Zinc Phthalocyanine Sensing Mechanism Quantification for Potential Application in Chemical Warfare Agent Detectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition of Zinc Phthalocyanine Layers
2.2. Exposure to DMMP
2.3. Thermal Desorption Spectroscopy Experiment
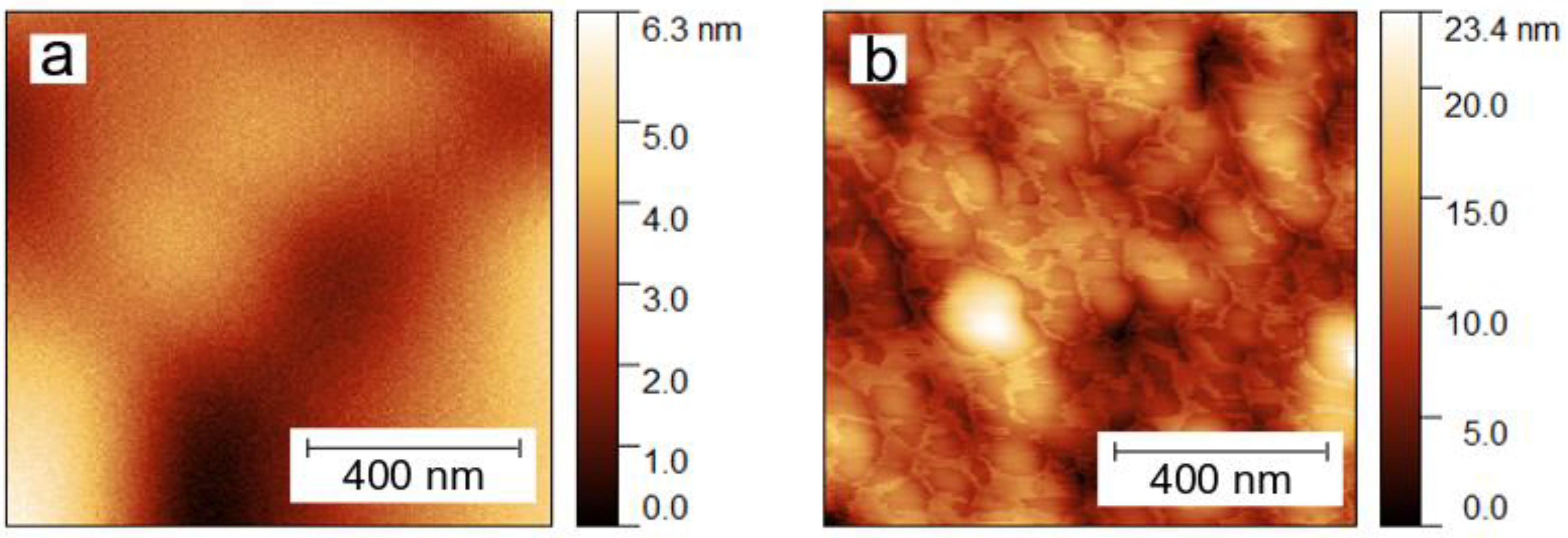
2.4. Characterization of Sample Topography
2.5. Computational Modeling
2.6. Sensor Response Measurements Using Impedance Spectroscopy
3. Results and Discussion
3.1. Thermal Desorption—Survey Approach
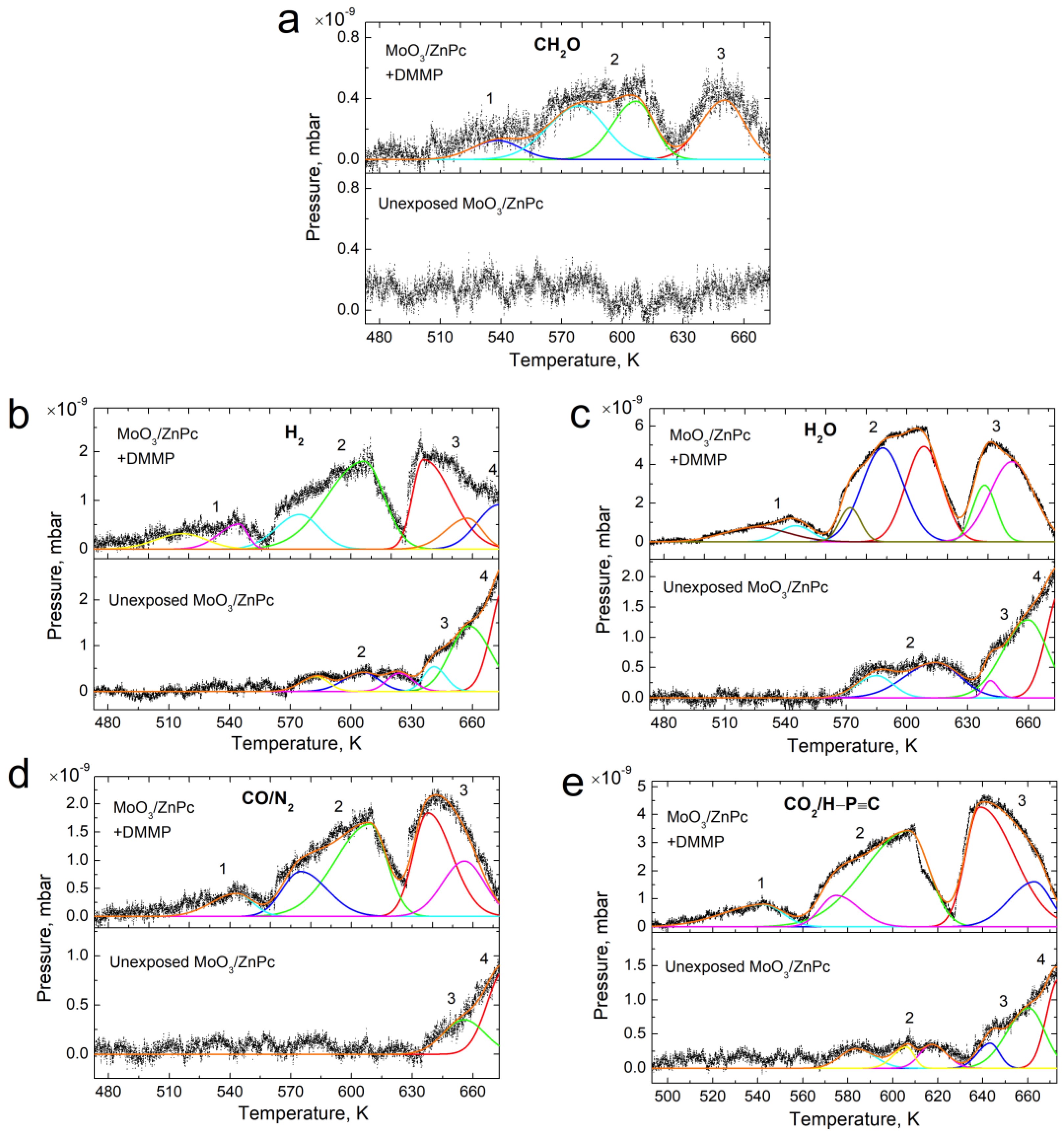
3.2. Thermal Desorption—Components Analysis
- R—gas constant, 8.314 J mol−1 K−1;
- ν—pre-exponential factor, s−1;
- Tmax—temperature at peak, K;
- β—heating rate, K s−1.
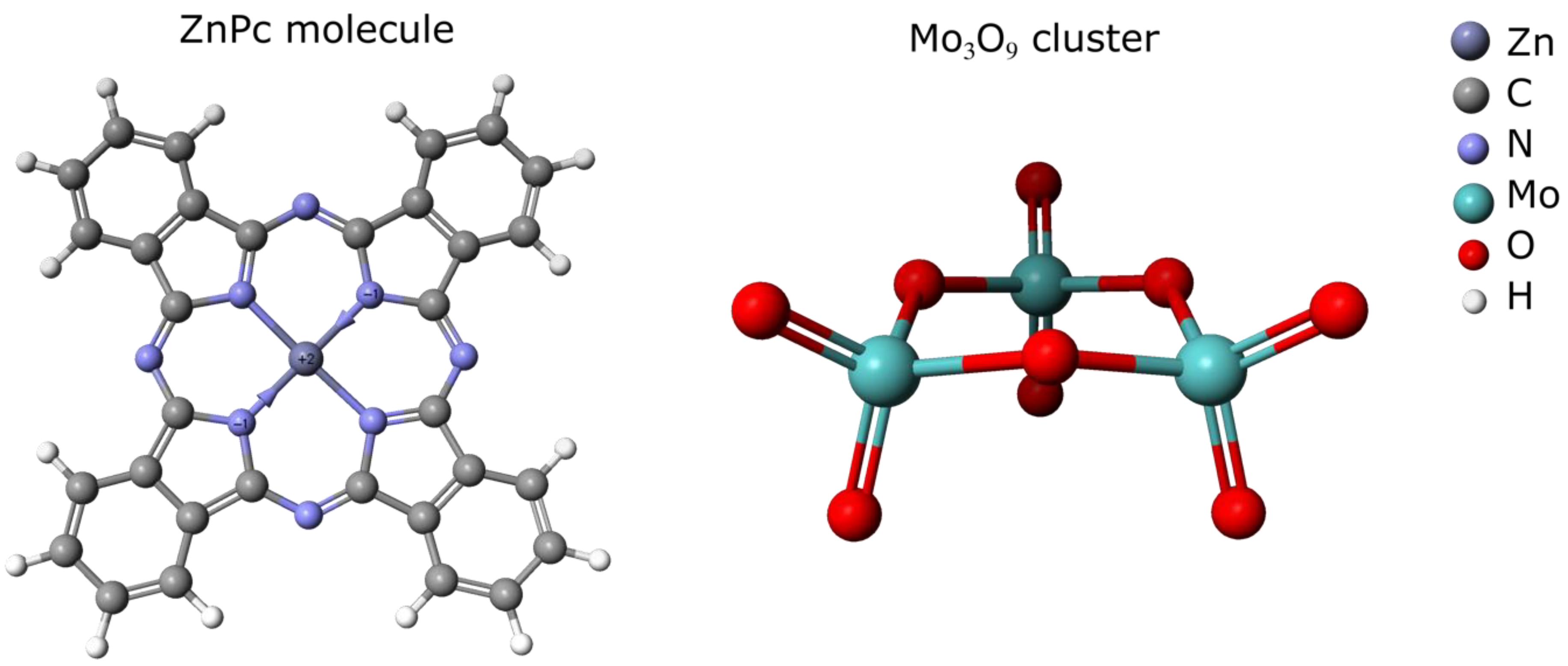
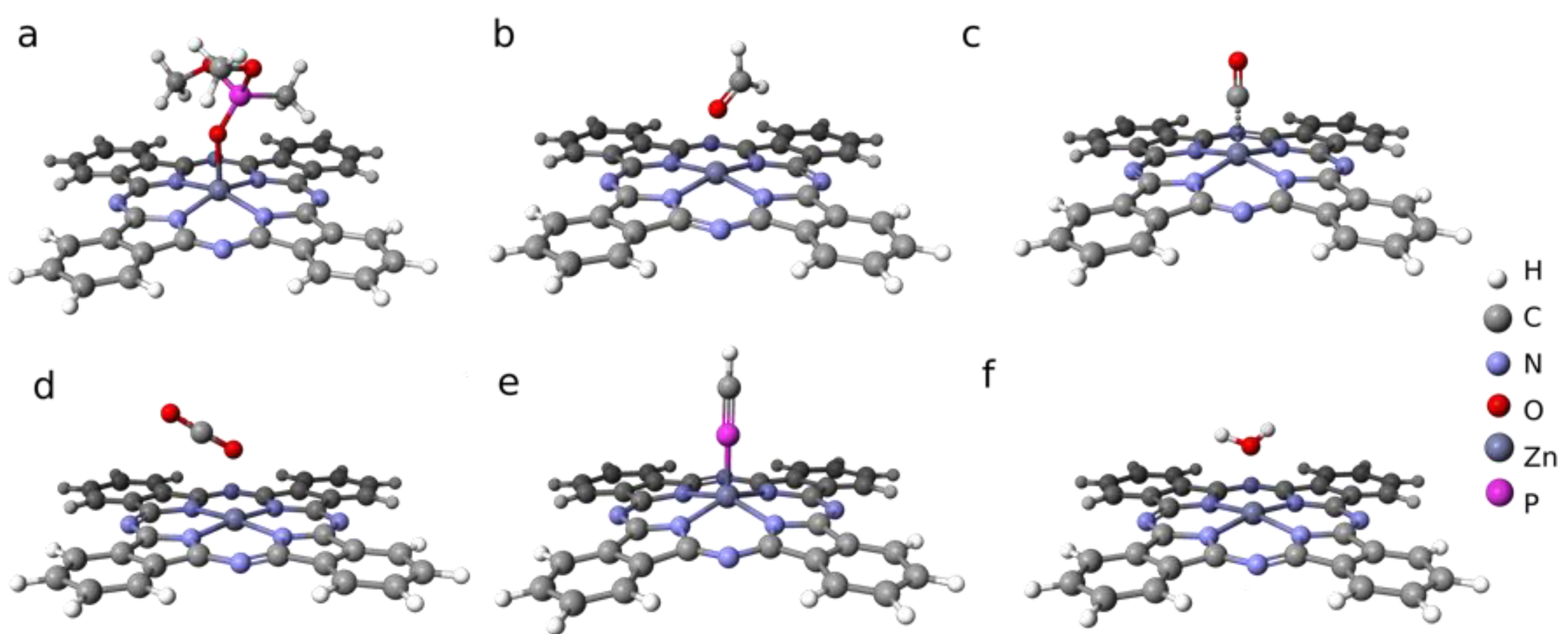
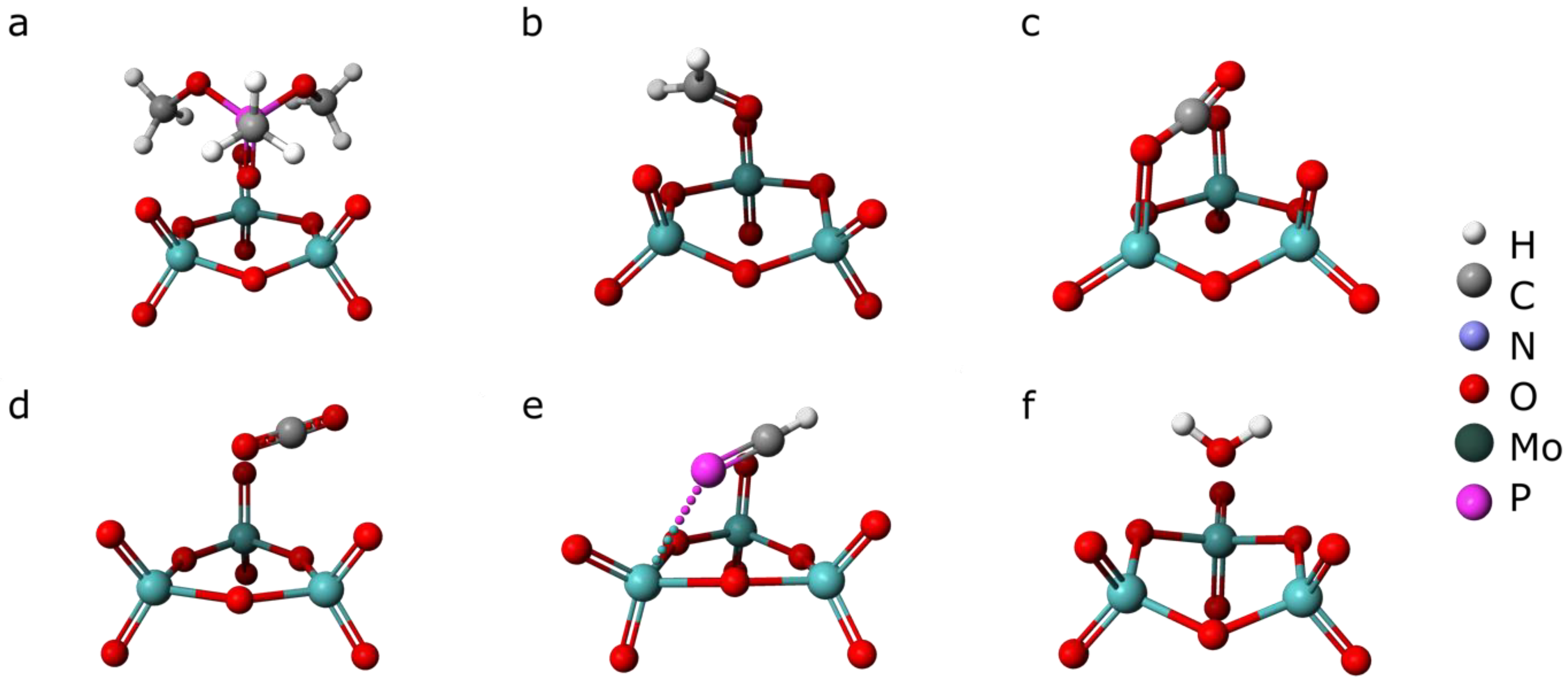
3.3. Semi-Empirical Modeling of Adsorption Energies
3.4. Implications for ZnPc Capability for DMMP Sensing
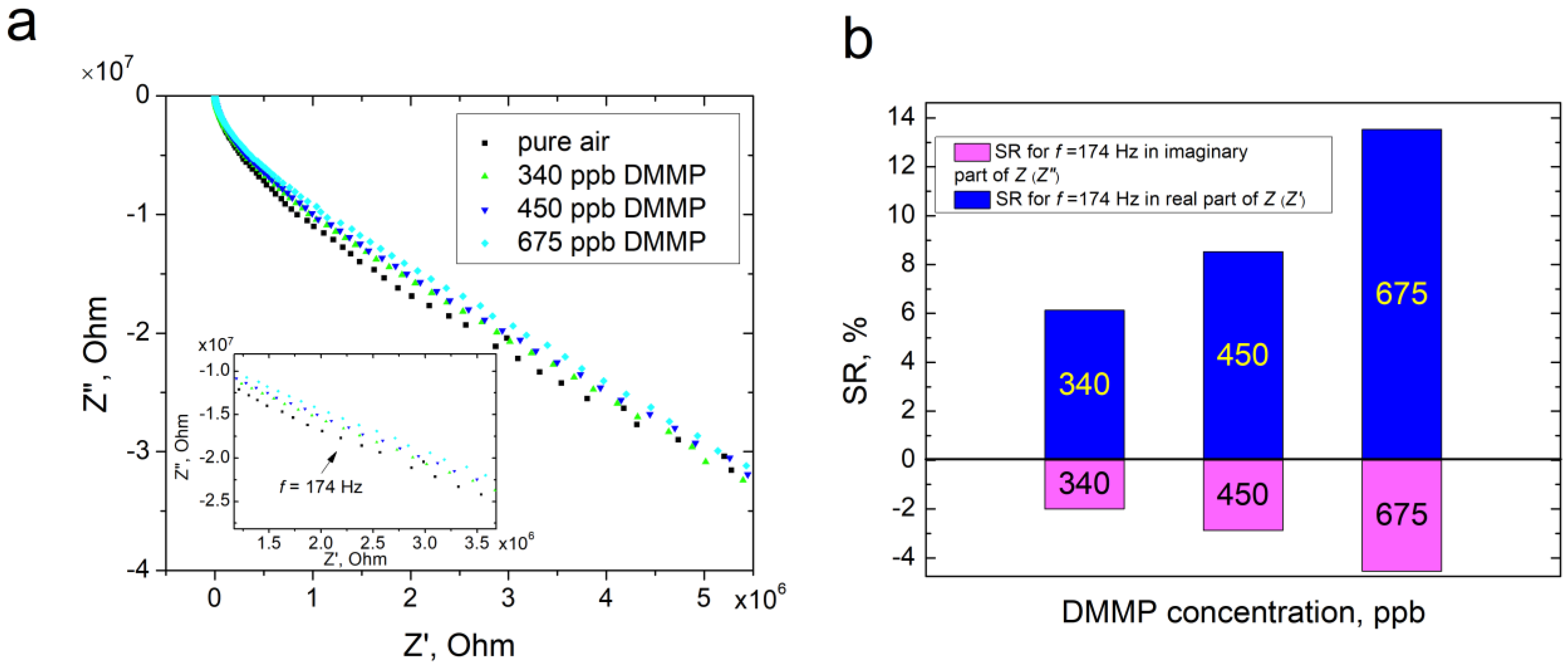
3.5. Verification of the Sensing Capability of ZnPc by Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catterall, R.W. Chemical Sensors (Oxford Chemistry Primers); Oxford Science Publications: Oxford, UK, 1997. [Google Scholar]
- Sizun, T.; Bouvet, M.; Chen, Y.; Suisse, J.-M.; Barochi, G.; Rossignol, J. Differential study of substituted and unsubstituted cobalt phthalocyanines for gas sensor applications. Sens. Actuators B Chem. 2011, 159, 163–170. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, B.; Wang, X.; Li, Y.; Gai, S.; Wu, Y.; Cheng, X. A high-sensitive room temperature gas sensor based on cobalt phthalocyanines and reduced graphene oxide nanohybrids for the ppb-levels of ammonia detection. RSC Adv. 2019, 9, 37518–37525. [Google Scholar] [CrossRef] [PubMed]
- Chia, L.S.; Du, Y.H.; Palale, S.; Lee, P.S. Interaction of Copper Phthalocyanine with Nitrogen Dioxide and Ammonia Investigation Using X-ray Absorption Spectroscopy and Chemiresistive Gas Measurements. ACS Omega 2019, 4, 10388–10395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, L.; Yang, G.; Xie, Q.; Zhong, S.; Su, X.; Zhang, B. Improvement of Sensing Properties for Copper Phthalocyanine Sensors Based on Polymer Nanofiber Scafolds. Langmuir 2020, 36, 4532–4539. [Google Scholar] [CrossRef]
- Prasongkit, J.; Tangsukworakhun, S.; Jaisutti, R.; Osothan, T. Highly sensitive and selective sensing of acetone and hydrogen sulfide using metal phthalocyanine—Carbon nanotube hybrids. Appl. Surf. Sci. 2020, 532, 147314. [Google Scholar] [CrossRef]
- Shu, J.H.; Wilke, H.C.; Chin, B.A. Passive chemiresistor sensor based on iron (II) phthalocyanine thin films for monitoring of nitrogen dioxide. Sens. Actuators B Chem. 2010, 148, 498–503. [Google Scholar] [CrossRef]
- Singh, A.; Samanta, S.; Kumar, A.; Debnath, A.K.; Prasad, R.; Veerender, P.; Balouria, V.; Aswal, D.K.; Gupta, S.K. Implication of molecular orientation on charge transport and gas sensing characteristics of cobalt–phthalocyanine thin films. Org. Electron. 2012, 13, 2600–2604. [Google Scholar] [CrossRef]
- Zou, D.; Zhao, W.; Cui, B.; Li, D.; Liu, D. Adsorption of gas molecules on a manganese phthalocyanine molecular device and its possibility as a gas sensor. Phys. Chem. Chem. Phys. 2018, 20, 2048–2056. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Escano, M.C.S.; Kasai, H. Nitric Oxide Adsorption Effects on Metal Phthalocyanines. J. Phys. Chem. B 2010, 114, 10017–10021. [Google Scholar] [CrossRef]
- Mukherjee, D.; Manjunatha, R.; Sampath, S.; Ray, A.K. Phthalocyanines as Sensitive Materials for Chemical Sensors. In Materials for Chemical Sensing; Cesar Paixão, T., Reddy, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Basova, T.V.; Ray, A.K. Review—Hybrid Materials Based on Phthalocyanines and Metal Nanoparticles for Chemiresistive and Electrochemical Sensors: A Mini-Review. ECS J. Solid State Sci. Technol. 2020, 9, 061001. [Google Scholar] [CrossRef]
- Parra, M.B.V.; Locatelli, C.; Xiong, H. Electrical transduction in phthalocyanine-based gas sensors: From classical chemiresistors to new functional structures. J. Porphyr. Phthalocyanines 2009, 13, 84–91. [Google Scholar]
- Oura, K.; Lifshits, V.G.; Saranin, A.A.; Zatov, A.V.; Katayama, M. Surface Science: An Introduction, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2003; p. 303. [Google Scholar]
- Zhou, J.; Varazo, K.; Reddic, J.E.; Myrick, M.L.; Chen, D.A. Decomposition of Dimethyl Methylphosphonate on TiO2(110): Principal Component Analysis Applied to X-ray Photoelectron Spectroscopy. Anal. Chim. Acta 2003, 496, 289–300. [Google Scholar] [CrossRef]
- Rusu, C.N.; Yates, J.T., Jr. Adsorption and Decomposition of Dimethyl Methylphosphonate on TiO2. J. Phys. Chem. B 2000, 104, 12292–12298. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Morris, J.R. Thermal Decomposition of a Chemical Warfare Agent Simulant (DMMP) on TiO2: Adsorbate Reactions with Lattice Oxygen as Studied by Infrared Spectroscopy. J. Phys. Chem. C 2009, 113, 15684–15691. [Google Scholar] [CrossRef]
- Moss, J.A.; Szczepankiewicz, S.H.; Park, E.; Hoffman, M.R. Adsorption and Photodegradation of Dimethyl Methylphosphonate Vapor at TiO2 Surfaces. J. Phys. Chem. B 2005, 109, 19779–19785. [Google Scholar] [CrossRef]
- Mitchell, M.B.; Sheinker, V.N.; Mintz, E.A. Adsorption and Decomposition of Dimethyl Methylphosphonate on Metal Oxides. J. Phys. Chem. B 1997, 101, 11192–11203. [Google Scholar] [CrossRef]
- Templeton, M.K.; Weinberg, W.H. Decomposition of Phosphonate Esters Adsorbed on Aluminum Oxide. J. Am. Chem. Soc. 1985, 107, 774–779. [Google Scholar] [CrossRef]
- Templeton, M.K.; Weinberg, W.H. Adsorption and Decomposition of Dimethyl Methylphosphonate on an Aluminum Oxide Surface. J. Am. Chem. Soc. 1985, 107, 97–108. [Google Scholar] [CrossRef]
- Henderson, M.A.; Jin, T.; White, J.M. A TPD/AES Study of the Interaction of Dimethyl Methylphosphonate with α-Fe2O3 and SiO2. J. Phys. Chem. 1986, 90, 4607–4611. [Google Scholar] [CrossRef]
- Tesfai, T.M.; Scheinker, V.N.; Mitchell, M.B. Decomposition of Dimethyl Methylphosphonate (DMMP) on Alumina-Supported Iron Oxide. J. Phys. Chem. B 1998, 102, 7299–7302. [Google Scholar] [CrossRef]
- Head, A.R.; Tsyshevsky, R.; Trotochaud, L.; Yu, Y.; Karslıoǧlu, O.; Eichhorn, B.; Kuklja, M.M.; Bluhm, H. Dimethyl methylphosphonate adsorption and decomposition on MoO2 as studied by ambient pressure X-ray photoelectron spectroscopy and DFT calculations. J. Phys. Condens. Matter 2018, 30, 134005. [Google Scholar] [CrossRef] [PubMed]
- Head, A.R.; Tsyshevsky, R.; Trotochaud, L.; Yu, Y.; Kyhl, L.; Karslıoǧlu, O.; Kuklja, M.M.; Bluhm, H. Adsorption of Dimethyl Methylphosphonate on MoO3: The Role of Oxygen Vacancies. J. Phys. Chem. C 2016, 120, 29077–29088. [Google Scholar] [CrossRef] [Green Version]
- Holdren, S.; Tsyshevsky, R.; Fears, K.; Owrutsky, J.; Wu, T.; Wang, X.; Eichhorn, B.W.; Kuklja, M.M.; Zachariah, M.R. Adsorption and Destruction of the G-Series Nerve Agent Simulant Dimethyl Methylphosphonate on Zinc Oxide. ACS Catal. 2019, 9, 902–911. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1–PR76. [Google Scholar] [CrossRef]
- Sheinker, V.N.; Mitchell, M.B. Quantitative Study of the Decomposition of Dimethyl Methylphosphonate (DMMP) on Metal Oxides at Room Temperature and Above. Chem. Mater. 2002, 14, 1257–1268. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, J.; Kang, Y.; Reddic, J.; Chen, D. Dimethyl Methylphosphonate Decomposition on Cu Surfaces: Supported Cu Nanoclusters and Films on TiO2 (110). Langmuir 2004, 20, 9686–9694. [Google Scholar] [CrossRef]
- Cao, L.; Segal, S.R.; Suib, S.L.; Tang, X.; Satyapal, S. Thermocatalytic oxidation of dimethyl methylphosphonate on supported metal oxides. J. Catal. 2000, 194, 61–70. [Google Scholar] [CrossRef]
- Chen, D.A.; Ratliff, J.S.; Hu, X.; Gordon, W.O.; Senanayake, S.D.; Mullins, D.R. Dimethyl Methylphosphonate Decomposition on Fully Oxidized and Partially Reduced Ceria Thin Films. Surf. Sci. 2010, 604, 574–587. [Google Scholar] [CrossRef]
- Aldahhak, H.; Powroźnik, P.; Pander, P.; Jakubik, W.; Dias, F.B.; Schmidt, W.G.; Gerstmann, U.; Krzywiecki, M. Toward Efficient Toxic-Gas Detectors: Exploring Molecular Interactions of Sarin and Dimethyl Methylphosphonate with Metal-Centered Phthalocyanine Structures. J. Phys. Chem. C 2020, 124, 6090–6102. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. Available online: www.gwyddion.net (accessed on 10 November 2022). [CrossRef]
- Contour, P.; Lenfant, P.; Vijh, A.K. Gas-phase chemisorption and electroreduction of oxygen on phthalocyanines. J. Catal. 1973, 29, 8–14. [Google Scholar] [CrossRef]
- Redhead, P.A. Thermal desorption of gases. Vacuum 1962, 12, 203–211. [Google Scholar] [CrossRef]
- Gates, B.C. Catalytic Chemistry; Wiley: New York, NY, USA, 1992. [Google Scholar]
- IUPAC. Compendium of Chemical Terminology (The “Gold Book”), 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar]
- Lüth, H. Surfaces and Interfaces of Solid Materials; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.A. FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes. J. Mol. Struct. 2005, 753, 119–126. [Google Scholar] [CrossRef]
- Broclawik, E.; Yamauchi, R.; Endou, A.; Kubo, M.; Miyamoto, A. Density functional study on the activation of methane over Pd2, PdO, and Pd2O clusters. Int. J. Quantum Chem. 1997, 61, 673–682. [Google Scholar] [CrossRef]
- Martinelli, G.; Carotta, M.C. Thick-film gas sensors. Sens. Actuators B Chem. 1995, 23, 157–161. [Google Scholar] [CrossRef]
- Sahm, T.; Gurlo, A.; Bârsan, N.; Weimar, U. Basics of oxygen and SnO2 interaction; work function change and conductivity measurements. Sens. Actuators B Chem. 2006, 118, 78–83. [Google Scholar] [CrossRef]
- Powroźnik, P.; Grządziel, L.; Jakubik, W.; Krzywiecki, M. Sarin-simulant detection by phthalocyanine/palladium structures: From modeling to real sensor response. Sens. Actuators B Chem. 2018, 273, 771–777. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, H.; Wang, Z.; Chen, C. Dimethyl methylphosphonate (DMMP) as an efficient flame retardant additive for the lithium-ion battery electrolytes. J. Power Sources 2007, 173, 562–564. [Google Scholar] [CrossRef]
- Lu, X. Design and Study of Lyotropic Liquid Crystal-Butyl Rubber Nanocomposites for Chemical Agent Vapor Barrier Applications. Ph.D. Thesis, The University of Colorado Boulder, Boulder, CO, USA, 2007. [Google Scholar]
- Snow, E.S.; Perkins, F.K.; Robinson, J.A. Chemical vapor detection using single-walled carbon nanotubes. Chem. Soc. Rev. 2006, 35, 790–798. [Google Scholar] [CrossRef]

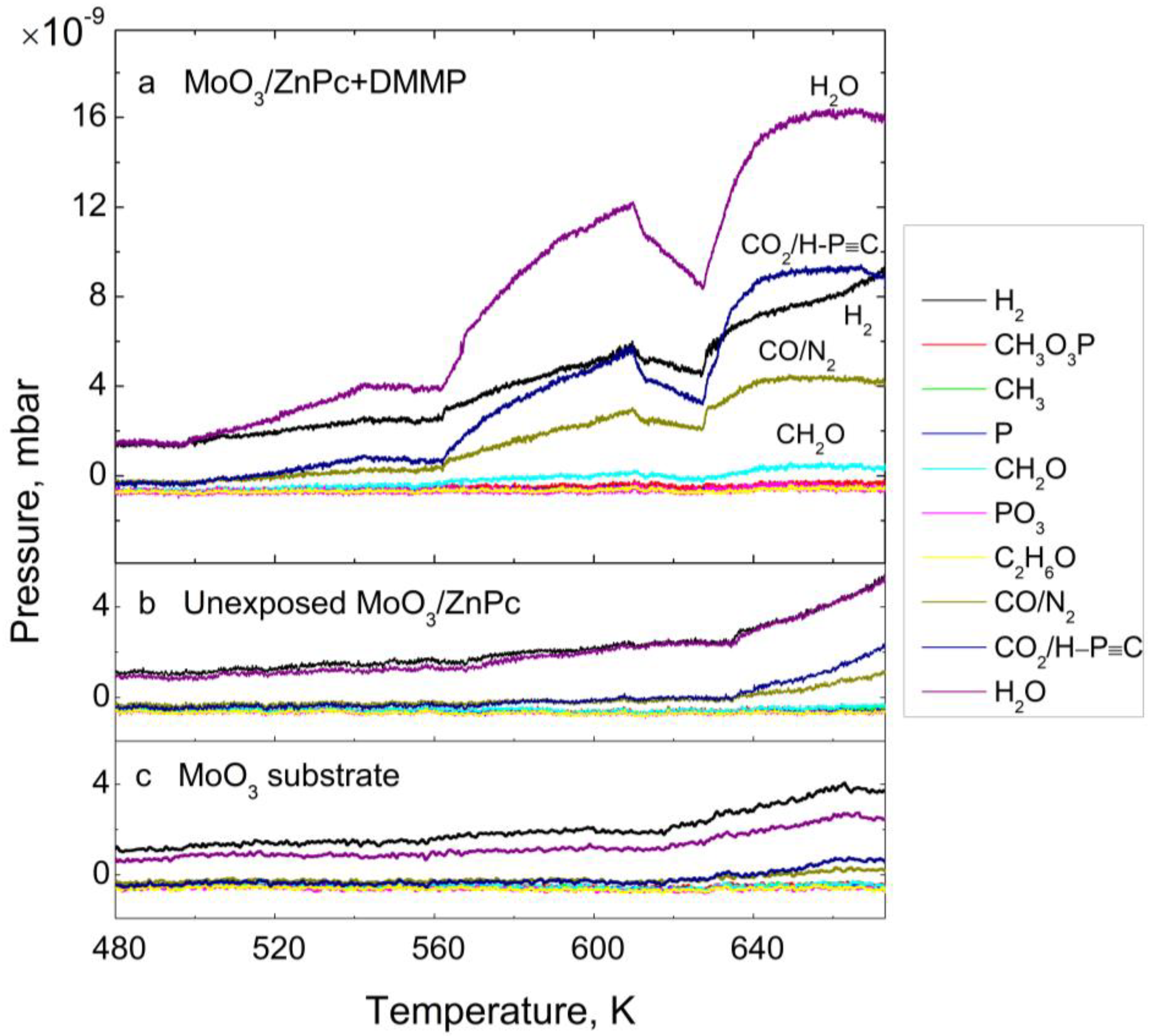

| Desorbing Species | No | Unexposed MoO3/ZnPc | MoO3/ZnPc with DMMP | ||||
|---|---|---|---|---|---|---|---|
| T, K | Edes, eV | Rel. Area, % | T, K | Edes, eV | Rel. Area, % | ||
| CH2O (30 amu) | 1 | - | 538.1 | 1.73 | 3.3 | ||
| 2 | 578.9 | 1.87 | |||||
| 606.9 | 1.96 | ||||||
| 3 | 650.3 | 2.10 | |||||
| H2 (2 amu) | 1 | - | 516.9 | 1.66 | 15.9 | ||
| 544.3 | 1.75 | ||||||
| 2 | 582.8 | 1.88 | 36.9 | 574.7 | 1.85 | ||
| 605.9 | 1.96 | 606.5 | 1.96 | ||||
| 623.8 | 2.02 | ||||||
| 3 | 640.9 | 2.07 | 635.7 | 2.06 | |||
| 658.4 | 2.13 | 657.7 | 2.13 | ||||
| 4 | 676.7 | 2.19 | 673.1 | 2.18 | |||
| CO/N2 (28 amu) | 1 | - | 543.2 | 1.75 | 13.9 | ||
| 2 | - | 574.8 | 1.85 | ||||
| 609.3 | 1.97 | ||||||
| 3 | 655.7 | 2.12 | 9.5 | 637.7 | 2.06 | ||
| 655.8 | 2.12 | ||||||
| 4 | 675.6 | 2.19 | - | ||||
| CO2/ H-C≡P (44 amu) | 1 | - | 543.7 | 1.75 | 28.9 | ||
| 2 | 583.6 | 1.88 | 18.9 | 578.2 | 1.86 | ||
| 606.2 | 1.96 | 607.1 | 1.96 | ||||
| 617.3 | 1.99 | ||||||
| 3 | 643.3 | 2.08 | 639.7 | 2.06 | |||
| 660.5 | 2.14 | 661.6 | 2.14 | ||||
| 4 | 673.8 | 2.18 | - | ||||
| H2O (18 amu) | 1 | - | 525.6 | 1.69 | 38.0 | ||
| 545.0 | 1.75 | ||||||
| 2 | 582.9 | 1.88 | 34.8 | 572.0 | 1.84 | ||
| 612.9 | 1.98 | 588.0 | 1.90 | ||||
| 608.4 | 1.96 | ||||||
| 3 | 641.5 | 2.07 | 638.4 | 2.07 | |||
| 657.2 | 2.13 | 651.8 | 2.11 | ||||
| 4 | 675.9 | 2.19 | - | ||||
| Adsorbed Species | Eads, eV (on ZnPc) from Theory | Eads, eV (on MoO3) from Theory | Edesmin, eV (Unexposed MoO3/ZnPc) from TDS | Edesmin, eV (MoO3/ZnPc with DMMP) from TDS |
|---|---|---|---|---|
| DMMP | 0.8 | 2.5 | - | - |
| CH2O | 0.5 | 0.8 | - | 1.7 |
| CO | 0.9 | 3.4 | 2.1 | 1.7 |
| CO2 | 0.1 | 0.4 | 1.9 | 1.7 |
| PCH | 1.8 | 1.9 | - | 1.7 |
| H2O | 0.4 | 0.7 | 1.9 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powroźnik, P.; Solecka, B.; Pander, P.; Jakubik, W.; Dias, F.B.; Krzywiecki, M. Zinc Phthalocyanine Sensing Mechanism Quantification for Potential Application in Chemical Warfare Agent Detectors. Sensors 2022, 22, 9947. https://doi.org/10.3390/s22249947
Powroźnik P, Solecka B, Pander P, Jakubik W, Dias FB, Krzywiecki M. Zinc Phthalocyanine Sensing Mechanism Quantification for Potential Application in Chemical Warfare Agent Detectors. Sensors. 2022; 22(24):9947. https://doi.org/10.3390/s22249947
Chicago/Turabian StylePowroźnik, Paulina, Barbara Solecka, Piotr Pander, Wiesław Jakubik, Fernando B. Dias, and Maciej Krzywiecki. 2022. "Zinc Phthalocyanine Sensing Mechanism Quantification for Potential Application in Chemical Warfare Agent Detectors" Sensors 22, no. 24: 9947. https://doi.org/10.3390/s22249947








