISBN 978-0-912035-65-9. [Google Scholar]
- Sliney, D.; Aron-Rosa, D.; DeLori, F.; Fankhauser, F.; Landry, R.; Mainster, M.; Marshall, J.; Rassow, B.; Stuck, B.; Trokel, S.; et al. Adjustment of guidelines for exposure of the eye to optical radiation from ocular instruments: Statement from a task group of the International Commission on Non-Ionizing Radiation Protection (ICNIRP). Appl. Opt. 2005, 44, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Ávila, F.J.; Gambín, A.; Artal, P.; Bueno, J.M. In vivo two-photon microscopy of the human eye. Sci. Rep. 2019, 9, 10121. [Google Scholar] [CrossRef]
- Xu, C.; Wise, F.W. Recent advances in fibre lasers for nonlinear microscopy. Nat. Photonics 2013, 7, 875–882. [Google Scholar] [CrossRef]
- Kieu, K.; Renninger, W.H.; Chong, A.; Wise, F.W. Sub-100 fs pulses at watt-level powers from a dissipative-soliton fiber laser. Opt. Lett. 2009, 34, 593. [Google Scholar] [CrossRef]
- Liu, G.; Kieu, K.; Wise, F.W.; Chen, Z. Multiphoton microscopy system with a compact fiber-based femtosecond-pulse laser and handheld probe. J. Biophotonics 2011, 4, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Krolopp, Á.; Csákányi, A.; Haluszka, D.; Csáti, D.; Vass, L.; Kolonics, A.; Wikonkál, N.; Szipőcs, R. Handheld nonlinear microscope system comprising a 2 MHz repetition rate, mode-locked Yb-fiber laser for in vivo biomedical imaging. Biomed. Opt. Express 2016, 7, 3531–3542. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, F.; Qin, Y.; Peyghambarian, N.; Barton, J.K.; Kieu, K. Compact fiber-based multi-photon endoscope working at 1700 nm. Biomed. Opt. Express 2018, 9, 2326. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, X.; Liu, Q.; MacAulay, C.E.; Tang, S. Miniaturized multimodal multiphoton microscope for simultaneous two-photon and three-photon imaging with a dual-wavelength Er-doped fiber laser. Biomed. Opt. Express 2020, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Batista, A.; König, A.; Breunig, G. Multimodal multiphoton tomograph using a compact femtosecond fiber laser. In Proceedings of the Multiphoton Microscopy in the Biomedical Sciences XIX; Periasamy, A., So, P.T., König, K., Eds.; SPIE: Bellingham, WA, USA, 2019; p. 43. [Google Scholar]
- König, K.; Batista, A.; Zieger, M.; Kaatz, M.; Hänßle, H.; Fink, C.; Breunig, G. Clinical multimodal multiphoton tomography of pigmented skin lesions with an ultracompact femtosecond fiber laser. Proc. SPIE 2020, 11211, 29–36. [Google Scholar]
- Wang, K.; Liu, T.; Wu, J.; Horton, N.G.; Lin, C.P.; Xu, C. Three-color femtosecond source for simultaneous excitation of three fluorescent proteins in two-photon fluorescence microscopy. Biomed. Opt. Express 2012, 3, 1972–1977. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Greinert, R.; Kärtner, F.X.; Chang, G. Multimodal imaging platform for optical virtual skin biopsy enabled by a fiber-based two-color ultrafast laser source. Biomed. Opt. Express 2019, 10, 514–525. [Google Scholar] [CrossRef]
- Akhoundi, F.; Peyghambarian, N. Single-cavity dual-wavelength all-fiber femtosecond laser for multimodal multiphoton microscopy. Biomed. Opt. Express 2020, 11, 2761. [Google Scholar] [CrossRef]
- Becker, W.; Su, B.; Holub, O.; Weisshart, K. FLIM and FCS detection in laser-scanning microscopes: Increased efficiency by GaAsP hybrid detectors. Microsc. Res. Tech. 2010, 74, 804–811. [Google Scholar] [CrossRef]
- Hirvonen, L.M.; Suhling, K. Fast Timing Techniques in FLIM Applications. Front. Phys. 2020, 8, 161. [Google Scholar] [CrossRef]
- Yokota, H.; Fukasawa, A.; Hirano, M.; Ide, T. Low-Light Photodetectors for Fluorescence Microscopy. Appl. Sci. 2021, 11, 2773. [Google Scholar] [CrossRef]
- Caccia, M.; Nardo, L.; Santoro, R.; Schaffhauser, D. Silicon Photomultipliers and SPAD imagers in biophotonics: Advances and perspectives. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2019, 926, 101–117. [Google Scholar] [CrossRef]
- Bruschini, C.; Homulle, H.; Antolovic, I.M.; Burri, S.; Charbon, E. Single-photon avalanche diode imagers in biophotonics: Review and outlook. Light Sci. Appl. 2019, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, D.; Becker, W.; Niu, J.; Yu, B.; Liu, L.; Qu, J. Fast fluorescence lifetime imaging techniques: A review on challenge and development. J. Innov. Opt. Health Sci. 2019, 12, 1930003. [Google Scholar] [CrossRef]
- Korzh, B.; Zhao, Q.Y.; Allmaras, J.P.; Frasca, S.; Autry, T.M.; Bersin, E.A.; Beyer, A.D.; Briggs, R.M.; Bumble, B.; Colangelo, M.; et al. Demonstration of sub-3 ps temporal resolution with a superconducting nanowire single-photon detector. Nat. Photonics 2020, 14, 250–255. [Google Scholar] [CrossRef]
- Wu, G.; Nowotny, T.; Zhang, Y.; Yu, H.-Q.; Li, D.D.-U. Artificial neural network approaches for fluorescence lifetime imaging techniques. Opt. Lett. 2016, 41, 2561. [Google Scholar] [CrossRef]
- Yao, R.; Ochoa, M.; Yan, P.; Intes, X. Net-FLICS: Fast quantitative wide-field fluorescence lifetime imaging with compressed sensing—A deep learning approach. Light Sci. Appl. 2019, 8, 26. [Google Scholar] [CrossRef]
- Smith, J.T.; Yao, R.; Sinsuebphon, N.; Rudkouskaya, A.; Un, N.; Mazurkiewicz, J.; Barroso, M.; Yan, P.; Intes, X. Fast fit-free analysis of fluorescence lifetime imaging via deep learning. Proc. Natl. Acad. Sci. USA 2019, 116, 24019–24030. [Google Scholar] [CrossRef]
- Smith, J.T.; Ochoa, M.; Intes, X. UNMIX-ME: Spectral and lifetime fluorescence unmixing via deep learning. Biomed. Opt. Express 2020, 11, 3857. [Google Scholar] [CrossRef]
- Guimarães, P.; Batista, A.; Zieger, M.; Kaatz, M.; König, K. Artificial Intelligence in Multiphoton Tomography: Atopic Dermatitis Diagnosis. Sci. Rep. 2020, 10, 7968. [Google Scholar] [CrossRef]
- Liang, L.; Liu, M.; Sun, W. A deep learning approach to estimate chemically-treated collagenous tissue nonlinear anisotropic stress-strain responses from microscopy images. Acta Biomater. 2017, 63, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Judd, N.B.; Smith, J.T.; Icaza, M.; Mukherjee, S.; Jain, M.; Gallagher, R.M.; Szeligowski, R.V.; Wu, B. A pilot study for distinguishing chromophobe renal cell carcinoma and oncocytoma using second harmonic generation imaging and convolutional neural network analysis of collagen fibrillar structure. In Proceedings of the Optical Biopsy XVI: Toward Real-Time Spectroscopic Imaging and Diagnosis; Alfano, R.R., Demos, S.G., Eds.; SPIE: Bellingham, WA, USA, 2018; Volume 10489, p. 44. [Google Scholar]
- Mirsanaye, K.; Uribe Castaño, L.; Kamaliddin, Y.; Golaraei, A.; Augulis, R.; Kontenis, L.; Done, S.J.; Žurauskas, E.; Stambolic, V.; Wilson, B.C.; et al. Machine learning-enabled cancer diagnostics with widefield polarimetric second-harmonic generation microscopy. Sci. Rep. 2022, 12, 10290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, W.; Chen, X.; Wang, X.; Chen, G.; Zhu, X. Quantification of scar collagen texture and prediction of scar development via second harmonic generation images and a generative adversarial network. Biomed. Opt. Express 2021, 12, 5305. [Google Scholar] [CrossRef] [PubMed]
- Woessner, A.E.; Quinn, K.P. Improved Segmentation of Collagen Second Harmonic Generation Images with a Deep Learning Convolutional Neural Network. J. Biophotonics 2022, e202200191. [Google Scholar] [CrossRef]
- Piston, D.W.; Masters, B.R.; Webb, W.W. Three-dimensionally resolved NAD(P)H cellular metabolic redox imaging of the in situ cornea with two-photon excitation laser scanning microscopy. J. Microsc. 1995, 178, 20–27. [Google Scholar] [CrossRef]
- Nissen, P.; Lieberman, M.; Fischbarg, J.; Chance, B. Altered redox states in corneal epithelium and endothelium: NADH fluorescence in rat and rabbit ocular tissue. Exp. Eye Res. 1980, 30, 691–697. [Google Scholar] [CrossRef]
- Laing, R.A.; Fischbarg, J.; Chance, B. Noninvasive measurements of pyridine nucleotide fluorescence from the cornea. Invest. Ophthalmol. Vis. Sci. 1980, 19, 96–102. [Google Scholar]
- Masters, B.R. Noninvasive redox fluorometry: How light can be used to monitor alterations of corneal mitochondrial function. Curr. Eye Res. 1984, 3, 23–26. [Google Scholar] [CrossRef]
- Shimazaki, J.; Tsubota, K.; Hayashi, K.; Kenyon, K.R.; Laing, R.A. Distribution of autofluorescence in the rabbit corneal epithelium. Ophthalmic Res. 1993, 25, 220–225. [Google Scholar] [CrossRef]
- Stolwijk, T.R.; van Best, J.A.; Boot, J.P.; Oosterhuis, J.A. Corneal autofluorescence in diabetic and penetrating keratoplasty patients as measured by fluorophotometry. Exp. Eye Res. 1990, 51, 403–409. [Google Scholar] [CrossRef]
- Van Schaik, H.J.; Coppens, J.; Van Den Berg, T.J.T.P.; Van Best, J.A. Autofluorescence distribution along the corneal axis in diabetic and healthy humans. Exp. Eye Res. 1999, 69, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Masters, B.R.; Kriete, A.; Kukulies, J. Ultraviolet confocal fluorescence microscopy of the in vitro cornea: Redox metabolic imaging. Appl. Opt. 1993, 32, 592–596. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Krauss, O.; Riemann, I. Intratissue surgery with 80 MHz nanojoule femtosecond laser pulses in the near infrared. Opt. Express 2002, 10, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Yeh, A.T.; Nassif, N.; Zoumi, A.; Tromberg, B.J. Selective corneal imaging using combined second-harmonic generation and two-photon excited fluorescence. Opt. Lett. 2002, 27, 2082–2084. [Google Scholar] [CrossRef]
- König, K. High-resolution multiphoton imaging and nanosurgery of the cornea using femtosecond laser pulses. In Lasers in Ophthalmology: Basic, Diagnostic, and Surgical Aspects: A Review; Fankhauser, F., Kwasniewska, S., Eds.; Kugler Publications: Amsterdam, The Netherlands, 2003; ISBN 9789062991891. [Google Scholar]
- Teng, S.W.; Tan, H.Y.; Peng, J.L.; Lin, H.H.; Kim, K.H.; Lo, W.; Sun, Y.; Lin, W.C.; Lin, S.J.; Jee, S.H.; et al. Multiphoton autofluorescence and second-harmonic generation imaging of the ex vivo porcine eye. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1216–1224. [Google Scholar] [CrossRef]
- Vohnsen, B.; Artal, P. Second-harmonic microscopy of ex vivo porcine corneas. J. Microsc. 2008, 232, 158–163. [Google Scholar] [CrossRef]
- Masihzadeh, O.; Lei, T.C.; Ammar, D.A.; Kahook, M.Y.; Gibson, E.A. A multiphoton microscope platform for imaging the mouse eye. Mol. Vis. 2012, 18, 1840–1848. [Google Scholar]
- König, K.; Wang, B.; Krauss, O.; Riemann, I.; Schubert, H.; Kirste, S.; Fischer, P. First in vivo animal studies on intraocular nanosurgery and multiphoton tomography with low-energy 80-MHz near-infrared femtosecond laser pulses. Proc. SPIE 2004, 5314, 262–269. [Google Scholar]
- Wang, B.; Halbhuber, K.-J.; Riemann, I.; König, K. In-vivo corneal nonlinear optical tomography based on second harmonic and multiphoton autofluorescence imaging induced by near-infrared femtosecond lasers with rabbits. Proc. SPIE 2005, 5964, 199–209. [Google Scholar]
- Wang, B.G.; Halbhuber, K.J. Corneal multiphoton microscopy and intratissue optical nanosurgery by nanojoule femtosecond near-infrared pulsed lasers. Ann. Anat. 2006, 188, 395–409. [Google Scholar] [CrossRef]
- Robertson, D.M.; Rogers, N.A.; Petroll, W.M.; Zhu, M. Second harmonic generation imaging of corneal stroma after infection by Pseudomonas aeruginosa. Sci. Rep. 2017, 7, 46116. [Google Scholar] [CrossRef] [PubMed]
- Steven, P.; Bock, F.; Hüttmann, G.; Cursiefen, C. Intravital two-photon microscopy of immune cell dynamics in corneal lymphatic vessels. PLoS ONE 2011, 6, e26253. [Google Scholar] [CrossRef] [PubMed]
- Aptel, F.; Olivier, N.; Deniset-Besseau, A.; Legeais, J.M.; Plamann, K.; Schanne-Klein, M.C.; Beaurepaire, E. Multimodal nonlinear imaging of the human cornea. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-Y.; Sun, Y.; Lo, W.; Teng, S.-W.; Wu, R.-J.; Jee, S.-H.; Lin, W.-C.; Hsiao, C.-H.; Lin, H.-C.; Chen, Y.-F.; et al. Multiphoton fluorescence and second harmonic generation microscopy for imaging infectious keratitis. J. Biomed. Opt. 2007, 12, 024013. [Google Scholar] [CrossRef]
- Hsueh, C.M.; Lo, W.; Chen, W.L.; Hovhannisyan, V.A.; Liu, G.Y.; Wang, S.S.; Tan, H.Y.; Dong, C.Y. Structural characterization of edematous corneas by forward and backward second harmonic generation imaging. Biophys. J. 2009, 97, 1198–1205. [Google Scholar] [CrossRef]
- Gehlsen, U.; Oetke, A.; Szaszak, M.; Koop, N.; Paulsen, F.; Gebert, A.; Huettmann, G.; Steven, P. Two-photon fluorescence lifetime imaging monitors metabolic changes during wound healing of corneal epithelial cells in vitro. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1293–1302. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sun, Y.; Lo, W.; Lin, S.J.; Hsiao, C.H.; Chen, Y.F.; Huang, S.C.M.; Lin, W.C.; Jee, S.H.; Yu, H.S.; et al. Multiphoton fluorescence and second harmonic generation imaging of the structural alterations in keratoconus ex vivo. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5251–5259. [Google Scholar] [CrossRef]
- Morishige, N.; Shin-Gyou-Uchi, R.; Azumi, H.; Ohta, H.; Morita, Y.; Yamada, N.; Kimura, K.; Takahara, A.; Sonoda, K.H. Quantitative analysis of collagen lamellae in the normal and keratoconic human cornea by second harmonic generation imaging microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8377–8385. [Google Scholar] [CrossRef]
- Mercatelli, R.; Ratto, F.; Rossi, F.; Tatini, F.; Menabuoni, L.; Malandrini, A.; Nicoletti, R.; Pini, R.; Pavone, F.S.; Cicchi, R. Three-dimensional mapping of the orientation of collagen corneal lamellae in healthy and keratoconic human corneas using SHG microscopy. J. Biophotonics 2016, 83, 75–83. [Google Scholar] [CrossRef]
- Morishige, N.; Wahlert, A.J.; Kenney, M.C.; Brown, D.J.; Kawamoto, K.; Chikama, T.; Nishida, T.; Jester, J. V Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest. Ophthalmol. Vis. Sci. 2007, 48, 1087–1094. [Google Scholar] [CrossRef]
- Bueno, J.; Gualda, E.J.; Giakoumaki, A.; Perez-Merino, P.; Marcos, S.; Artal, P. Multiphoton microscopy of ex vivo corneas after collagen cross-linking. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5325–5331. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, R.; Li, J.; Cummings, A.; Mrochen, M.; Vohnsen, B. Second-harmonic reflection imaging of normal and accelerated corneal crosslinking using porcine corneas and the role of intraocular pressure. Cornea 2014, 33, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Mercatelli, R.; Ratto, F.; Tatini, F.; Rossi, F.; Menabuoni, L.; Nicoletti, R.; Pini, R.; Pavone, F.S.; Cicchi, R. Characterization of the lamellar rearrangement induced by cross-linking treatment in keratoconic corneal samples imaged by SHG microscopy. Proc. SPIE 2017, 10045, 48–53. [Google Scholar]
- Zyablitskaya, M.; Takaoka, A.; Munteanu, E.L.; Nagasaki, T.; Trokel, S.L.; Paik, D.C. Evaluation of therapeutic tissue crosslinking (TXL) for myopia using second harmonic generation signal microscopy in rabbit sclera. Investig. Opthalmology Vis. Sci. 2017, 58, 21–29. [Google Scholar] [CrossRef][Green Version]
- Steven, P.; Hovakimyan, M.; Guthoff, R.F.; Hüttmann, G.; Stachs, O. Imaging corneal crosslinking by autofluorescence 2-photon microscopy, second harmonic generation, and fluorescence lifetime measurements. J. Cataract Refract. Surg. 2010, 36, 2150–2159. [Google Scholar] [CrossRef]
- Batista, A.; Breunig, G.; Hager, T.; Seitz, B.; König, K. Early evaluation of corneal collagen crosslinking in ex-vivo human corneas using two-photon imaging. Sci. Rep. 2019, 9, 10241. [Google Scholar] [CrossRef]
- Kwok, S.J.J.; Kuznetsov, I.A.; Kim, M.; Choi, M.; Scarcelli, G.; Yun, S.H.; Wok, S.H.J.J.K.; Uznetsov, I.V.A.N.A.K.; Im, M.O.K.; Hoi, M.Y.C.; et al. Selective two-photon collagen crosslinking in situ measured by Brillouin microscopy. Optica 2016, 3, 469. [Google Scholar] [CrossRef]
- Bradford, S.; Brown, D.; Juhasz, T.; Mikula, E.; Jester, J. Nonlinear optical corneal collagen crosslinking of ex vivo rabbit eyes. J. Cataract Refract. Surg. 2016, 42, 1660–1665. [Google Scholar] [CrossRef]
- Bradford, S.; Mikula, E.; Chai, D.; Brown, D.; Juhasz, T.; Jester, J. Custom built nonlinear optical crosslinking (NLO CXL) device capable of producing mechanical stiffening in ex vivo rabbit corneas. Biomed. Opt. Express 2017, 8, 4788–4797. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, N.; Chang, L.; Qi, P.; Zhang, L.; Lin, L.; Wang, Y.; Liu, W. Two-photon collagen crosslinking in ex vivo human corneal lenticules induced by near-infrared femtosecond laser. J. Biophotonics 2022, e202200160. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, L.; Cheng, Z.; Zhang, N.; Wang, C.; Wang, Y.; Liu, W. Effectiveness of collagen cross-linking induced by two-photon absorption properties of a femtosecond laser in ex vivo human corneal stroma. Biomed. Opt. Express 2022, 13, 5067. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, N.S. Epidemiology of keratoconus. Indian J. Ophthalmol. 2013, 61, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, E.; Huhle, M.; Seiler, T. Induction of cross-links in corneal tissue. Exp. Eye Res. 1998, 66, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Seiler, T.G.; Batista, A.; Frueh, B.E.; Koenig, K. Riboflavin Concentrations at the Endothelium During Corneal Cross-Linking in Humans. Investig. Opthalmology Vis. Sci. 2019, 60, 2140. [Google Scholar] [CrossRef]
- Bradford, S.; Mikula, E.; Xie, Y.; Juhasz, T.; Brown, D.; Jester, J. Enhanced transepithelial riboflavin delivery using femtosecond laser-machined epithelial microchannels. Transl. Vis. Sci. Technol. 2020, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuetemeyer, K.; Kensah, G.; Heidrich, M.; Meyer, H.; Martin, U.; Gruh, I.; Heisterkamp, A. Two-photon induced collagen cross-linking in bioartificial cardiac tissue. Opt. Express 2011, 19, 15996. [Google Scholar] [CrossRef]
- Chai, D.; Juhasz, T.; Brown, D.; Jester, J. Nonlinear optical collagen cross-linking and mechanical stiffening: A possible photodynamic therapeutic approach to treating corneal ectasia. J. Biomed. Opt. 2013, 18, 038003. [Google Scholar] [CrossRef]
- Latour, G.; Kowalczuk, L.; Savoldelli, M.; Bourges, J.L.; Plamann, K.; Behar-Cohen, F.; Schanne-Klein, M.C. Hyperglycemia-induced abnormalities in rat and human corneas: The potential of second harmonic generation microscopy. PLoS ONE 2012, 7, e48388. [Google Scholar] [CrossRef]
- Kowalczuk, L.; Latour, G.; Bourges, J.-L.; Savoldelli, M.; Jeanny, J.-C.; Plamann, K.; Schanne-Klein, M.-C.; Behar-Cohen, F. Multimodal Highlighting of Structural Abnormalities in Diabetic Rat and Human Corneas. Transl. Vis. Sci. Technol. 2013, 2, 3. [Google Scholar] [CrossRef]
- Lin, C.-J.; Kang, J.W.; So, P.T.C.; Dong, C.-Y. Multiphoton autofluorescence imaging of advanced glycation end products in glycated tissues. Proc. SPIE 2020, 11244, 91–95. [Google Scholar]
- Sasaki, N.; Fukatsu, R.; Tsuzuki, K.; Hayashi, Y.; Yoshida, T.; Fujii, N.; Koike, T.; Wakayama, I.; Yanagihara, R.; Garruto, R.; et al. Advanced Glycation End Products in Alzheimer’s Disease and Other Neurodegenerative Diseases. Am. J. Pathol. 1998, 153, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diab. Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef] [PubMed]




| Condition | Key Findings | Reference Examples |
|---|---|---|
| Corneal Infections |
| [114,174,177] |
| Corneal Edema |
| [105,176,178] |
| Corneal Neovascularization |
| [175] |
| Wound Healing |
| [103,179] |
| Keratoconus |
| [99,105,114,180,181,182,183] |
| CXL Evaluation |
| [108,184,185,186,187,188,189] |
| Two-photon induced CXL |
| [190,191,192,193,194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, A.; Guimarães, P.; Domingues, J.P.; Quadrado, M.J.; Morgado, A.M. Two-Photon Imaging for Non-Invasive Corneal Examination. Sensors 2022, 22, 9699. https://doi.org/10.3390/s22249699
Batista A, Guimarães P, Domingues JP, Quadrado MJ, Morgado AM. Two-Photon Imaging for Non-Invasive Corneal Examination. Sensors. 2022; 22(24):9699. https://doi.org/10.3390/s22249699
Chicago/Turabian StyleBatista, Ana, Pedro Guimarães, José Paulo Domingues, Maria João Quadrado, and António Miguel Morgado. 2022. "Two-Photon Imaging for Non-Invasive Corneal Examination" Sensors 22, no. 24: 9699. https://doi.org/10.3390/s22249699
APA StyleBatista, A., Guimarães, P., Domingues, J. P., Quadrado, M. J., & Morgado, A. M. (2022). Two-Photon Imaging for Non-Invasive Corneal Examination. Sensors, 22(24), 9699. https://doi.org/10.3390/s22249699







