A Differential Inertial Wearable Device for Breathing Parameter Detection: Hardware and Firmware Development, Experimental Characterization
Abstract
:1. Introduction
- The development of a novel chest band for monitoring breathing parameters based on a differential inertial approach; this includes a low-power microcontroller section that acquires inertial data from both inertial sensors, processes them to extract breathing data, and coordinates the transmission of data to a custom mobile app.
- Firmware development for acquiring and processing inertial data from the two IMUs. In detail, the processing relies on digitally filtering the acquired data to remove the undesired signal components and applying a peak detection algorithm to detect the inhalation and exhalation times, useful for deriving other breathing parameters.
- A comprehensive characterization of the developed inertia-based chest band for determining its performances in terms of the correlation coefficient, mean difference (MD), limits of agreement (LoA), and mean absolute error (MAE), compared to the reference measurements. In detail, correlation and Bland–Altman analyses were performed on the measures gathered by eight users varying in age, gender, and physical constitution. Additionally, a comparison between the performances of the developed chest band with another one based on a piezoresistive strain sensor and an IMU, presented in our previous work, was carried out [28].
Overview of Wearable Devices for Monitoring Cardiorespiratory Parameters Based on Inertial Sensors
2. Materials and Methods
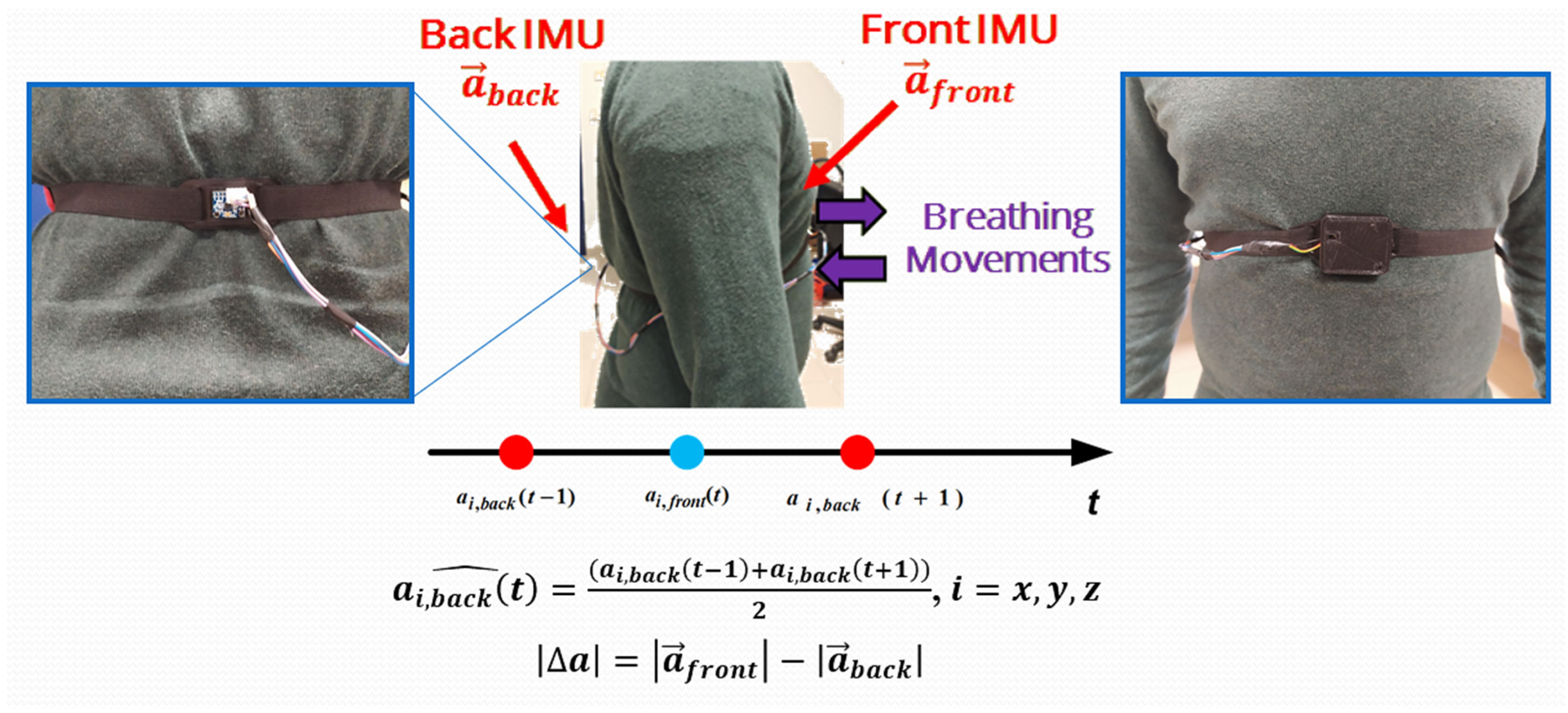
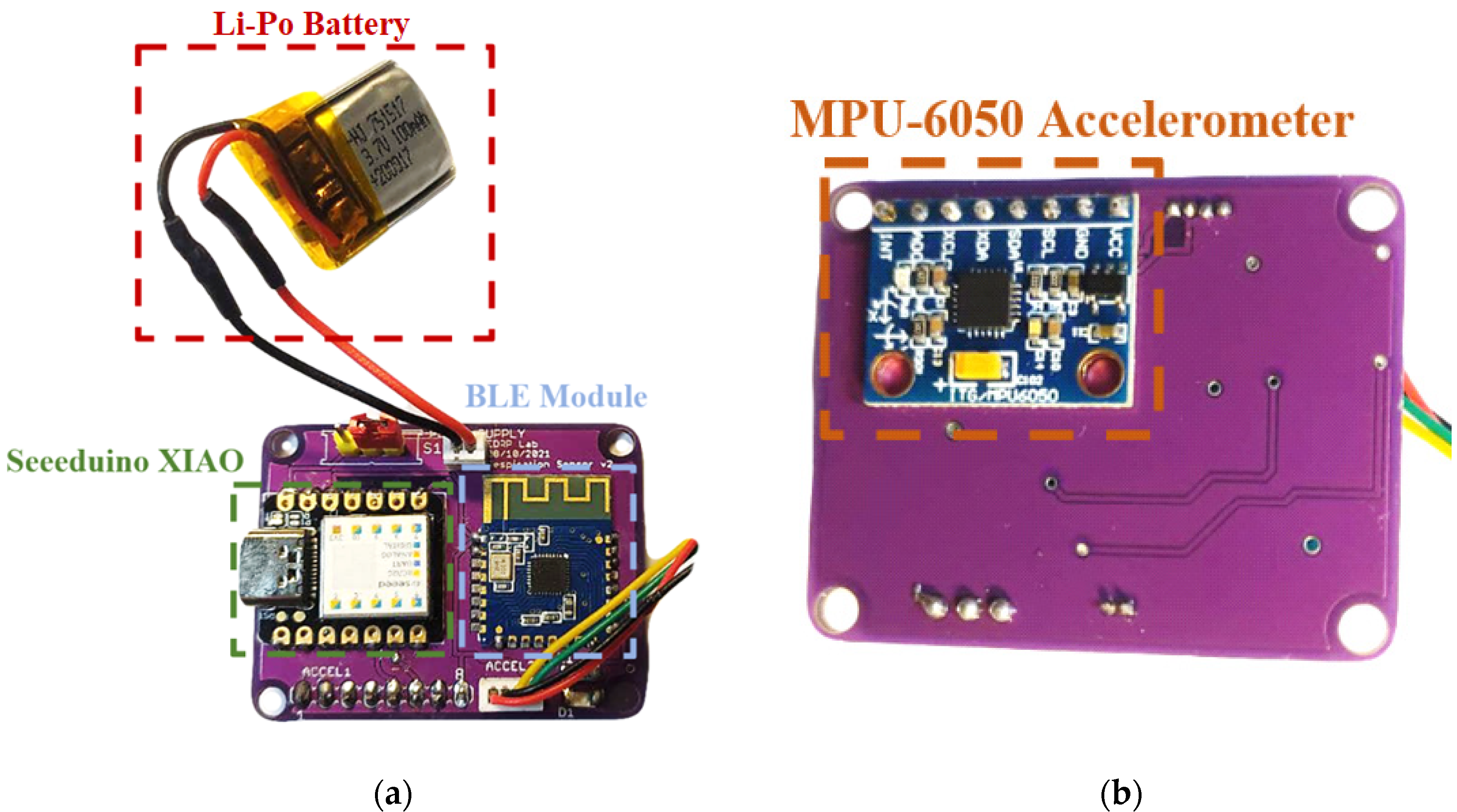
2.1. Architecture of the Developed Inertia-Based Chest Band
- Low dimensions and weight: the chest band was compact (6 cm × 2.8 cm × 1.4 cm front section and 4 cm × 2 cm × 0.1 cm rear section) and lightweight (35 g), making it discreet and nonobtrusive.
- Complete and ready-to-use: the development covered both the hardware and firmware sections, leading to a complete monitoring system applicable in real operative scenarios.
- Real-time monitoring: the developed system provided real-time measurements of the breathing parameters without postprocessing through an external host device (e.g., PC).
- High immunity to motion artifacts: thanks to the deployed differential inertial approach, the presented wearable device was relatively immune to artifacts induced by extraneous body movements.
- The better integration of the rear IMU inside the chest band, improving the wiring, or providing a wireless connection with the front section; in this way, the device wearability would be further enhanced.
- The deployment of advanced digital filtering methods, enabling a better rejection of noise and artifacts induced by body motions.
2.2. Assembly of the Developed Chest Band
2.3. Firmware of Developed Inertia-Based Chest Band
3. Results
3.1. Firmware Optimization for Enhancing the Chest Band’s Accuracy
3.2. Characterization of the Developed Inertia-Based Chest Band
4. Discussion
4.1. Characterization of the Chest Band for Multiple Users
4.2. Performance Comparison of the Presented Chest Band with the Scientific Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Exercise. Sensors 2020, 20, 6396. [Google Scholar] [CrossRef] [PubMed]
- Chourpiliadis, C.; Bhardwaj, A. Physiology, Respiratory Rate; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cretikos, M.A.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory Rate: The Neglected Vital Sign. Med. J. Aust. 2008, 188, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Capodilupo, J.V.; Lastella, M.; Sargent, C.; Roach, G.D.; Lee, V.H.; Capodilupo, E.R. Analyzing Changes in Respiratory Rate to Predict the Risk of COVID-19 Infection. PLoS ONE 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Talaminos Barroso, A.; Márquez Martín, E.; Roa Romero, L.M.; Ortega Ruiz, F. Factors Affecting Lung Function: A Review of the Literature. Arch. Bronconeumol. 2018, 54, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.W.; Richards, J.C.; Foster, G.E.; Guenette, J.A. Sex Differences in Respiratory Exercise Physiology. Sports Med. 2004, 34, 567–579. [Google Scholar] [CrossRef]
- Hopkins, E.; Sanvictores, T.; Sharma, S. Physiology, Acid Base Balance; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bae, D.; Matthews, J.J.L.; Chen, J.J.; Mah, L. Increased Exhalation to Inhalation Ratio during Breathing Enhances High-Frequency Heart Rate Variability in Healthy Adults. Psychophysiology 2021, 58, e13905. [Google Scholar] [CrossRef]
- Van Diest, I.; Verstappen, K.; Aubert, A.E.; Widjaja, D.; Vansteenwegen, D.; Vlemincx, E. Inhalation/Exhalation Ratio Modulates the Effect of Slow Breathing on Heart Rate Variability and Relaxation. Appl. Psychophysiol. Biofeedback 2014, 39, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Wickboldt, A.T.; Bowen, A.F.; Kaye, A.J.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Sleep Physiology, Abnormal States, and Therapeutic Interventions. Ochsner J. 2012, 12, 123–134. [Google Scholar]
- Anderson, M.; Collison, K.; Drummond, M.B.; Hamilton, M.; Jain, R.; Martin, N.; Mularski, R.A.; Thomas, M.; Zhu, C.-Q.; Ferguson, G.T. Peak Inspiratory Flow Rate in COPD: An Analysis of Clinical Trial and Real-World Data. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 933–943. [Google Scholar] [CrossRef]
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent Development of Respiratory Rate Measurement Technologies. Physiol. Meas. 2019, 40, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Massaroni, C.; Nicolò, A.; Lo Presti, D.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-Based Methods for Measuring Respiratory Rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [PubMed]
- De Fazio, R.; De Vittorio, M.; Visconti, P. Innovative IoT Solutions and Wearable Sensing Systems for Monitoring Human Biophysical Parameters: A Review. Electronics 2021, 10, 1660. [Google Scholar] [CrossRef]
- Smith, I.; Mackay, J.; Fahrid, N.; Krucheck, D. Respiratory Rate Measurement: A Comparison of Methods. Br. J. Healthc. Assist. 2011, 5, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Postolache, O.; Girao, P.S.; Mendes, J.; Postolache, G. Unobstrusive Heart Rate and Respiratory Rate Monitor Embedded on a Wheelchair. In Proceedings of the 2009 IEEE International Workshop on Medical Measurements and Applications, Cetraro, Italy, 29–30 May 2009; pp. 83–88. [Google Scholar]
- Ciuti, G.; Ricotti, L.; Menciassi, A.; Dario, P. MEMS Sensor Technologies for Human Centred Applications in Healthcare, Physical Activities, Safety and Environmental Sensing: A Review on Research Activities in Italy. Sensors 2015, 15, 6441–6468. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.E.; Cretu, E. A Wireless, Real-Time Respiratory Effort and Body Position Monitoring System for Sleep. Biomed. Signal Process. Control. 2020, 61, 102023. [Google Scholar] [CrossRef]
- De Fazio, R.; Al-Hinnawi, A.-R.; De Vittorio, M.; Visconti, P. An Energy-Autonomous Smart Shirt Employing Wearable Sensors for Users’ Safety and Protection in Hazardous Workplaces. Appl. Sci. 2022, 12, 2926. [Google Scholar] [CrossRef]
- Milici, S.; Lázaro, A.; Villarino, R.; Girbau, D.; Magnarosa, M. Wireless Wearable Magnetometer-Based Sensor for Sleep Quality Monitoring. IEEE Sens. J. 2018, 18, 2145–2152. [Google Scholar] [CrossRef]
- Yüzer, A.H.; Sümbül, H.; Polat, K. A Novel Wearable Real-Time Sleep Apnea Detection System Based on the Acceleration Sensor. IRBM 2020, 41, 39–47. [Google Scholar] [CrossRef]
- Kempe, V. Inertial MEMS: Principles and Practice; Cambridge University Press: Cambridge, MA, USA, 2011; ISBN 978-1-139-49482-3. [Google Scholar]
- Kok, M.; Hol, J.D.; Schön, T.B. Using Inertial Sensors for Position and Orientation Estimation. FNT Signal Process 2017, 11, 1–89. [Google Scholar] [CrossRef] [Green Version]
- Cardarelli, D. An Integrated MEMS Inertial Measurement Unit. In Proceedings of the 2002 IEEE Position Location and Navigation Symposium (IEEE Cat. No.02CH37284), Palm Springs, CA, USA, 15–18 April 2002; pp. 314–319. [Google Scholar]
- Fitzgerald, A.M. MEMS Inertial Sensors. In Position, Navigation, and Timing Technologies in the 21st Century; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 1435–1446. ISBN 978-1-119-45855-5. [Google Scholar]
- Alandry, B.; Latorre, L.; Mailly, F.; Nouet, P. A CMOS-MEMS Inertial Measurement Unit. In Proceedings of the 2010 IEEE SENSORS, Waikoloa, HI, USA, 1–4 November 2010; pp. 1033–1036. [Google Scholar]
- Chatterjee, G.; Latorre, L.; Mailly, F.; Nouet, P.; Hachelef, N.; Oudea, C. MEMS Based Inertial Measurement Units. In Proceedings of the 2015 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), Montpellier, France, 27–30 April 2015; pp. 1–5. [Google Scholar]
- De Fazio, R.; De Vittorio, M.; Visconti, P. A BLE-Connected Piezoresistive and Inertial Chest Band for Remote Monitoring of the Respiratory Activity by an Android Application: Hardware Design and Software Optimization. Future Internet 2022, 14, 183. [Google Scholar] [CrossRef]
- De Fazio, R.; Stabile, M.; De Vittorio, M.; Velázquez, R.; Visconti, P. An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring. Electronics 2021, 10, 2178. [Google Scholar] [CrossRef]
- Karacocuk, G.; Höflinger, F.; Zhang, R.; Reindl, L.M.; Laufer, B.; Möller, K.; Röell, M.; Zdzieblik, D. Inertial Sensor-Based Respiration Analysis. IEEE Trans. Instrum. Meas. 2019, 68, 4268–4275. [Google Scholar] [CrossRef]
- Lo Presti, D.; Carnevale, A.; D’Abbraccio, J.; Massari, L.; Massaroni, C.; Sabbadini, R.; Zaltieri, M.; Di Tocco, J.; Bravi, M.; Miccinilli, S.; et al. A Multi-Parametric Wearable System to Monitor Neck Movements and Respiratory Frequency of Computer Workers. Sensors 2020, 20, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of Breathing Parameters Using an Inertial Measurement Unit (IMU)-Based System. Sensors 2019, 19, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani, M.H.; Berkvens, R.; Weyn, M. Chest-Worn Inertial Sensors: A Survey of Applications and Methods. Sensors 2021, 21, 2875. [Google Scholar] [CrossRef]
- De Fazio, R.; Visconti, P.; Perrone, E.; Greco, M.R.; Velazquez, R. Development and Testing of Piezoresistive and Inertial-Based Chest Bands for Breathing Monitoring. In Proceedings of the 2022 7th International Conference on Smart and Sustainable Technologies (SpliTech), Split and Bol, Croatia, 5–8 July 2022; pp. 1–6. [Google Scholar]
- Doheny, E.P.; Lowery, M.M.; Russell, A.; Ryan, S. Estimation of Respiration Rate and Sleeping Position Using a Wearable Accelerometer. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4668–4671. [Google Scholar]
- Dash, S.; Shelley, K.H.; Silverman, D.G.; Chon, K.H. Estimation of Respiratory Rate From ECG, Photoplethysmogram, and Piezoelectric Pulse Transducer Signals: A Comparative Study of Time–Frequency Methods. IEEE Trans. Biomed. Eng. 2010, 57, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Bawua, L.K.; Miaskowski, C.; Hu, X.; Rodway, G.W.; Pelter, M.M. A Review of the Literature on the Accuracy, Strengths, and Limitations of Visual, Thoracic Impedance, and Electrocardiographic Methods Used to Measure Respiratory Rate in Hospitalized Patients. Ann. Noninvasive Electrocardiol. 2021, 26, 1–14. [Google Scholar] [CrossRef]
- Helfenbein, E.; Firoozabadi, R.; Chien, S.; Carlson, E.; Babaeizadeh, S. Development of Three Methods for Extracting Respiration from the Surface ECG: A Review. J. Electrocardiol. 2014, 47, 819–825. [Google Scholar] [CrossRef]
- Lee, J.; Chon, K.H. An Autoregressive Model-Based Particle Filtering Algorithms for Extraction of Respiratory Rates as High as 90 Breaths per Minute from Pulse Oximeter. IEEE Trans. Biomed. Eng. 2010, 57, 2158–2167. [Google Scholar] [CrossRef]
- Lázaro, J.; Nam, Y.; Gil, E.; Laguna, P.; Chon, K.H. Respiratory Rate Derived from Smartphone-Camera-Acquired Pulse Photoplethysmographic Signals. Physiol. Meas. 2015, 36, 2317–2333. [Google Scholar] [CrossRef] [Green Version]
- Rehouma, H.; Noumeir, R.; Essouri, S.; Jouvet, P. Advancements in Methods and Camera-Based Sensors for the Quantification of Respiration. Sensors 2020, 20, 7252. [Google Scholar] [CrossRef] [PubMed]
- Janik, P.; Janik, M.A.; Pielka, M. Monitoring Breathing and Heart Rate Using Episodic Broadcast Data Transmission. Sensors 2022, 22, 6019. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Laufer, B.; Krueger-Ziolek, S.; Moeller, K. Measurement of Respiratory Rate with Inertial Measurement Units. Curr. Dir. Biomed. Eng. 2020, 6, 237–240. [Google Scholar] [CrossRef]
- Singh, R.E.; Fleury, J.M.; Gupta, S.; Bachman, N.P.; Alumbaugh, B.; White, G. Involuntary Breathing Movement Pattern Recognition and Classification via Force-Based Sensors. Biomechanics 2022, 2, 525–537. [Google Scholar] [CrossRef]
- Hafezi, M.; Montazeri, N.; Saha, S.; Zhu, K.; Gavrilovic, B.; Yadollahi, A.; Taati, B. Sleep Apnea Severity Estimation From Tracheal Movements Using a Deep Learning Model. IEEE Access 2020, 8, 22641–22649. [Google Scholar] [CrossRef]
- Bucklin, C.L.; Das, M.; Luo, S.L. An Inexpensive Accelerometer-Based Sleep-Apnea Screening Technique. In Proceedings of the IEEE 2010 National Aerospace Electronics Conference, Fairborn, OH, USA, 14–16 July 2010; pp. 396–399. [Google Scholar]
- Fekr, A.R.; Radecka, K.; Zilic, Z. Tidal Volume Variability and Respiration Rate Estimation Using a Wearable Accelerometer Sensor. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare—Transforming Healthcare through Innovations in Mobile and Wireless Technologies (MOBIHEALTH), Athens, Greece, 3–5 November 2014; pp. 1–6. [Google Scholar]
- Elfaramawy, T.; Latyr Fall, C.; Morissette, M.; Lellouche, F.; Gosselin, B. Wireless Respiratory Monitoring and Coughing Detection Using a Wearable Patch Sensor Network. In Proceedings of the 2017 15th IEEE International New Circuits and Systems Conference (NEWCAS), Strasbourg, France, 25–28 June 2017; pp. 197–200. [Google Scholar]
- Gravina, R.; Alinia, P.; Ghasemzadeh, H.; Fortino, G. Multi-Sensor Fusion in Body Sensor Networks: State-of-the-Art and Research Challenges. Inf. Fusion 2017, 35, 68–80. [Google Scholar] [CrossRef]
- Johnson, G.; Pianosi, P.; Rajamani, R. Estimation of Three-Dimensional Thoracoabdominal Displacements During Respiration Using Inertial Measurement Units. IEEE/ASME Trans. Mechatron. 2022, 27, 4224–4234. [Google Scholar] [CrossRef]
- Forner-Cordero, A.; Mateu-Arce, M.; Forner-Cordero, I.; Alcántara, E.; Moreno, J.C.; Pons, J.L. Study of the Motion Artefacts of Skin-Mounted Inertial Sensors under Different Attachment Conditions. Physiol. Meas. 2008, 29, N21–N31. [Google Scholar] [CrossRef]
- Vertens, J.; Fischer, F.; Heyde, C.; Hoeflinger, F.; Zhang, R.; Reindl, L.; Gollhofer, A. Measuring Respiration and Heart Rate Using Two Acceleration Sensors on a Fully Embedded Platform. In Proceedings of the 3rd International Congress on Sport Sciences Research and Technology Support; SCITEPRESS—Science and Technology Publications: Lisbon, Portugal, 2015; pp. 15–23. [Google Scholar]
- Lapi, S.; Lavorini, F.; Borgioli, G.; Calzolai, M.; Masotti, L.; Pistolesi, M.; Fontana, G.A. Respiratory Rate Assessments Using a Dual-Accelerometer Device. Respir. Physiol. Neurobiol. 2014, 191, 60–66. [Google Scholar] [CrossRef]
- West, J.B.; Luks, A.M. West’s Respiratory Physiology: The Essentials, 11th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2020; ISBN 978-1-975139-26-1. [Google Scholar]
- McCarron, M.M.; Devine, B.J. Clinical Pharmacy: Case Studies: Case Number 25 Gentamicin Therapy. Drug Intell. Clin. Pharm. 1974, 8, 650–655. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman Analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoo, S.K. Respiration Rate Estimation Based on Independent Component Analysis of Accelerometer Data: Pilot Single-Arm Intervention Study. JMIR mHealth uHealth 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Liu, H.; Zheng, D. Influences of Sensor Placement Site and Subject Posture on Measurement of Respiratory Frequency Using Triaxial Accelerometers. Front. Physiol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Jacobs, F.; Scheerhoorn, J.; Mestrom, E.; van der Stam, J.; Bouwman, R.A.; Nienhuijs, S. Reliability of Heart Rate and Respiration Rate Measurements with a Wireless Accelerometer in Postbariatric Recovery. PLoS ONE 2021, 16, e0247903. [Google Scholar] [CrossRef] [PubMed]
- Jarchi, D.; Rodgers, S.J.; Tarassenko, L.; Clifton, D. Accelerometry-Based Estimation of Respiratory Rate for Post-Intensive Care Patient Monitoring. IEEE Sens. J. 2018, 18, 4981–4989. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.; Li, Y.; Rehg, J.M.; Picard, R.W. Cardiac and Respiratory Parameter Estimation Using Head-Mounted Motion-Sensitive Sensors. EAI Endorsed Trans. Pervasive Health Technol. 2015, 1, e2. [Google Scholar] [CrossRef]
- De Fazio, R.; Mattei, V.; Al-Naami, B.; De Vittorio, M.; Visconti, P. Methodologies and Wearable Devices to Monitor Biophysical Parameters Related to Sleep Dysfunctions: An Overview. Micromachines 2022, 13, 1335. [Google Scholar] [CrossRef]
- Ryser, F.; Hanassab, S.; Lambercy, O.; Werth, E.; Gassert, R. Respiratory Analysis during Sleep Using a Chest-Worn Accelerometer: A Machine Learning Approach. Biomed. Signal Process. Control. 2022, 78, 104014. [Google Scholar] [CrossRef]
- Pham, H.-T.; Nguyen, M.-A.; Sun, C.-C. AIoT Solution Survey and Comparison in Machine Learning on Low-Cost Microcontroller. In Proceedings of the 2019 International Symposium on Intelligent Signal Processing and Communication Systems (ISPACS), Taipei, Taiwan, 3–6 December 2019; pp. 1–2. [Google Scholar]












| Work | N° of Inertial Sensor | Installation Position | Processing | Complexity | Application |
|---|---|---|---|---|---|
| P. Janik et al. [42] | 1 (LSM9DS1) | Abdominal wall | Digital filtering and peak detection | Low | Breathing and heartbeat monitoring |
| S. Beck et al. [43] | 2 (MPU-6050) | Abdomen and thorax | Digital filtering and frequency domain analysis | Low | Breathing monitoring |
| M. Hafezi et al. [45] | 1 | Suprasternal notch | Time domain features extraction and deep learning classifier | High | Breathing monitoring and OSA and hypopnea event detection |
| C. L. Bucklin et al. [46] | 1 | Suprasternal notch | Frequency domain analysis, digital filtering, and PCA | Medium | Breathing monitoring and OSA and hypopnea event detection |
| Doheny et al. [35] | 1 (BiostampRC) | Torso | Digital filtering and time domain analysis | Low | Breathing monitoring |
| T. Eferamawy et al. [48] | 2 (LSM9DS0) | Abdomen and thorax | Digital filtering and time domain analysis | Low | Breathing monitoring |
| J. Vertens et al. [52] | 2 (LIS3DSH) | Chest and back | Digital filtering and time domain analysis | Low | Breathing monitoring |
| S. Lapi et al. [53] | 2 (T100) | Sternum | Digital filtering and time domain analysis | Low | Breathing monitoring |
| User | Gender | Age (Years) | Height (m) | Weight (Kg) |
|---|---|---|---|---|
| 1 | Male | 24 | 1.82 | 78 |
| 2 | Male | 25 | 1.89 | 91 |
| 3 | Male | 25 | 1.83 | 82 |
| 4 | Male | 27 | 1.78 | 78 |
| 5 | Female | 22 | 1.71 | 62 |
| 6 | Female | 23 | 1.67 | 56 |
| 7 | Female | 24 | 1.65 | 61 |
| 8 | Female | 25 | 1.70 | 69 |
| Parameters Set # | Cut-off Frequency (Hz) | Lag | Threshold | Influence | Minimum Peaks Time Distance (ms) |
|---|---|---|---|---|---|
| 0 | 0.5 | 56 | 2.95 | 0.58 | 1100 |
| 1 | 0.6 | 52 | 2.95 | 0.58 | 1100 |
| 2 | 0.6 | 56 | 2.90 | 0.58 | 1100 |
| 3 | 0.6 | 56 | 2.85 | 0.58 | 1000 |
| 4 | 0.6 | 58 | 2.85 | 0.58 | 1000 |
| 5 | 0.6 | 60 | 2.87 | 0.58 | 1000 |
| First Set (BrPM) | Second Set (BrPM) | Third Set (BrPM) | Fourth Set (BrPM) | Fifth Set (BrPM) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Band | Reference | Band | Reference | Band | Reference | Band | Reference | Band | Reference |
| 18 | 22 | 20 | 24 | 26 | 26 | 32 | 30 | 26 | 26 |
| 24 | 24 | 24 | 26 | 26 | 26 | 20 | 20 | 26 | 26 |
| 20 | 24 | 22 | 24 | 20 | 20 | 30 | 26 | 30 | 30 |
| 30 | 28 | 22 | 24 | 24 | 24 | 30 | 28 | 30 | 30 |
| 28 | 28 | 22 | 24 | 20 | 22 | 30 | 30 | 32 | 32 |
| 24 | 26 | 24 | 24 | 22 | 22 | 28 | 28 | 30 | 30 |
| 24 | 26 | 24 | 24 | 26 | 24 | 24 | 24 | 30 | 32 |
| 20 | 20 | 22 | 24 | 20 | 20 | 26 | 26 | 26 | 28 |
| 20 | 22 | 22 | 22 | 22 | 22 | 26 | 26 | 30 | 30 |
| 22 | 22 | 20 | 22 | 28 | 26 | 32 | 32 | 26 | 28 |
| Parameter | Pearson Coefficient (r) | MAE (%) |
|---|---|---|
| TI (ms) | 0.97 | 4.3 |
| TE (ms) | 0.98 | 3.8 |
| IER (Adim) | 0.70 | 5.0 |
| V (l/min) | 0.98 | 4.3 |
| Seated | Standing | Walking | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
(BrPM) | (BrPM) | (%) | (BrPM) | (BrPM) | (%) | (BrPM) | (BrPM) | (%) | ||||
| Piezoresistive/inertial chest band [28] | 0.96 | +0.68 | +3.20/ −1.75 | 3.13 | 0.95 | +0.40 | +3.34/−2.56 | 3.56 | 0.93 | +0.23 | 3.65/ −3.28 | 3.56 |
| Proposed differential inertial chest band | 0.92 | −0.27 | +1.16/ −1.75 | 1.15 | 0.92 | +0.56 | +3.48/ −2.53 | 2.69 | 0.94 | +0.48 | 3.02/ −1.8 | 3.26 |
| Work | N° of Inertial Sensors | Sensor Position | Processing Unit | Acquisition Frequency (Hz) | (%) | Wireless Connectivity |
|---|---|---|---|---|---|---|
| E. P. Doheny et al. [35] | 2 (BiostampRC, MC10 Inc.) | Chest and Abdomen (b) | PC | 125 | 11.13 | No |
| S. Beck et al. [43] | 2 (MPU-6050) | Thorax and Back (c) | Arduino MKR1010 | N.A. (a) | 0.83 | No |
| J. Lee et al. [57] | 1 (Biopac MP 150TM) | Chest (b) | PC | 500 | 4.42 | No |
| S. Hughes et al. [58] | 1 (Biopac TSD109C2) | Clavicular (b) | PC | 500 | 13.33 | No |
| F. Jacobs et al. [59] | 1 (Philips Healthdot) | Lower Rib (c) | Healthdot | 100 | <25 | LoRa |
| D. Jarchi et al. [60] | 1 | Chest (c) | PC | 25 | 4.28 | No |
| J. Hernandez et al. [61] | 1 (Google Glass) | Head (c) | N.A. (a) | 256 | 10.57 | WiFi/BLE |
| Proposed chest band | 2 (MPU-6050) | Chest and Back (d) | SAMD21G18A | 100 | 1.15 | BLE 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Fazio, R.; Greco, M.R.; De Vittorio, M.; Visconti, P. A Differential Inertial Wearable Device for Breathing Parameter Detection: Hardware and Firmware Development, Experimental Characterization. Sensors 2022, 22, 9953. https://doi.org/10.3390/s22249953
De Fazio R, Greco MR, De Vittorio M, Visconti P. A Differential Inertial Wearable Device for Breathing Parameter Detection: Hardware and Firmware Development, Experimental Characterization. Sensors. 2022; 22(24):9953. https://doi.org/10.3390/s22249953
Chicago/Turabian StyleDe Fazio, Roberto, Maria Rosaria Greco, Massimo De Vittorio, and Paolo Visconti. 2022. "A Differential Inertial Wearable Device for Breathing Parameter Detection: Hardware and Firmware Development, Experimental Characterization" Sensors 22, no. 24: 9953. https://doi.org/10.3390/s22249953








