High Spatial Resolution Ion Imaging by Focused Electron-Beam Excitation with Nanometric Thin Sensor Substrate
Abstract
:1. Introduction
2. Materials and Methods
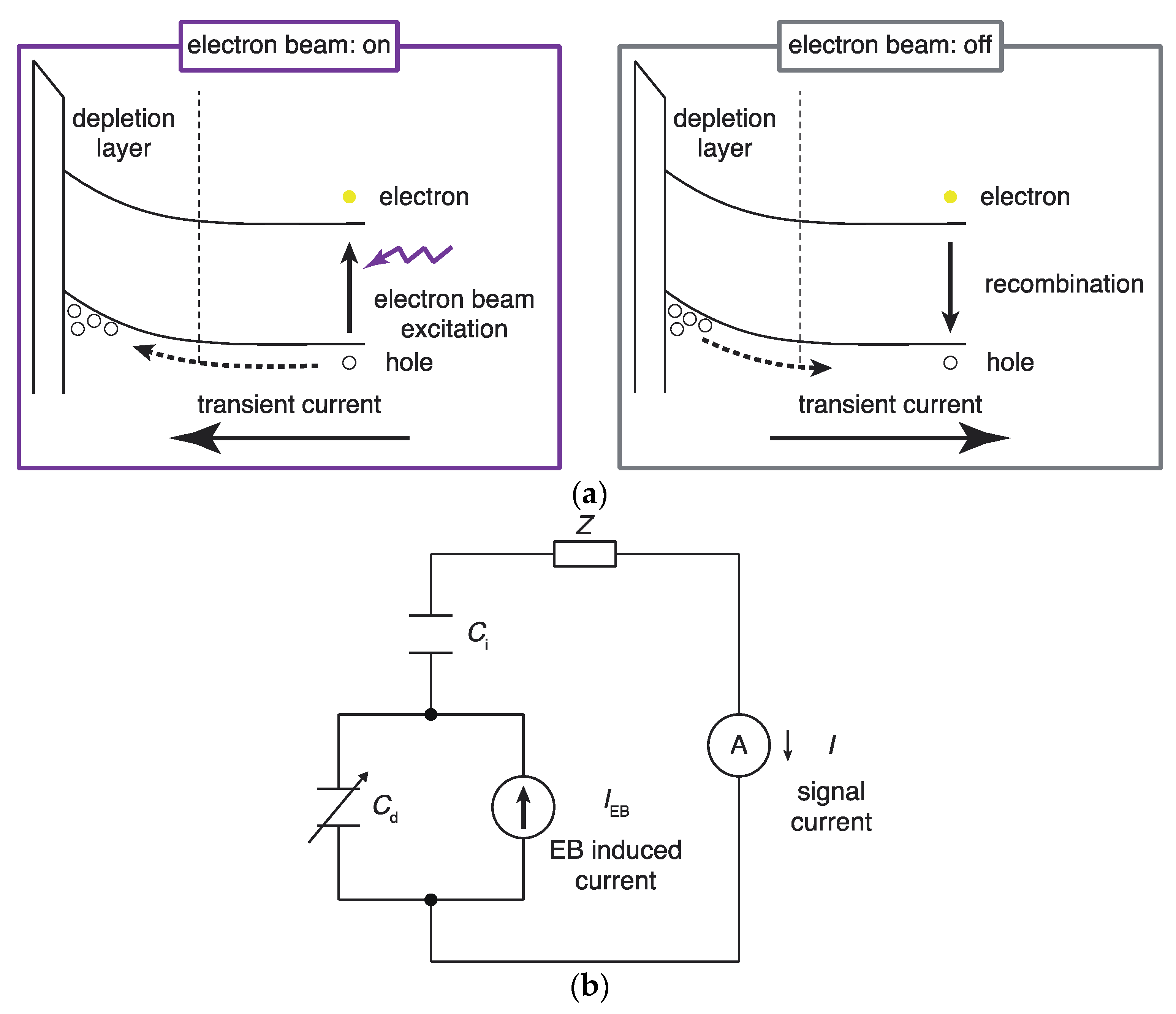
2.1. Principle of Operation of an Electron-Beam-Addressable Potentiometric Sensor
2.1.1. Method for Measuring the Distribution of the Ion Concentration
2.1.2. Measurement of the Depletion Layer Width with a Circuit Model and Electron Beam Irradiation
2.1.3. Relationship between the Ion Sensor Substrate and Spatial Resolution
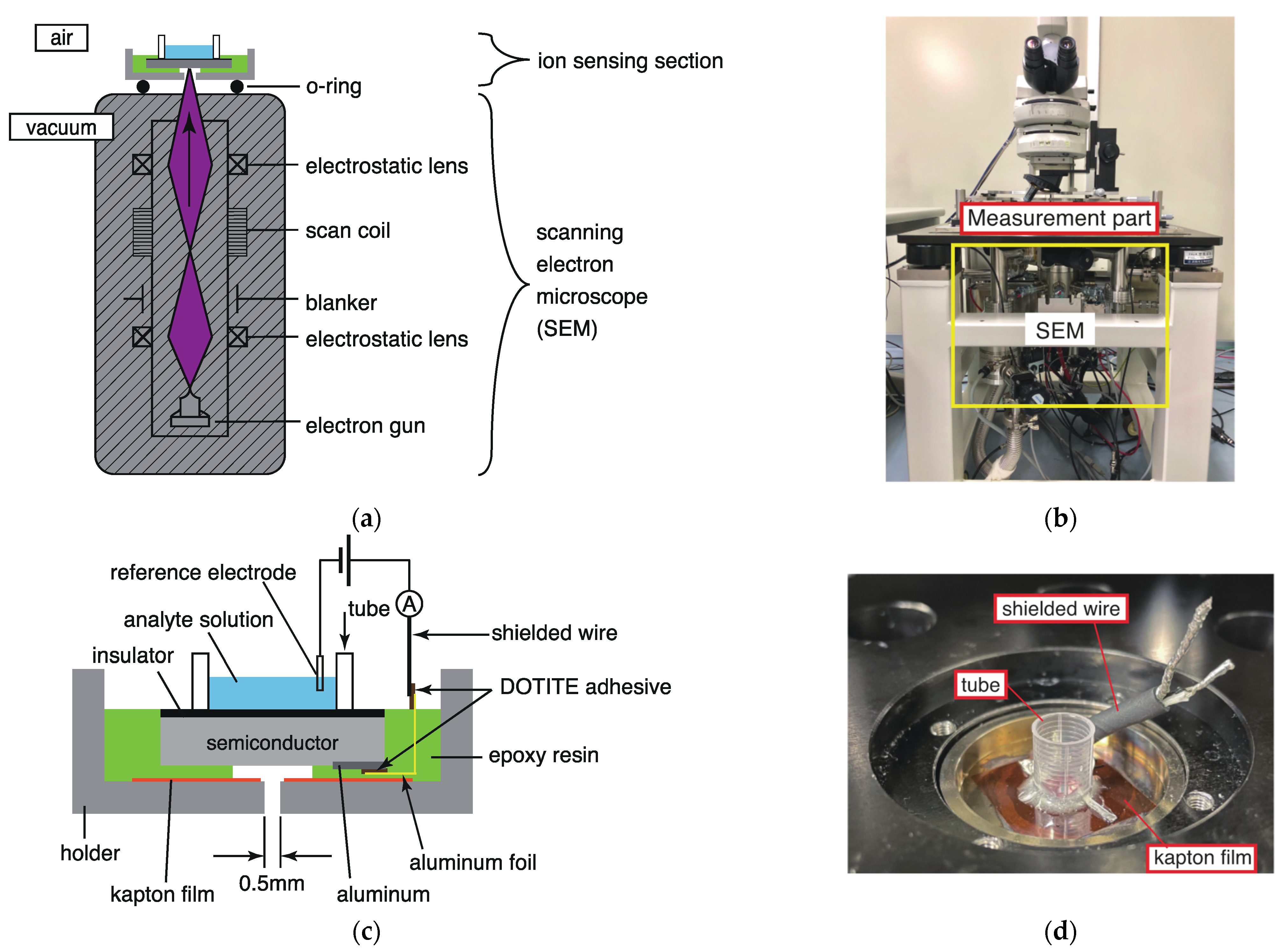
2.2. Ion-Imaging System Based on Electron Beam Excitation
2.3. Fabrication of the Thin Ion Sensor Substrate
3. Results and Discussion
3.1. Measurement Results for Solutions with Various Values of pH
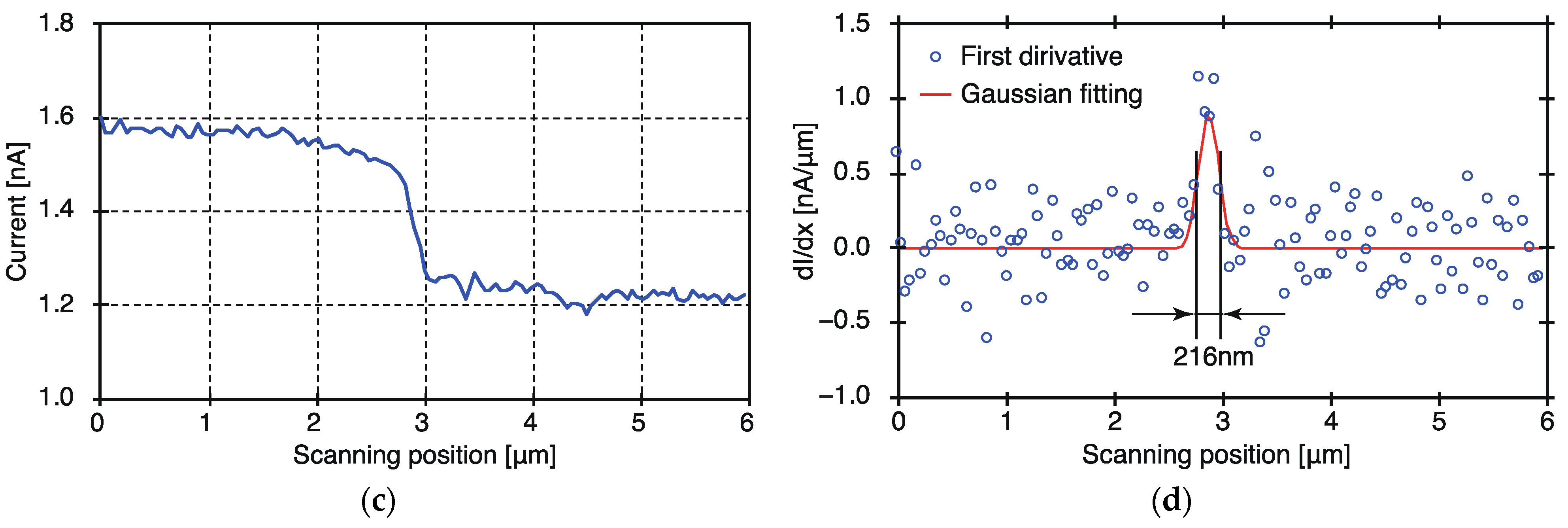
3.2. Evaluation of the Spatial Resolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Wise, K.D. An Integrated Field-Effect Electrode for Biopotential Recording. IEEE Trans. Biomed. Eng. 1974, BME-21, 485–487. [Google Scholar] [CrossRef]
- Nose, K.; Miyamoto, K.; Yoshinobu, T. Estimation of Potential Distribution during Crevice Corrosion through Analysis of I–V Curves Obtained by LAPS. Sensors 2020, 20, 2873. [Google Scholar] [CrossRef] [PubMed]
- Kono, A.; Sakurai, T.; Hattori, T.; Okumura, K.; Ishida, M.; Sawada, K. Label Free Bio Image Sensor for Real Time Monitoring of Potassium Ion Released from Hippocampal Slices. Sens. Actuators B 2014, 201, 439–443. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Wu, F.; Wang, J.; Watkinson, M.; Krause, S. Image Detection of Yeast Saccharomyces Cerevisiae by Light-Addressable Potentiometric Sensors (LAPS). Electrochem. Commun. 2016, 72, 41–45. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, B.; Wang, J.; Zhong, M.; Das, A.; Watkinson, M.; Hing, K.; Zhang, D.-W.; Krause, S. Photoelectrochemical Imaging System for the Mapping of Cell Surface Charges. Anal. Chem. 2019, 91, 5896–5903. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Das, A.; Kappers, M.J.; Oliver, R.A.; Humphreys, C.J.; Krause, S. InGaN as a Substrate for AC Photoelectrochemical Imaging. Sensors 2019, 19, 4386. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Werner, C.F.; Handa, S.; Wang, M.; Ohshiro, T.; Mushiake, H.; Yoshinobu, T. Miniature Multiplexed Label-Free pH Probe in Vivo. Biosens. Bioelectron. 2021, 174, 112870. [Google Scholar] [CrossRef]
- Doi, H.; Parajuli, B.; Horio, T.; Shigetomi, E.; Shinozaki, Y.; Noda, T.; Takahashi, K.; Hattori, T.; Koizumi, S.; Sawada, K. Development of a Label-Free ATP Image Sensor for Analyzing Spatiotemporal Patterns of ATP Release from Biological Tissues. Sens. Actuators B 2021, 335, 129686. [Google Scholar] [CrossRef]
- Wagner, T.; Molina, R.; Yoshinobu, T.; Kloock, J.P.; Biselli, M.; Canzoneri, M.; Schnitzler, T.; Schöning, M.J. Handheld Multi-Channel LAPS Device as a Transducer Platform for Possible Biological and Chemical Multi-Sensor Applications. Electrochim. Acta 2007, 53, 305–311. [Google Scholar] [CrossRef]
- Zeng, J.; Kuang, L.; Cacho-Soblechero, M.; Georgiou, P. An Ultra-High Frame Rate Ion Imaging Platform Using ISFET Arrays With Real-Time Compression. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Zhong, X.; Xu, J.; Lee, Y.-K.; Bermak, A. A High Offset Distribution Tolerance High Resolution ISFET Array with Auto-Compensation for Long-Term Bacterial Metabolism Monitoring. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Fakih, I.; Durnan, O.; Mahvash, F.; Napal, I.; Centeno, A.; Zurutuza, A.; Yargeau, V.; Szkopek, T. Selective Ion Sensing with High Resolution Large Area Graphene Field Effect Transistor Arrays. Nat. Commun. 2020, 11, 3226. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, K. An Integrated ISFET Sensor Array. Sensors 2009, 9, 8831–8851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, T.; Dasai, F.; Sato, H.; Kato, R.; Sawada, K. CCD Multi-Ion Image Sensor with Four 128 × 128 Pixels Array. Sensors 2019, 19, 1582. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, H.; Agetsuma, M.; Ishida, J.; Nakamura, Y.; Lawrence Cheung, D.; Nanasaki, S.; Kimura, Y.; Iwata, T.; Takahashi, K.; Sawada, K.; et al. CMOS-Based Bio-Image Sensor Spatially Resolves Neural Activity-Dependent Proton Dynamics in the Living Brain. Nat. Commun. 2020, 11, 712. [Google Scholar] [CrossRef] [Green Version]
- Edo, Y.; Tamai, Y.; Yamazaki, S.; Inoue, Y.; Kanazawa, Y.; Nakashima, Y.; Yoshida, T.; Arakawa, T.; Saitoh, S.; Maegawa, M.; et al. 1.3 Mega Pixels CCD pH Imaging Sensor with 3.75 μm Spatial Resolution. In Proceedings of the 2015 IEEE International Electron Devices Meeting (IEDM), Washington, DC, USA, 7–9 December 2015. [Google Scholar] [CrossRef]
- Zhou, B.; Das, A.; Zhong, M.; Guo, Q.; Zhang, D.-W.; Hing, K.A.; Sobrido, A.J.; Titirici, M.-M.; Krause, S. Photoelectrochemical Imaging System with High Spatiotemporal Resolution for Visualizing Dynamic Cellular Responses. Biosens. Bioelectron. 2021, 180, 113121. [Google Scholar] [CrossRef]
- George, M.; Parak, W.J.; Gerhardt, I.; Moritz, W.; Kaesen, F.; Geiger, H.; Eisele, I.; Gaub, H.E. Investigation of the Spatial Resolution of the Light-Addressable Potentiometric Sensor. Sens. Actuators A 2000, 86, 187–196. [Google Scholar] [CrossRef]
- Parak, W.J.; Hofmann, U.G.; Gaub, H.E.; Owicki, J.C. Lateral Resolution of Light-Addressable Potentiometric Sensors: An Experimental and Theoretical Investigation. Sens. Actuators A 1997, 63, 47–57. [Google Scholar] [CrossRef]
- Wu, F.; Campos, I.; Zhang, D.-W.; Krause, S. Biological Imaging Using Light-Addressable Potentiometric Sensors and Scanning Photo-Induced Impedance Microscopy. Proc. R. Soc. A 2017, 473. [Google Scholar] [CrossRef] [Green Version]
- Yoshinobu, T.; Miyamoto, K.; Wagner, T.; Schöning, M.J. Recent Developments of Chemical Imaging Sensor Systems Based on the Principle of the Light-Addressable Potentiometric Sensor. Sens. Actuators B 2015, 207, 926–932. [Google Scholar] [CrossRef]
- Yang, C.-M.; Chen, C.-H.; Akuli, N.; Yen, T.-H.; Lai, C.-S. A Revised Manuscript Submitted to Sensors and Actuators B: Chemical Illumination Modification from an LED to a Laser to Improve the Spatial Resolution of IGZO Thin Film Light-Addressable Potentiometric Sensors in pH Detections. Sens. Actuators B 2021, 329, 128953. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; Jiang, S.; Kunze, J.; Schmuki, P.; Krause, S. High Resolution LAPS and SPIM. Electrochem. Commun. 2010, 12, 758–760. [Google Scholar] [CrossRef]
- Shibano, S.; Kawata, Y.; Inami, W. Evaluation of pH Measurement Using Electron-Beam-Induced Current Detection. Phys. Status Solidi A 2021, 218, 2100147. [Google Scholar] [CrossRef]
- Inami, W.; Nii, K.; Shibano, S.; Tomita, H.; Kawata, Y. Improvement of the Spatial Resolution of Ion Imaging System Using Thinned Sensor Substrate. In Proceedings of the Biomedical Imaging and Sensing Conference 2020, Yokohama, Japan, 20–24 April 2020; Matoba, O., Awatsuji, Y., Luo, Y., Yatagai, T., Aizu, Y., Eds.; SPIE: New York, NY, USA, 15 June 2020; p. 115210. [Google Scholar]
- Yates, D.E.; Levine, S.; Healy, T.W. Site-Binding Model of the Electrical Double Layer at the Oxide/Water Interface. J. Chem. Soc. Faraday Trans. 1 1974, 70, 1807–1818. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Schöning, M.J. Light-Addressable Potentiometric Sensors for Cell Monitoring and Biosensing. Curr. Opin. Electrochem. 2021, 28, 100727. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Miyamoto, K.; Werner, C.F.; Poghossian, A.; Wagner, T.; Schöning, M.J. Light-Addressable Potentiometric Sensors for Quantitative Spatial Imaging of Chemical Species. Annu. Rev. Anal. Chem. 2017, 10, 225–246. [Google Scholar] [CrossRef]
- Sartore, M.; Adami, M.; Nicolini, C.; Bousse, L.; Mostarshed, S.; Hafeman, D. Minority Carrier Diffusion Length Effects on Light-Addressable Potentiometric Sensor (LAPS) Devices. Sens. Actuators A 1992, 32, 431–436. [Google Scholar] [CrossRef]
- Nakao, M.; Yoshinobu, T.; Iwasaki, H. Improvement of Spatial Resolution of a Laser-Scanning pH-Imaging Sensor. Jpn. J. Appl. Phys. 1994, 33, L394–L397. [Google Scholar] [CrossRef]
- Truong, H.A.; Werner, C.F.; Miyamoto, K.; Yoshinobu, T. A Partially Etched Structure of Light-Addressable Potentiometric Sensor for High-Spatial-Resolution and High-Speed Chemical Imaging. Phys. Status Solidi A 2018, 215, 1700964. [Google Scholar] [CrossRef]
- Moritz, W.; Gerhardt, I.; Roden, D.; Xu, M.; Krause, S. Photocurrent Measurements for Laterally Resolved Interface Characterization. Fresenius J. Anal. Chem. 2000, 367, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Hovington, P.; Drouin, D.; Gauvin, R. CASINO: A New Monte Carlo Code in C Language for Electron Beam Interaction -Part I: Description of the Program. Scanning 2006, 19, 1–14. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nii, K.; Inami, W.; Kawata, Y. High Spatial Resolution Ion Imaging by Focused Electron-Beam Excitation with Nanometric Thin Sensor Substrate. Sensors 2022, 22, 1112. https://doi.org/10.3390/s22031112
Nii K, Inami W, Kawata Y. High Spatial Resolution Ion Imaging by Focused Electron-Beam Excitation with Nanometric Thin Sensor Substrate. Sensors. 2022; 22(3):1112. https://doi.org/10.3390/s22031112
Chicago/Turabian StyleNii, Kiyohisa, Wataru Inami, and Yoshimasa Kawata. 2022. "High Spatial Resolution Ion Imaging by Focused Electron-Beam Excitation with Nanometric Thin Sensor Substrate" Sensors 22, no. 3: 1112. https://doi.org/10.3390/s22031112
APA StyleNii, K., Inami, W., & Kawata, Y. (2022). High Spatial Resolution Ion Imaging by Focused Electron-Beam Excitation with Nanometric Thin Sensor Substrate. Sensors, 22(3), 1112. https://doi.org/10.3390/s22031112






