Single Camera-Based Dual-Channel Near-Infrared Fluorescence Imaging system
Abstract
:1. Introduction
2. Materials and Methods
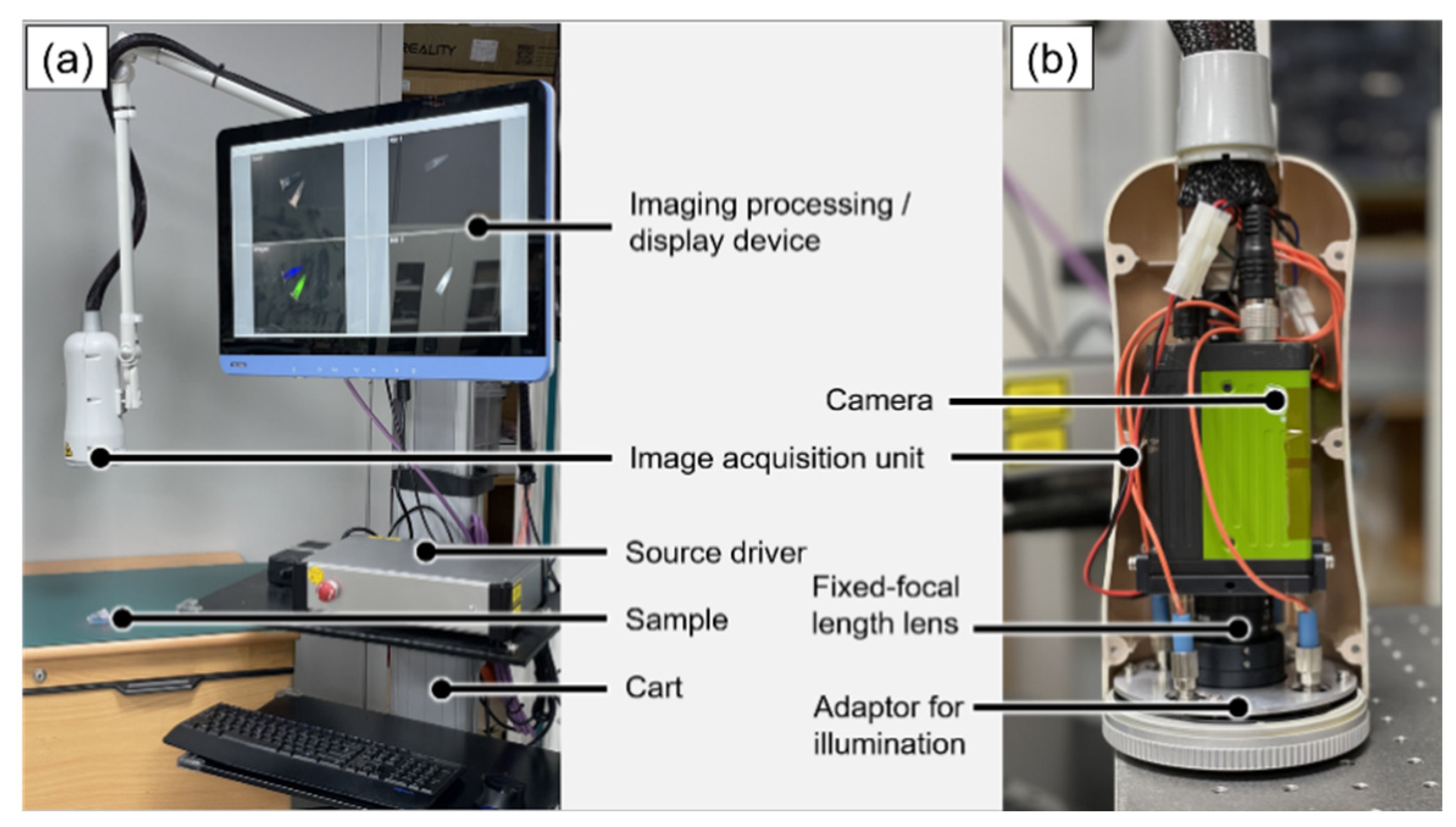
2.1. System Design
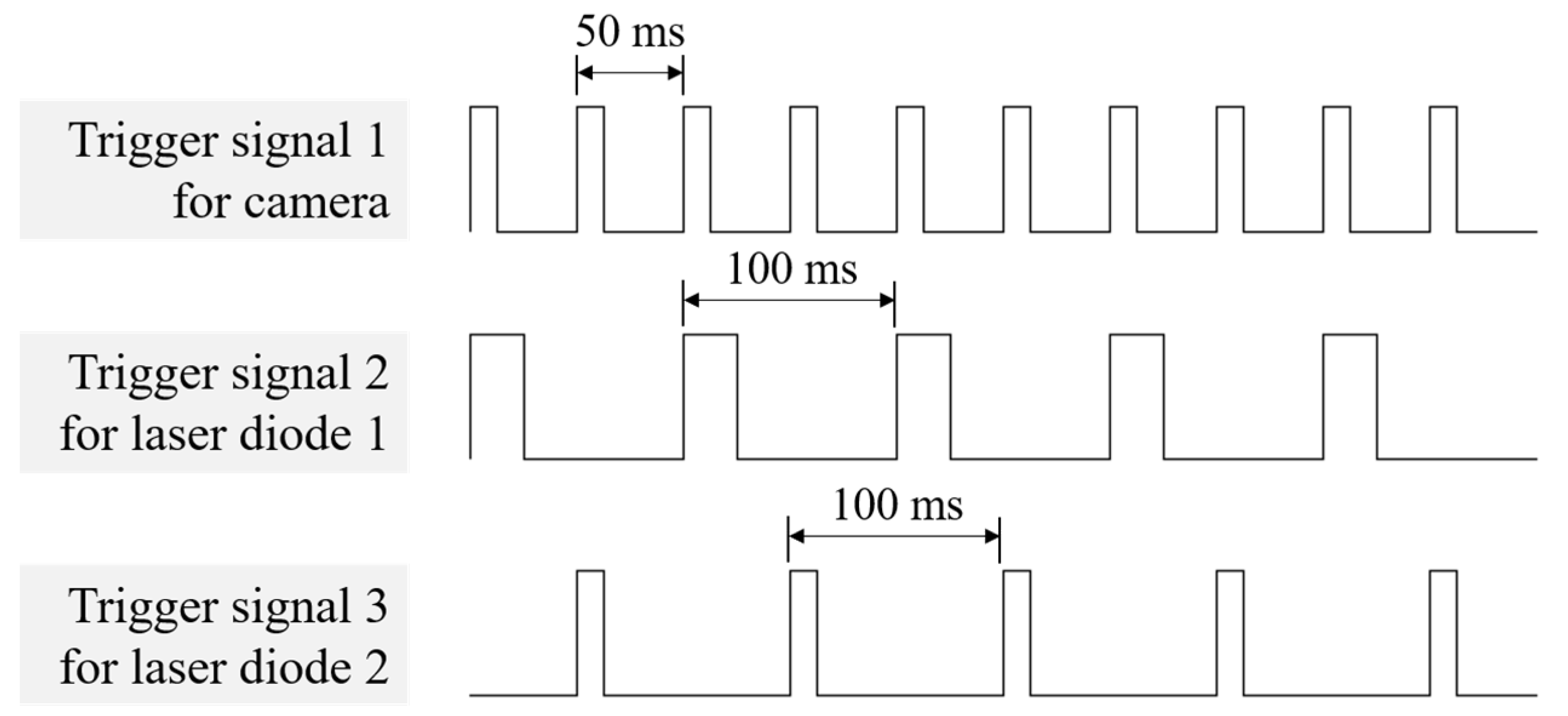
2.2. System Characterization
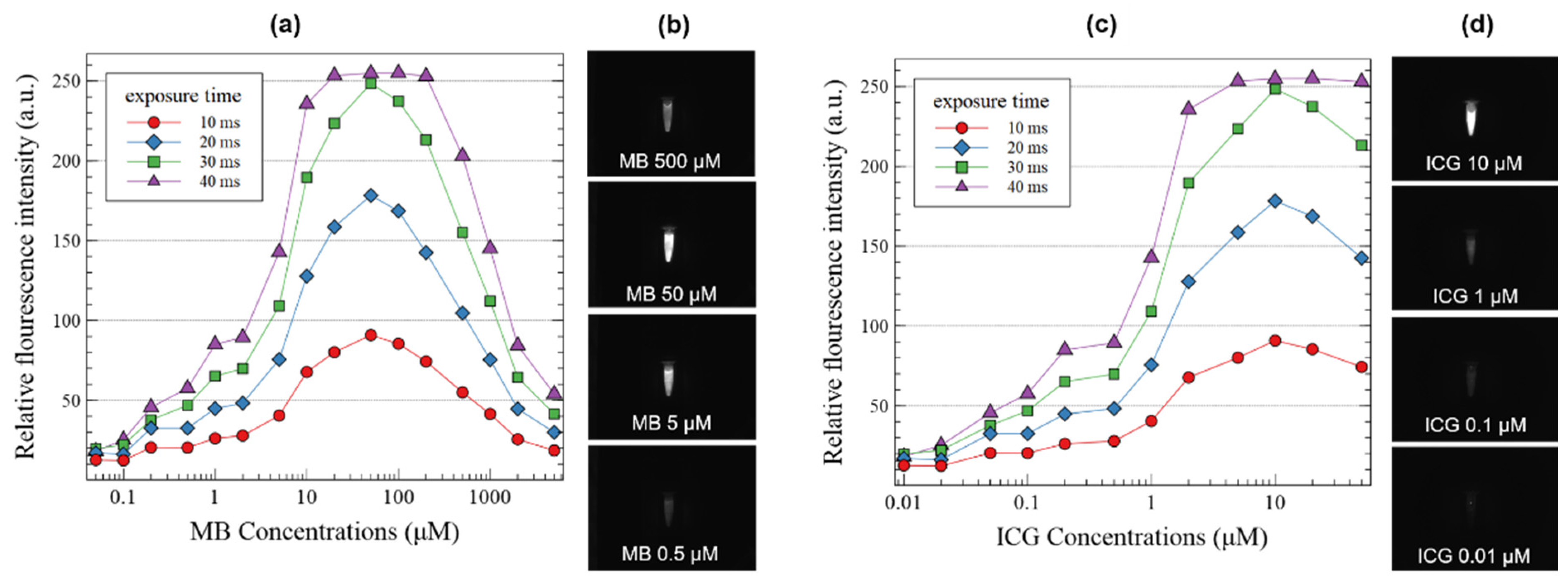
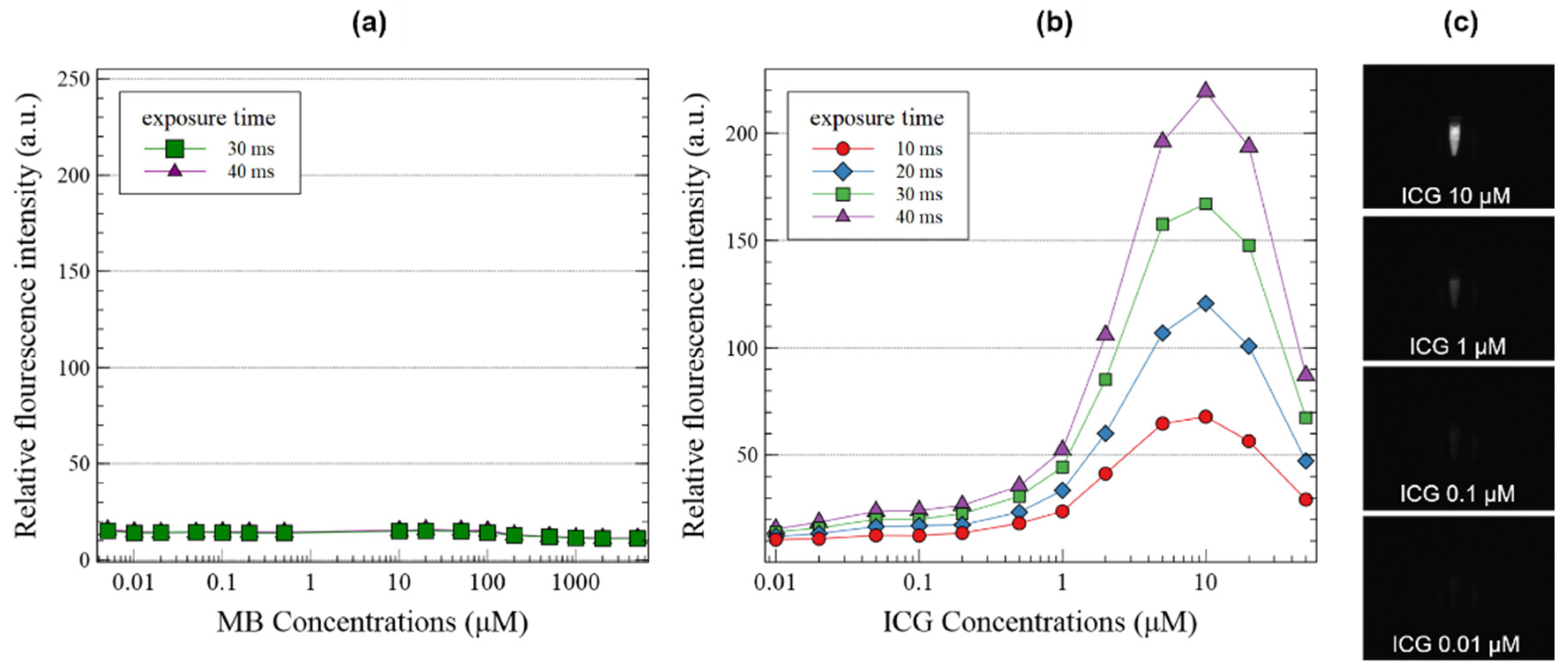
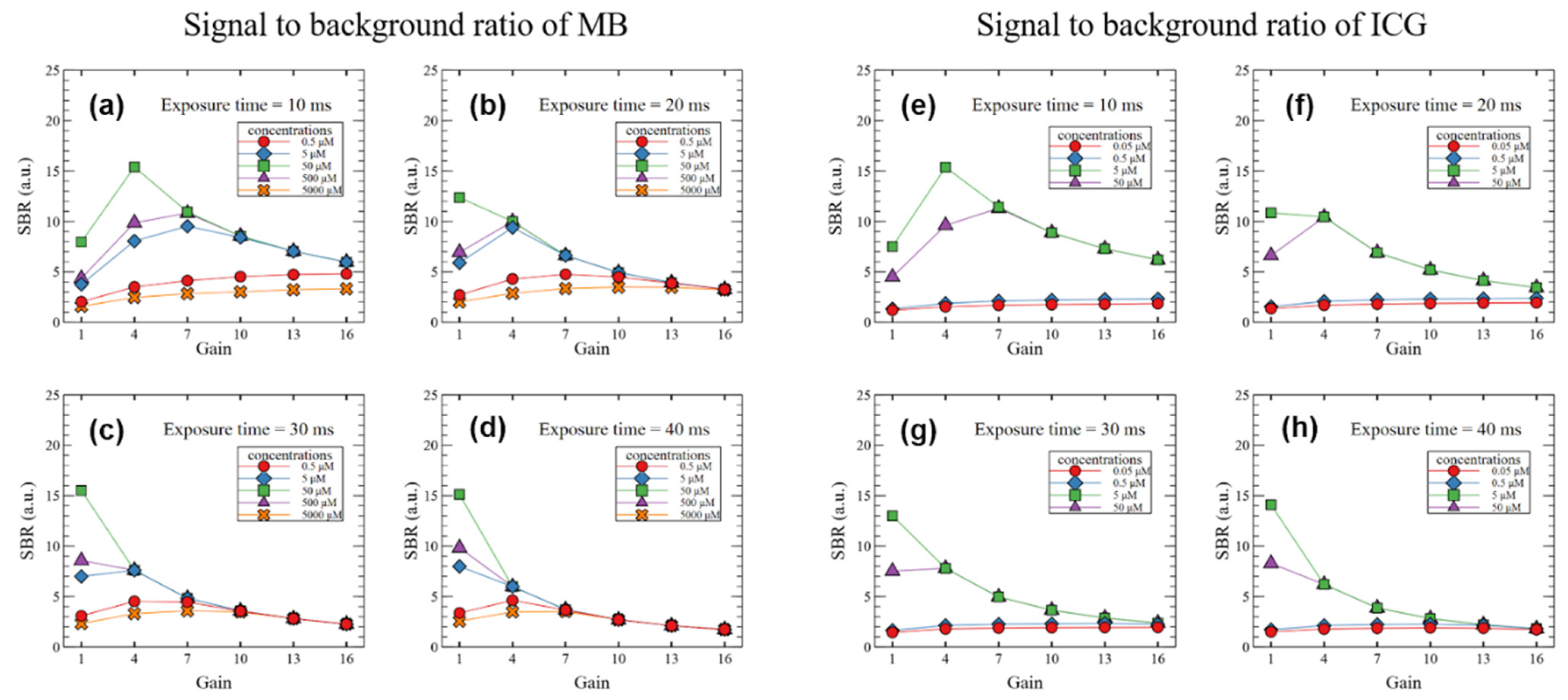
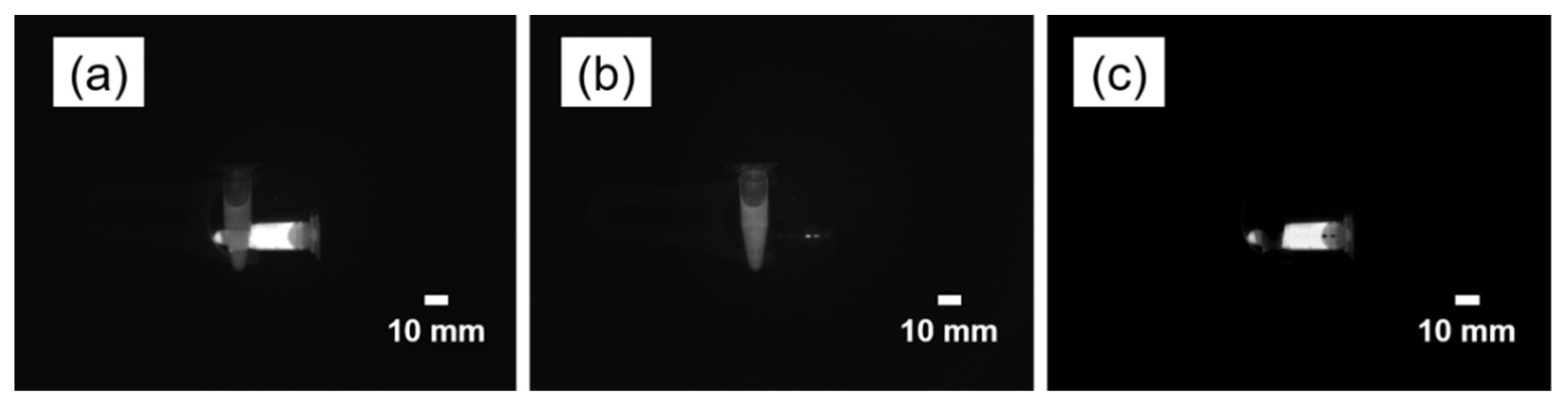
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.J.; Jett, J.R.; Sloan, J.A.; Midthun, D.E.; Hartman, T.E.; Sykes, A.-M.; Aughenbaugh, G.L.; Zink, F.E.; Hillman, S.L.; Noetzel, G.R.; et al. Screening for Lung Cancer with Low-Dose Spiral Computed Tomography. Am. J. Respir. Crit. Care Med. 2002, 165, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. New Technologies for Human Cancer Imaging. J. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef]
- Gallamini, A.; Zwarthoed, C.; Borra, A. Positron Emission Tomography (PET) in Oncology. Cancers 2014, 6, 1821–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Inomoto, T.; Miwa, M.; Shikayama, T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 2005, 12, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, N.; Yamazaki, R.; Nakagawa, A.; Abe, A.; Hamada, K.; Kubota, K.; Oyama, T. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am. J. Surg. 2008, 195, 850–853. [Google Scholar] [CrossRef]
- Verbeek, F.P.R.; Troyan, S.L.; Mieog, J.S.D.; Liefers, G.-J.; Moffitt, L.A.; Rosenberg, M.; Hirshfield-Bartek, J.; Gioux, S.; Van De Velde, C.J.H.; Vahrmeijer, A.L.; et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: A multicenter experience. Breast Cancer Res. Treat. 2013, 143, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Hutteman, M.; van der Vorst, J.R.; Gaarenstroom, K.; Peters, A.A.; Mieog, J.S.D.; Schaafsma, B.E.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Optimization of near-infrared fluorescent sentinel lymph node mapping for vulvar cancer. Am. J. Obstet. Gynecol. 2012, 206, 89.e1–89.e5. [Google Scholar] [CrossRef] [Green Version]
- Schaafsma, B.; Verbeek, F.; Elzevier, H.; Tummers, Q.; Van Der Vorst, J.; Frangioni, J.; Van De Velde, C.; Pelger, R.; Vahrmeijer, A. Optimization of sentinel lymph node mapping in bladder cancer using near-infrared fluorescence imaging. J. Surg. Oncol. 2014, 110, 845–850. [Google Scholar] [CrossRef]
- Van Der Vorst, J.; Schaafsma, B.; Verbeek, F.; Swijnenburg, R.; Hutteman, M.; Liefers, G.; Van De Velde, C.; Frangioni, J.; Vahrmeijer, A. Dose optimization for near-infrared fluorescence sentinel lymph node mapping in patients with melanoma. Br. J. Dermatol. 2012, 168, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demarchi, M.; Seeliger, B.; Lifante, J.-C.; Alesina, P.; Triponez, F. Fluorescence Image-Guided Surgery for Thyroid Cancer: Utility for Preventing Hypoparathyroidism. Cancers 2021, 13, 3792. [Google Scholar] [CrossRef] [PubMed]
- Sound, S.; Okoh, A.; Yigitbas, H.; Yazici, P.; Berber, E. Utility of Indocyanine Green Fluorescence Imaging for Intraoperative Localization in Reoperative Parathyroid Surgery. Surg. Innov. 2015, 26, 774–779. [Google Scholar] [CrossRef]
- Angell, J.E.; Khemees, T.A.; Abaza, R. Optimization of Near Infrared Fluorescence Tumor Localization during Robotic Partial Nephrectomy. J. Urol. 2013, 190, 1668–1673. [Google Scholar] [CrossRef]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; Berg, N.S.V.D.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neuro-Oncol. 2018, 139, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeek, F.P.R.; Schaafsma, B.E.; Tummers, Q.R.J.G.; Van Der Vorst, J.R.; Van Der Made, W.J.; Baeten, C.I.M.; Bonsing, B.A.; Frangioni, J.V.; Van De Velde, C.J.H.; Vahrmeijer, A.L.; et al. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg. Endosc. 2013, 28, 1076–1082. [Google Scholar] [CrossRef]
- Lee, B.; Matsui, A.; Hutteman, M.; Lin, S.; Winer, J.; Laurence, R.; Frangioni, J. Intraoperative Near-infrared Fluorescence Imaging in Perforator Flap Reconstruction: Current Research and Early Clinical Experience. J. Reconstr. Microsurg. 2009, 26, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Wang, H.; Xu, Y.; Chen, M.; Duan, W.; Lu, S. Indocyanine green fluorescence image-guided total laparoscopic living donor right hepatectomy: The first case report from Mainland China. Int. J. Surg. Case Rep. 2018, 53, 406–409. [Google Scholar] [CrossRef]
- Yin, R.; Ding, L.-Y.; Wei, Q.-Z.; Zhou, Y.; Tang, G.-Y.; Zhu, X. Comparisons of ICG-fluorescence with conventional tracers in sentinel lymph node biopsy for patients with early-stage breast cancer: A meta-analysis. Oncol. Lett. 2020, 21, 114. [Google Scholar] [CrossRef]
- Tummers, Q.; Verbeek, F.; Schaafsma, B.; Boonstra, M.; Van der Vorst, J.; Liefers, G.-J.; Van de Velde, C.; Frangioni, J.; Vahrmeijer, A. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and Methylene Blue. Eur. J. Surg. Oncol. 2014, 40, 850–858. [Google Scholar] [CrossRef]
- Budner, O.; Cwalinski, T.; Skokowski, J.; Marano, L.; Resca, L.; Cwalina, N.; Kalinowski, L.; Hoveling, R.; Roviello, F.; Polom, K. Methylene Blue Near-Infrared Fluorescence Imaging in Breast Cancer Sentinel Node Biopsy. Cancers 2022, 14, 1817. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.; Schepers, A.; Hamming, J.F.; Kievit, J.; Frangioni, J.V.; Van de Velde, C.J.; Vahrmeijer, A.L. Intraoperative guidance in parathyroid surgery using near-infrared fluorescence imaging and low-dose Methylene Blue. Surgery 2015, 158, 1323–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tummers, Q.R.; Boonstra, M.C.; Frangioni, J.V.; Van de Velde, C.J.; Vahrmeijer, A.L.; Bonsing, B.A. Intraoperative near-infrared fluorescence imaging of a paraganglioma using methylene blue: A case report. Int. J. Surg. Case Rep. 2015, 6, 150–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, A.; Tanaka, E.; Choi, H.S.; Kianzad, V.; Gioux, S.; Lomnes, S.J.; Frangioni, J.V. Real-time, near-infrared, fluorescence-guided identification of the ureters using methylene blue. Surgery 2010, 148, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeek, F.P.; Van Der Vorst, J.R.; Schaafsma, B.E.; Swijnenburg, R.-J.; Gaarenstroom, K.; Elzevier, H.W.; Van De Velde, C.J.; Frangioni, J.V.; Vahrmeijer, A.L. Intraoperative Near Infrared Fluorescence Guided Identification of the Ureters Using Low Dose Methylene Blue: A First in Human Experience. J. Urol. 2013, 190, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Xue, Z.; Yasin, A.; He, Y.; Chai, Y.; Li, J.; Zhang, K. Colorectal cancer and adjacent normal mucosa differ in apoptotic and inflammatory protein expression. Eng. Regen. 2021, 2, 279–287. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.; Wang, J.; Dou, B.; Zhou, F.; Cao, J.; Qu, J.; Cao, Z.; Zhao, W.; Peng, X. Fluorescence Discrimination of Cancer from Inflammation by Molecular Response to COX-2 Enzymes. J. Am. Chem. Soc. 2013, 135, 17469–17475. [Google Scholar] [CrossRef]
- Tanaka, E.; Chen, F.Y.; Flaumenhaft, R.; Graham, G.J.; Laurence, R.G.; Frangioni, J.V. Real-time assessment of cardiac perfusion, coronary angiography, and acute intravascular thrombi using dual-channel near-infrared fluorescence imaging. J. Thorac. Cardiovasc. Surg. 2009, 138, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Miyashiro, I.; Kishi, K.; Yano, M.; Tanaka, K.; Motoori, M.; Ohue, M.; Ohigashi, H.; Takenaka, A.; Tomita, Y.; Ishikawa, O. Laparoscopic detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging. Surg. Endosc. 2010, 25, 1672–1676. [Google Scholar] [CrossRef]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azar, F.; Frangioni, J.V. The FLARE™ Intraoperative Near-Infrared Fluorescence Imaging System: A First-in-Human Clinical Trial in Breast Cancer Sentinel Lymph Node Mapping. Ann. Surg. Oncol. 2009, 16, 2943–2952. [Google Scholar] [CrossRef]
- Choi, J.; Shin, J.G.; Kwon, H.-S.; Tak, Y.-O.; Park, H.J.; Ahn, J.-C.; Eom, J.B.; Seo, Y.; Park, J.W.; Choi, Y.; et al. Development of Intraoperative Near-Infrared Fluorescence Imaging System Using a Dual-CMOS Single Camera. Sensors 2022, 22, 5597. [Google Scholar] [CrossRef] [PubMed]
- Hovi, A.; Forsström, P.; Mõttus, M.; Rautiainen, M. Evaluation of Accuracy and Practical Applicability of Methods for Measuring Leaf Reflectance and Transmittance Spectra. Remote Sens. 2017, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.L.; Williams, P.; Rheault, J.-P.; Prochaska, T.; Allen, R.D.; DePoy, D.L. Characterization of the Reflectivity of Various Black Materials. In Proceedings of the Ground-Based and Airborne Instrumentation for Astronomy V, Montréal, QC, Canada, 8 July 2014; Volume 9147, pp. 1459–1466. [Google Scholar]
- Borlan, R.; Focsan, M.; Maniu, D.; Astilean, S. Interventional NIR Fluorescence Imaging of Cancer: Review on Next Generation of Dye-Loaded Protein-Based Nanoparticles for Real-Time Feedback During Cancer Surgery. Int. J. Nanomed. 2021, 16, 2147–2171. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Shin, J.-G.; Tak, Y.-O.; Seo, Y.; Eom, J. Single Camera-Based Dual-Channel Near-Infrared Fluorescence Imaging system. Sensors 2022, 22, 9758. https://doi.org/10.3390/s22249758
Choi J, Shin J-G, Tak Y-O, Seo Y, Eom J. Single Camera-Based Dual-Channel Near-Infrared Fluorescence Imaging system. Sensors. 2022; 22(24):9758. https://doi.org/10.3390/s22249758
Chicago/Turabian StyleChoi, Janghoon, Jun-Geun Shin, Yoon-Oh Tak, Youngseok Seo, and Jonghyun Eom. 2022. "Single Camera-Based Dual-Channel Near-Infrared Fluorescence Imaging system" Sensors 22, no. 24: 9758. https://doi.org/10.3390/s22249758




