Intrinsic Blue Fluorescence of 2.0G PAMAM-DCM Polymer Dots and Its Applications for Fe3+ Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Methods
2.2.1. Synthesis of 0.5G PAMAM, 1.0G PAMAM and 2.0G PAMAM
2.2.2. Synthesis of 2.0G PAMAM-DCM PDs
2.3. Measurements
3. Results and Discussion
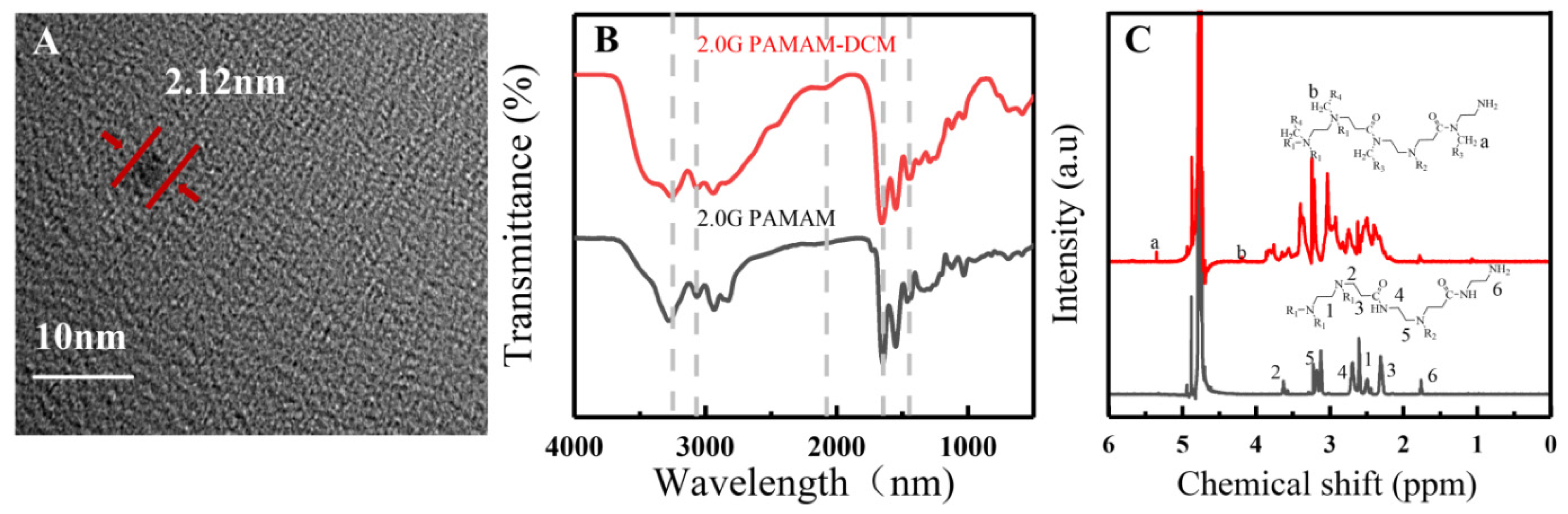
3.1. 2.0G PAMAM-DCM PDs
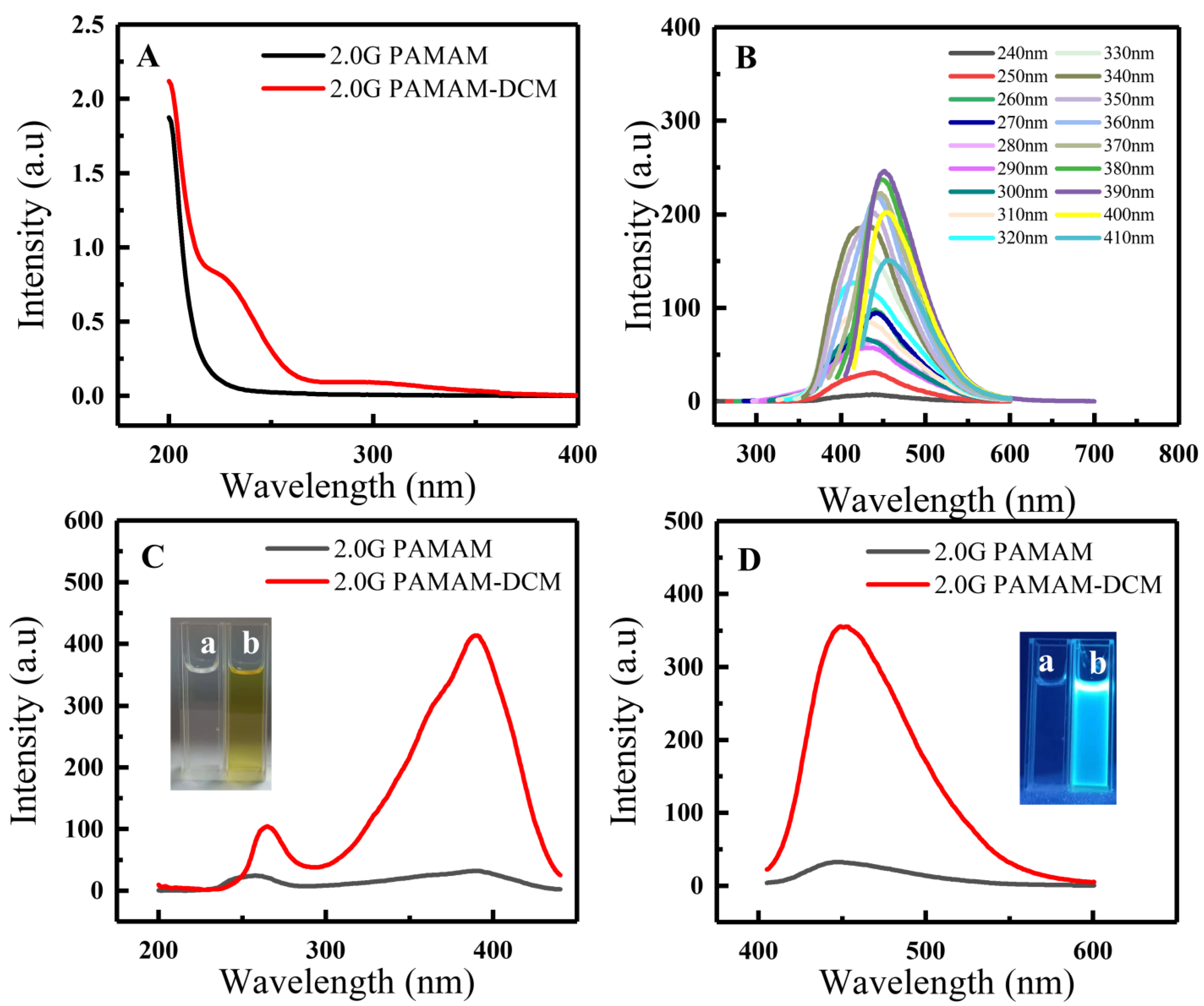
3.2. Fluorescence Properties of 2.0G PAMAM-DCM PDs
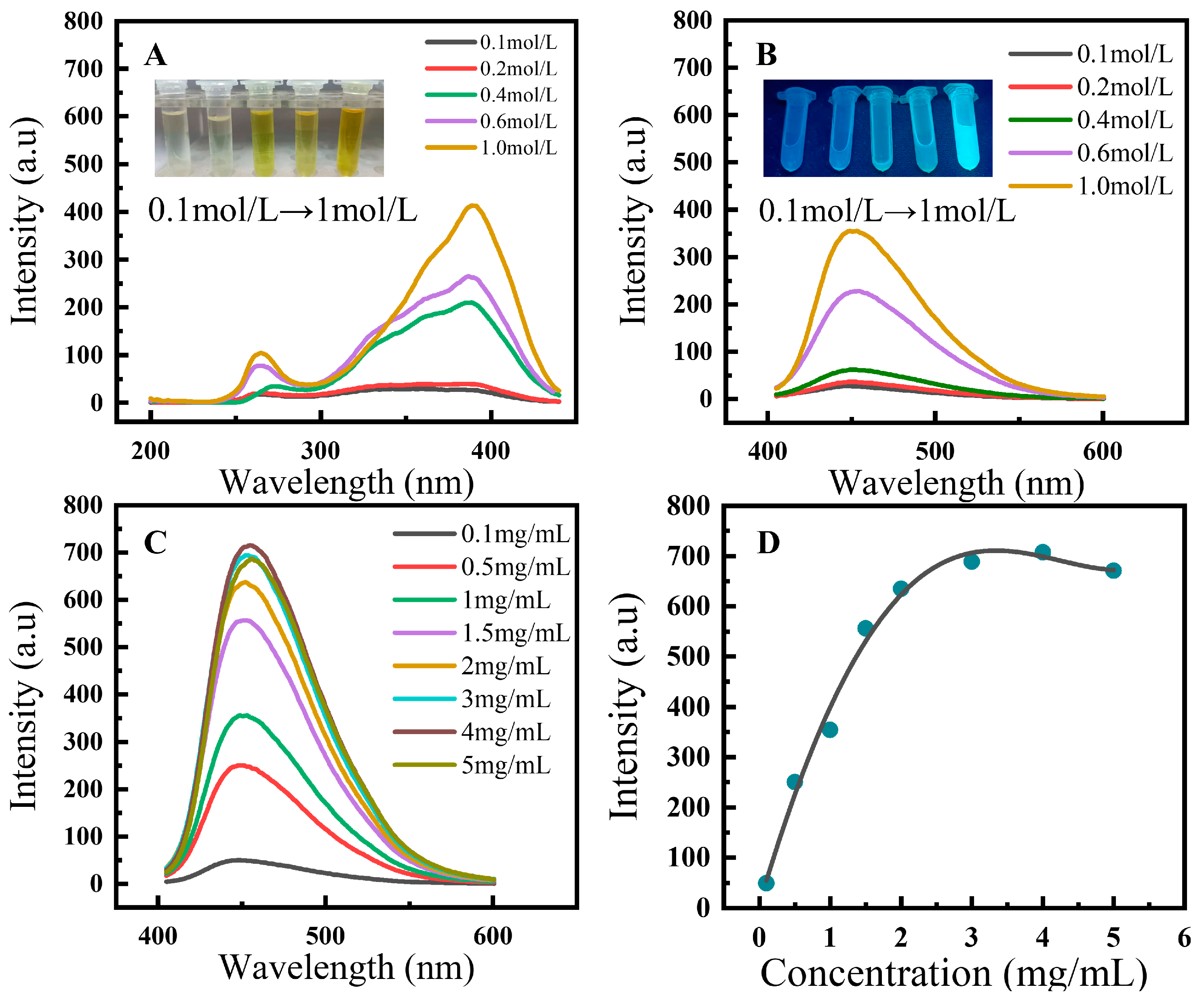
3.2.1. Effects of Concentrations on the Fluorescence Properties
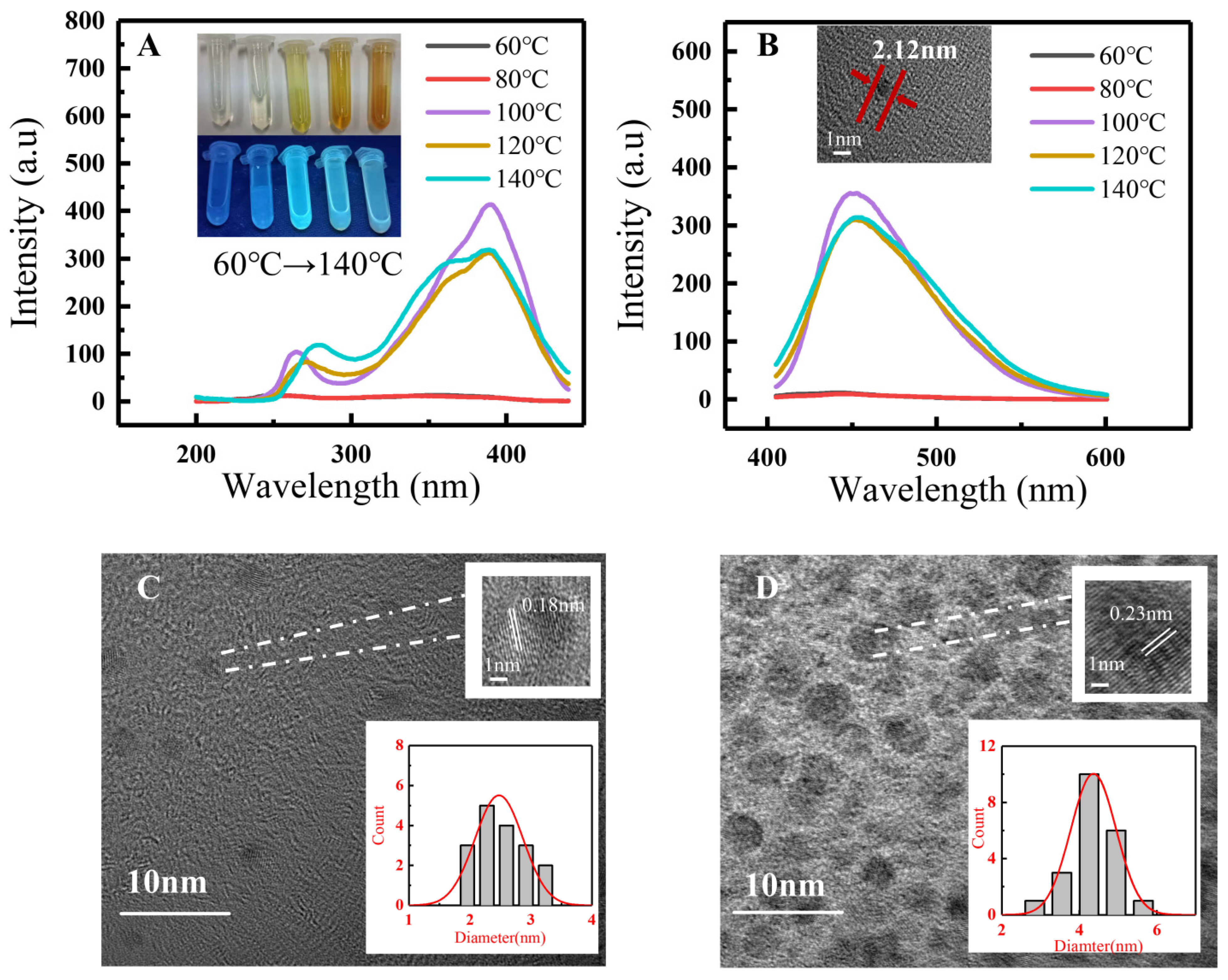
3.2.2. Effects of Temperature on the Fluorescence Properties
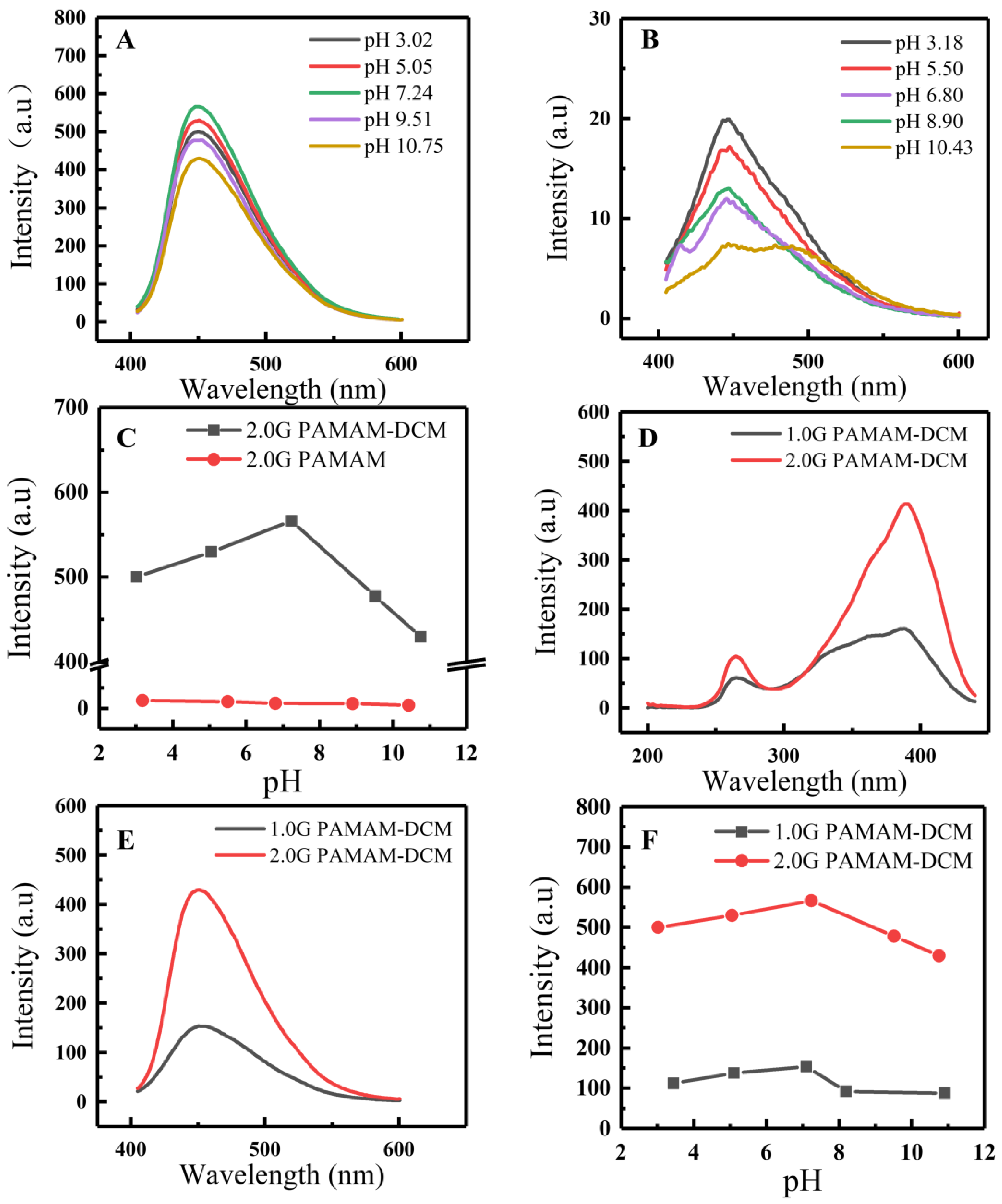
3.2.3. Effects of pH on the Fluorescence Properties
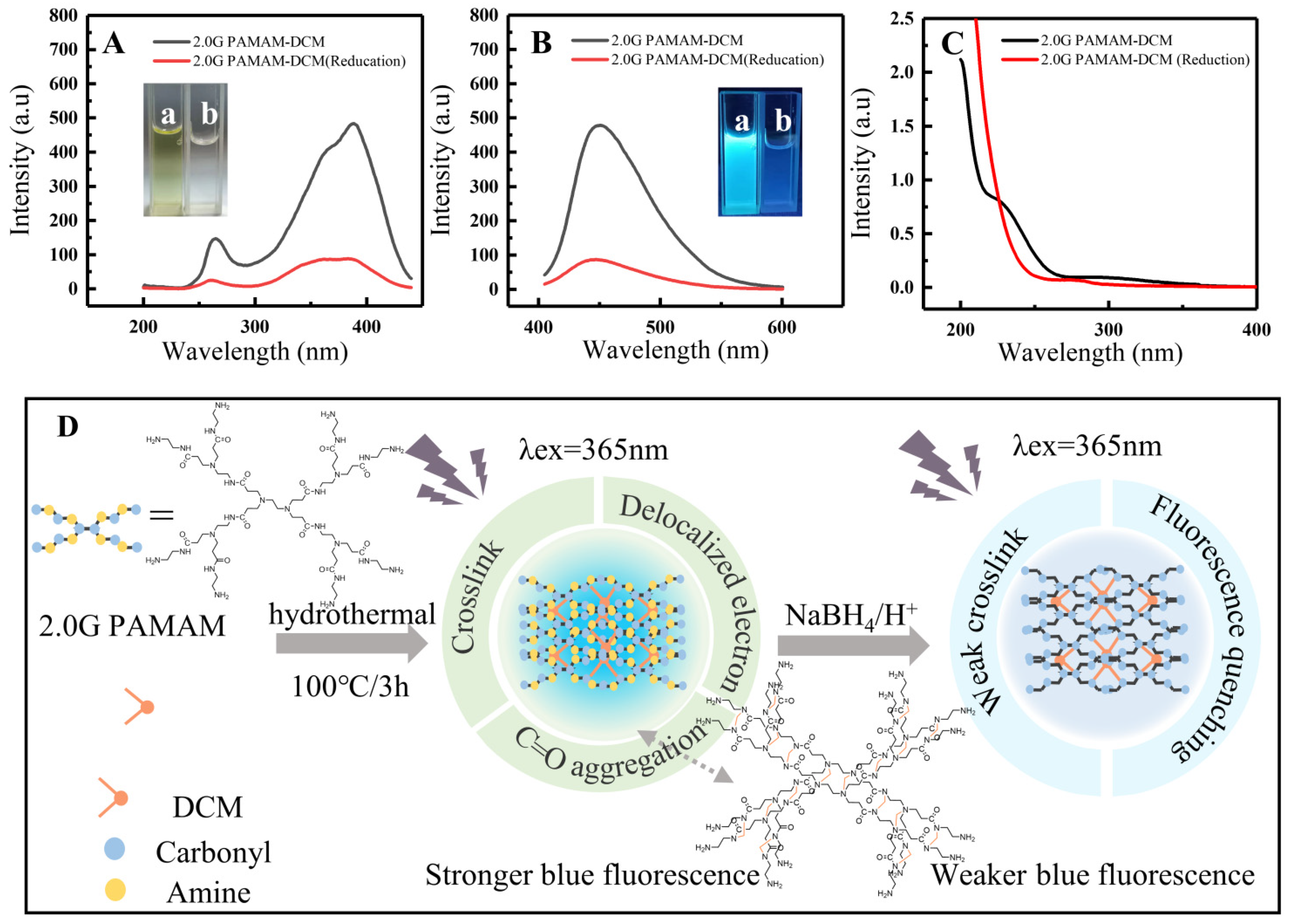
3.3. Possible Fluorescence Mechanism of 2.0G PAMAM-DCM PDs
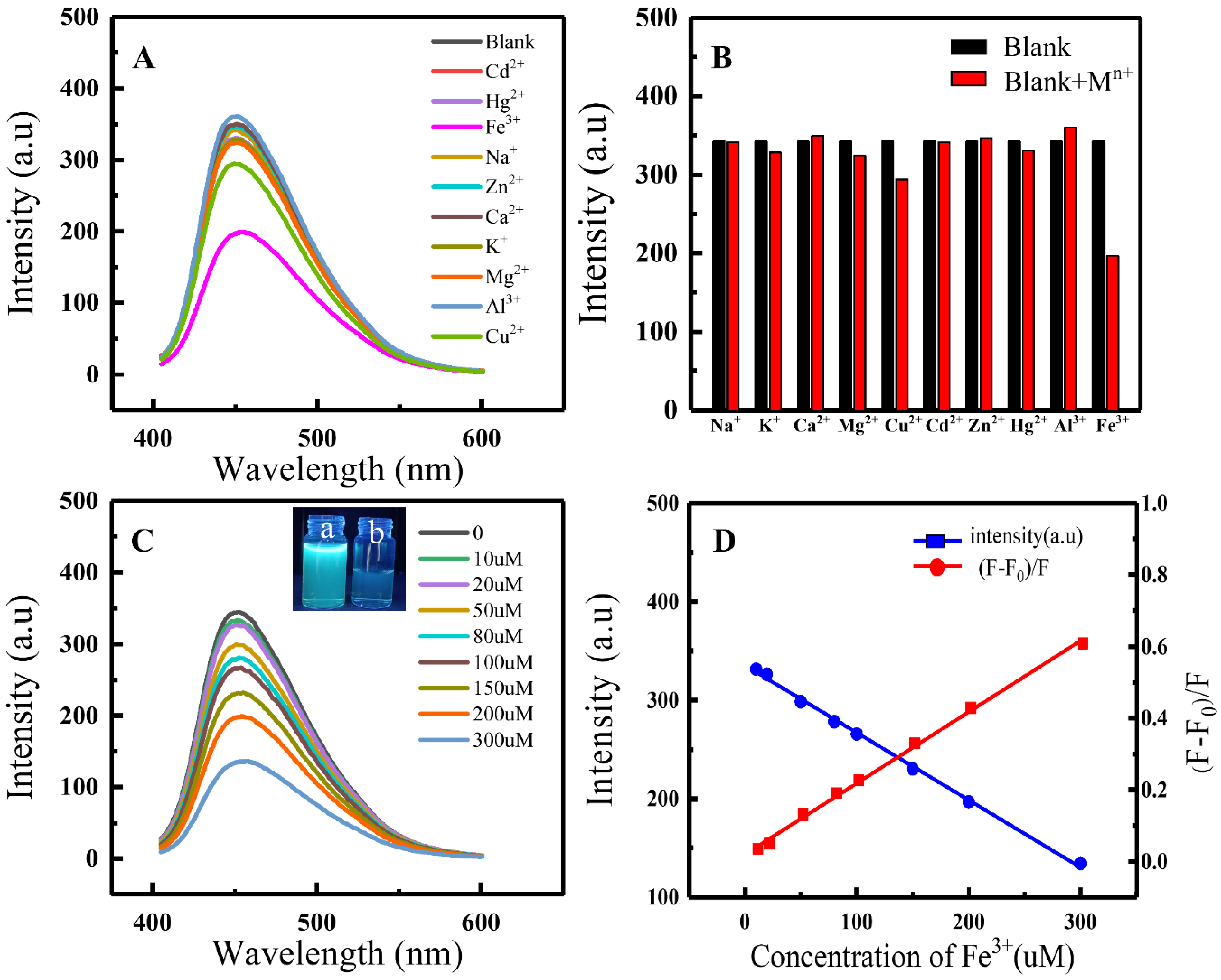
3.4. Metal Ion Sensing Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz, C.; Benitez, C.; Vidal, F.; Barraza, L.F.; Jiménez, V.A.; Guzman, L.; Fuentealba, J.; Yevenes, G.E.; Alderete, J.B. Cytotoxicity and in vivo plasma kinetic behavior of surface-functionalized PAMAM dendrimers. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Zhou, L.; Meng, Q.; Zhang, Y.; Yu, B.; Shen, Y.; Hu, H. Preparation and evaluation of PAMAM dendrimer-based polymer gels physically cross-linked by hydrogen bonding. Biomater. Sci. 2019, 7, 3918–3925. [Google Scholar] [CrossRef] [PubMed]
- Gholipour-Mahmoudalilou, M.; Roghani-Mamaqani, H.; Azimi, R.; Abdollahi, A. Preparation of hyperbranched poly (amidoamine)-grafted graphene nanolayers as a composite and curing agent for epoxy resin. Appl. Surf. Sci. 2018, 428, 1061–1069. [Google Scholar] [CrossRef]
- Martins, I.; Tomás, H.; Lahoz, F.; Rodrigues, J. Engineered fluorescent carbon dots and G4–G6 PAMAM dendrimer nanohybrids for bioimaging and gene delivery. Biomacromolecules 2021, 22, 2436–2450. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Li, D.; Li, L.; Lou, X.; Liu, H. Self-nucleation and self-assembly of highly fluorescent Au5 nanoclusters for bioimaging. Chem. Mater. 2018, 30, 5507–5515. [Google Scholar] [CrossRef]
- Kamil, Y.M.; Al-Rekabi, S.H.; Yaacob, M.H.; Syahir, A.; Chee, H.Y.; Mahdi, M.A.; Bakar, M.H.A. Detection of dengue using PAMAM dendrimer integrated tapered optical fiber sensor. Sci. Rep. 2019, 9, 13483. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Zhang, R.; Zhao, T.; Sun, T.; Chu, X.; Liu, S.; Tang, E.; Xu, X. Efficient reduction of 4-nitrophenol catalyzed by 4-carbo-methoxypyrrolidone modified PAMAM dendrimer–silver nanocomposites. Catal. Sci. Technol. 2019, 9, 6145–6151. [Google Scholar] [CrossRef]
- Guerra, J.; Cantillo, D.; Kappe, C.O. Visible-light photoredox catalysis using a macromolecular ruthenium complex: Reactivity and recovery by size-exclusion nanofiltration in continuous flow. Catal. Sci. Technol. 2016, 6, 4695–4699. [Google Scholar] [CrossRef] [Green Version]
- Fana, M.; Gallien, J.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. PAMAM dendrimer nanomolecules utilized as drug delivery systems for potential treatment of glioblastoma: A systematic review. Int. J. Nanomed. 2020, 15, 2789. [Google Scholar] [CrossRef] [Green Version]
- Vu, M.T.; Bach, L.G.; Nguyen, D.C.; Ho, M.N.; Nguyen, N.H.; Tran, N.Q.; Nguyen, D.H.; Nguyen, C.K.; Hoang Thi, T.T. Modified carboxyl-terminated PAMAM dendrimers as great cytocompatible nano-based drug delivery system. Int. J. Mol. Sci. 2019, 20, 2016. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Imae, T. Fluorescence emission from dendrimers and its pH dependence. J. Am. Chem. Soc. 2004, 126, 13204–13205. [Google Scholar] [CrossRef]
- Shi, W.; Lu, X.; Zhang, S.; Li, H.; Liu, M.; Dong, B. C=N based PAMAM polymer dots: Fluorescent property and Cu2+ sensing application. Colloid. Surface A 2020, 585, 124112. [Google Scholar] [CrossRef]
- Campos, B.B.; Oliva, M.M.; Contreras-Cáceres, R.; Rodriguez-Castellón, E.; Jiménez-Jiménez, J.; da Silva, J.C.E.; Algarra, M. Carbon dots on based folic acid coated with PAMAM dendrimer as platform for Pt (IV) detection. J. Colloid. Interface Sci. 2016, 465, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.B.; Contreras-Cáceres, R.; Bandosz, T.J.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E.; da Silva, J.C.E.; Algarra, M. Carbon dots as fluorescent sensor for detection of explosive nitrocompounds. Carbon 2016, 106, 171–178. [Google Scholar] [CrossRef]
- Wang, D.; Imae, T.; Miki, M. Fluorescence emission from PAMAM and PPI dendrimers. J. Colloid. Interface Sci. 2007, 306, 222–227. [Google Scholar] [CrossRef] [PubMed]
- El-Betany, A.M.; Kamoun, E.A.; James, C.; Jangher, A.; Aljayyoussi, G.; Griffiths, P.; McKeown, N.B.; Gumbleton, M. Auto-fluorescent PAMAM-based dendritic molecules and their potential application in pharmaceutical sciences. Int. J. Pharmaceut. 2020, 579, 119187. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Feng, L.; Li, S.; Zhang, J.; Lu, H.; Feng, S. Unexpected strong blue photoluminescence produced from the aggregation of unconventional chromophores in novel siloxane–poly (amidoamine) dendrimers. Macromolecules 2015, 48, 476–482. [Google Scholar] [CrossRef]
- Ji, Y.; Qian, Y. A study using quantum chemical theory methods on the intrinsic fluorescence emission and the possible emission mechanisms of PAMAM. RSC Adv. 2014, 4, 58788–58794. [Google Scholar] [CrossRef]
- Konopka, M.; Janaszewska, A. Klajnert-Maculewicz, B., Intrinsic fluorescence of PAMAM dendrimers—Quenching studies. Polymers 2018, 10, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, N.I.; Asiri, A.M.; Qusti, A.H.; Alamry, K.A.; Bojinov, V.B. Design and synthesis of pH-selective fluorescence sensing PAMAM light-harvesting dendrons based on 1, 8-naphthalimides. Sens. Actuat. B Chem. 2014, 190, 185–198. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Feng, S. Controllable photophysical properties and self-assembly of siloxane-poly (amidoamine) dendrimers. Phys. Chem. Chem. Phys. 2015, 17, 26783–26789. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25. [Google Scholar] [CrossRef]
- Feng, T.; Zhu, S.; Zeng, Q.; Lu, S.; Tao, S.; Liu, J.; Yang, B. Supramolecular cross-link-regulated emission and related applications in polymer carbon dots. ACS Appl. Mater. Inter. 2017, 10, 12262–12277. [Google Scholar] [CrossRef]
- Vallan, L.; Urriolabeitia, E.P.; Ruipérez, F.; Matxain, J.M.; Canton-Vitoria, R.; Tagmatarchis, N.; Benito, A.M.; Maser, W.K. Supramolecular-enhanced charge transfer within entangled polyamide chains as the origin of the universal blue fluorescence of polymer carbon dots. J. Am. Chem. Soc. 2018, 140, 12862–12869. [Google Scholar] [CrossRef] [Green Version]
- Noun, F.; Jury, E.A.; Naccache, R. Elucidating the quenching mechanism in carbon dot-metal interactions- designing sensitive and selective optical probes. Sensors 2021, 21, 1391. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, P.; Sekar, K.; Sivaraman, G.; Singaravadivel, S. Rhodamine–benzothiazole conjugate as an efficient multimodal sensor for Hg2+ ions and its application to imaging in living cells. New J. Chem. 2018, 42, 11665–11672. [Google Scholar] [CrossRef]
- Bendicho, C.; Lavilla, I.; Pena-Pereira, F.; De la Calle, I.; Romero, V. Nanomaterial-integrated cellulose platforms for optical sensing of trace metals and anionic species in the environment. Sensors 2021, 21, 604. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. Recent advances and future trends on molecularly imprinted polymer-based fluorescence sensors with luminescent carbon dots. Talanta 2021, 223, 121411. [Google Scholar] [CrossRef]
- Shangguan, J.; Huang, J.; He, D.; He, X.; Wang, K.; Ye, R.; Yang, X.; Qing, T.; Tang, J. Highly Fe3+-selective fluorescent nanoprobe based on ultrabright N/P codoped carbon dots and its application in biological samples. Anal. Chem. 2017, 89, 7477–7484. [Google Scholar] [CrossRef] [PubMed]
- Baluta, S.; Lesiak, A.; Cabaj, J. Simple and cost-effective electrochemical method for norepinephrine determination based on carbon dots and tyrosinase. Sensors 2020, 20, 4567. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.H.K.; Chan, K.K.; Tjin, S.C.; Yong, K.-T. Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review. Sensors 2020, 20, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Cheng, D.; Liu, X.; Han, A. Green hydrothermal synthesis of N-doped carbon dots from biomass highland barley for the detection of Hg2+. Sensors 2019, 19, 3169. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xiao, C.; Dai, X.; Li, J.; Gui, D.; Sheng, D.; Chen, L.; Zhou, R.; Chai, Z.; Albrecht-Schmitt, T.E. Exceptional perrhenate/pertechnetate uptake and subsequent immobilization by a low-dimensional cationic coordination polymer: Overcoming the hofmeister bias selectivity. Environ. Sci. Technol. Let. 2017, 4, 316–322. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Liu, B.; Zhang, H.; Liu, S.; Sun, J.; Zhang, X.; Tang, B.Z. Polymerization-induced emission. Mater. Horiz. 2020, 7, 987–998. [Google Scholar] [CrossRef]
- Herbani, Y.; Suliyanti, M.M. Concentration effect on optical properties of carbon dots at room temperature. J. Lumin. 2018, 198, 215–219. [Google Scholar]
- Adsetts, J.R.; Hoesterey, S.; Gao, C.; Love, D.A.; Ding, Z. Electrochemiluminescence and photoluminescence of carbon quantum dots controlled by aggregation-induced emission, aggregation-caused quenching, and interfacial reactions. Langmuir 2020, 36, 14432–14442. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Shao, J.; Zhao, X.; Yang, B. Non-conjugated polymer dots with crosslink-enhanced emission in the absence of fluorophore units. Angew. Chem. Int. Edit. 2015, 54, 14626–14637. [Google Scholar] [CrossRef]
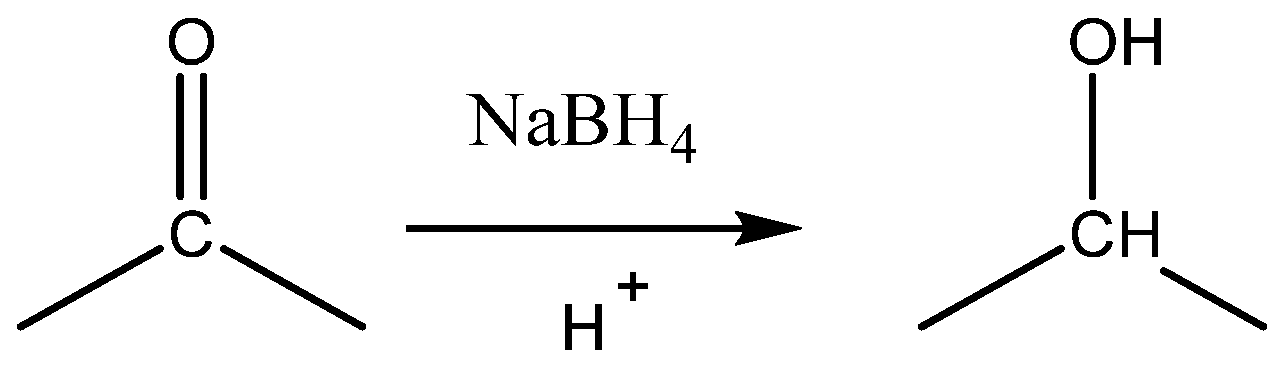
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shi, W.; Wang, Y.; Cheng, D.; Liu, J.; Xu, S.; Liu, W.; Dong, B.; Sun, J. Intrinsic Blue Fluorescence of 2.0G PAMAM-DCM Polymer Dots and Its Applications for Fe3+ Sensing. Sensors 2022, 22, 1075. https://doi.org/10.3390/s22031075
Wang X, Shi W, Wang Y, Cheng D, Liu J, Xu S, Liu W, Dong B, Sun J. Intrinsic Blue Fluorescence of 2.0G PAMAM-DCM Polymer Dots and Its Applications for Fe3+ Sensing. Sensors. 2022; 22(3):1075. https://doi.org/10.3390/s22031075
Chicago/Turabian StyleWang, Xin, Weiguang Shi, Yuda Wang, Dan Cheng, Jiahui Liu, Shihan Xu, Wei Liu, Biao Dong, and Jiao Sun. 2022. "Intrinsic Blue Fluorescence of 2.0G PAMAM-DCM Polymer Dots and Its Applications for Fe3+ Sensing" Sensors 22, no. 3: 1075. https://doi.org/10.3390/s22031075
APA StyleWang, X., Shi, W., Wang, Y., Cheng, D., Liu, J., Xu, S., Liu, W., Dong, B., & Sun, J. (2022). Intrinsic Blue Fluorescence of 2.0G PAMAM-DCM Polymer Dots and Its Applications for Fe3+ Sensing. Sensors, 22(3), 1075. https://doi.org/10.3390/s22031075






