Constant Potential Coulometric Measurements with Ca2+-Selective Electrode: Analysis Using Calibration Plot vs. Analysis Using the Charge Curve Fitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrode Preparation
2.3. Measurements
3. Results
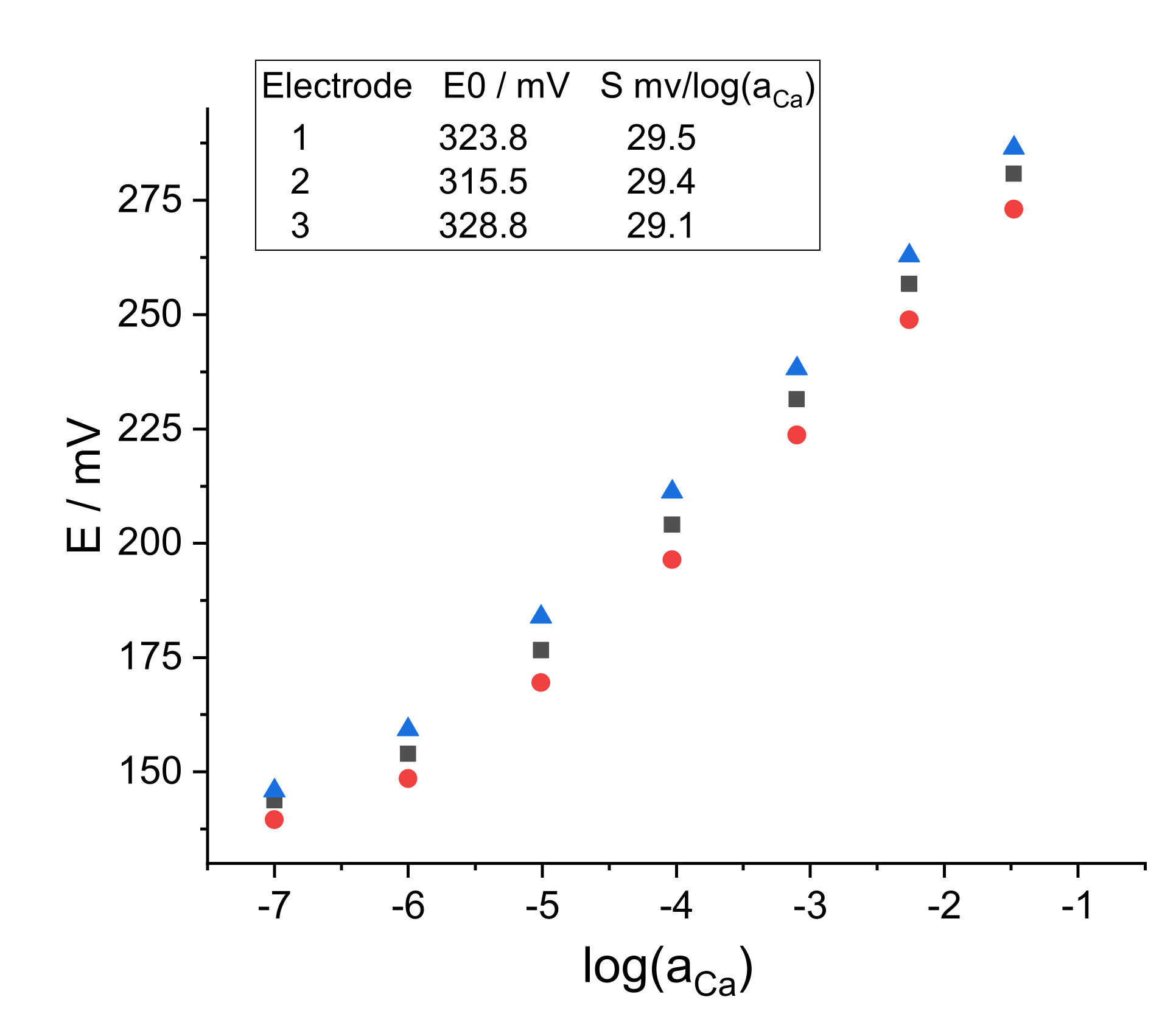
3.1. Control of the Quality of the ISEs
3.2. Chronoamperometric/Coulometric Measurements
3.2.1. Electrodes without Electronic Capacitor in Series
3.2.2. Electrodes with Electronic Capacitor in Series
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hupa, E.; Vanamo, U.; Bobacka, J. Novel Ion-to-Electron Transduction Principle for Solid-Contact ISEs. Electroanalysis 2015, 27, 591–594. [Google Scholar] [CrossRef]
- Vanamo, U.; Hupa, E.; Yrjänä, V.; Bobacka, J. New Signal Readout Principle for Solid-Contact Ion-Selective Electrodes. Anal. Chem. 2016, 88, 4369–4374. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Vanamo, U.; Bobacka, J. Influence of Electrode Geometry on the Response of Solid-Contact Ion-Selective Electrodes when Utilizing a New Coulometric Signal Readout Method. ChemElectroChem 2016, 8, 2071–2077. [Google Scholar] [CrossRef]
- Jaworska, E.; Pawłowski, P.; Michalska, A.; Maksymiuk, K. Advantages of amperometric readout mode of ion-selective electrodes under potentiostatic conditions. Electroanalysis 2019, 31, 343–349. [Google Scholar] [CrossRef]
- Han, T.; Mousavi, Z.; Mattinen, U.; Bobacka, J. Coulometric response characteristics of solid contact ion-selective electrodes for divalent cations. J. Sol. State Electrochem. 2020, 24, 2975–2983. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Qi, L.; Ding, J.; Qin, W. Light-driven ion extraction of polymeric membranes for on-demand Cu(II) sensing. Anal. Chim. Acta 2021, 1176, 338756. [Google Scholar] [CrossRef]
- Bondar, A.V.; Keresten, V.M.; Mikhelson, K.N. Registration of small (below 1%) changes of calcium ion concentration in aqueous solutions and in serum by the constant potential coulometric method. Sens. Actuators B Chem. 2022, 354, 131231. [Google Scholar] [CrossRef]
- Han, T.; Mattinen, U.; Mousavi, Z.; Bobacka, J. Coulometric response of solid-contact anion-sensitive electrodes. Electrochim. Acta 2021, 367, 137566. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, B.; Yin, T.; Qin, W. Alternative coulometric signal readout based on a solid-contact ionselective electrode for detection of nitrate. Anal. Chim. Acta 2020, 1129, 136–142. [Google Scholar] [CrossRef]
- Han, T.; Mattinen, U.; Bobacka, J. Improving the sensitivity of solid-contact ion-selective electrodes by using coulometric signal transduction. ACS Sens. 2019, 4, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Kraikaew, P.; Sailapu, S.K.; Bakker, E. Rapid Constant Potential Capacitive Measurements with SolidContact Ion-Selective Electrodes Coupled to Electronic Capacitor. Anal. Chem. 2020, 92, 14174–14180. [Google Scholar] [CrossRef] [PubMed]
- Kraikaew, P.; Jeanneret, S.; Soda, Y.; Cherubini, T.; Bakker, E. Ultrasensitive Seawater pH Measurement by Capacitive Readout of Potentiometric Sensors. ACS Sens. 2020, 5, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Kraikaew, P.; Sailapu, S.K.; Bakker, E. Electronic control of constant potential capacitive readout of ion-selective electrodes for high precision sensing. Sens. Actuators B Chem. 2021, 344, 130282. [Google Scholar] [CrossRef]
- Jarolímova, Z.; Han, T.; Mattinen, U.; Bobacka, J.; Bakker, E. Capacitive Model for Coulometric Readout of Ion-Selective Electrodes. Anal. Chem. 2018, 90, 8700–8707. [Google Scholar] [CrossRef] [PubMed]
- Kondratyeva, Y.O.; Tolstopjatova, E.G.; Kirsanov, D.O.; Mikhelson, K.N. Chronoamperometric and coulometric analysis with ionophore-based ion-selective electrodes: A modified theory and the potassium ion assay in serum samples. Sens. Actuators B Chem. 2020, 310, 127894. [Google Scholar] [CrossRef]
- Mikhelson, K.N. Ion-Selective Electrodes; Lecture Notes in Chemistry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2013; Volume 81, 162p. [Google Scholar] [CrossRef]
- Lewenstam, A. Routines and Challenges in Clinical Application of Electrochemical Ion-Sensors. Electroanalysis 2014, 26, 1171–1181. [Google Scholar] [CrossRef]
- Bard, L.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA; Chichester, UK; Weinheim, Germany; Brisbane, Australia; Singapore; Toronto, OT, Canada, 2001; 864p. [Google Scholar]
- Kondratyeva, Y.O.; Solovyeva, E.V.; Khripoun, G.A.; Mikhelson, K.N. Non-constancy of the bulk resistance of ionophore-based ion-selective electrode: A result of electrolyte co-extraction or of something else? Electrochim. Acta 2018, 259, 458–465. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhelson, K. Electrochemical Properties of Nitrate-Selective Electrodes: The Dependence of Resistance on the Solution Concentration. Sensors 2018, 18, 2062. [Google Scholar] [CrossRef] [Green Version]
- Kondratyeva, Y.O.; Solovyeva, E.V.; Khripoun, G.A.; Mikhelson, K.N. Paradox of the variation of the bulk resistance of potassium ion-selective electrode membranes within nernstian potentiometric response range. Russ. J. Electrochem. 2019, 55, 1118–1126. [Google Scholar] [CrossRef]
- Kalinichev, A.V.; Solovyeva, E.V.; Ivanova, A.R.; Khripoun, G.A.; Mikhelson, K.N. Non-constancy of the bulk resistance of ionophore-based Cd2+-selective electrode: A correlation with the water uptake by the electrode membrane. Electrochim. Acta 2020, 334, 135541. [Google Scholar] [CrossRef]
- Solovyeva, E.V.; Lu, H.; Khripoun, G.A.; Mikhelson, K.N.; Kazarian, S.G. In situ ATR-FTIR spectroscopic imaging of PVC, plasticizer and water in solvent-polymeric ion-selective membrane containing Cd2+-selective neutral ionophore. J. Membr. Sci. 2020, 619, 118798. [Google Scholar] [CrossRef]
- Keresten, V.; Solovyeva, E.; Mikhelson, K. The Origin of the Non-Constancy of the Bulk Resistance of Ion-Selective Electrode Membranes within the Nernstian Response Range. Membranes 2021, 11, 344. [Google Scholar] [CrossRef] [PubMed]






| Electrode | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Without capacitor in series | |||
| Q0 (C) | −1.413·10−5 | −1.219·10−5 | −1.244·10−5 |
| Sq (C/log(aCa) | −3.907·10−6 | −3.358·10−6 | −3.408·10−6 |
| With electronic capacitor of 10 µF in series with the electrode | |||
| Q0 (C) | −1.227·10−6 | −1.178·10−6 | −9.153·10−6 |
| Sq (C/log(aCa) | −3.363·10−7 | −3.217·10−7 | −2.483·10−7 |
| Using Calibration Plot | Using Curve Fitting | |||||
|---|---|---|---|---|---|---|
| Target, mM | Measured, mM | SD, mM | Recovery, % | Measured, mM | SD, mM | Recovery, % |
| 0.400 | 0.403 | 0.004 | 101.8 | 0.387 | 0.003 | 96.7 |
| 0.600 | 0.612 | 0.003 | 102 | 0.589 | 0.004 | 98.1 |
| 0.800 | 0.802 | 0.013 | 100.2 | 0.870 | 0.006 | 109 |
| 1.200 | 1.15 | 0.05 | 95.5 | 1.17 | 0.08 | 97.7 |
| 3.500 | 4.14 | 0.06 | 118 | 4.38 | 0.20 | 125 |
| Using Calibration Plot | Using Curve Fitting | |||||
|---|---|---|---|---|---|---|
| Target, mM | Measured, mM | SD, mM | Recovery, % | Measured, mM | SD, mM | Recovery, % |
| 0.400 | 0.395 | 0.007 | 98.2 | 0.368 | 92.1 | |
| 0.600 | 0.612 | 0.032 | 102 | 0.650 | 0.006 | 108 |
| 0.800 | 0.819 | 0.009 | 102 | 0.853 | 107 | |
| 1.200 | 1.28 | 0.07 | 107 | 1.33 | 0.35 | 111 |
| 3.500 | 3.93 | 0.10 | 112 | 4.02 | 0.80 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondar, A.; Mikhelson, K. Constant Potential Coulometric Measurements with Ca2+-Selective Electrode: Analysis Using Calibration Plot vs. Analysis Using the Charge Curve Fitting. Sensors 2022, 22, 1145. https://doi.org/10.3390/s22031145
Bondar A, Mikhelson K. Constant Potential Coulometric Measurements with Ca2+-Selective Electrode: Analysis Using Calibration Plot vs. Analysis Using the Charge Curve Fitting. Sensors. 2022; 22(3):1145. https://doi.org/10.3390/s22031145
Chicago/Turabian StyleBondar, Anna, and Konstantin Mikhelson. 2022. "Constant Potential Coulometric Measurements with Ca2+-Selective Electrode: Analysis Using Calibration Plot vs. Analysis Using the Charge Curve Fitting" Sensors 22, no. 3: 1145. https://doi.org/10.3390/s22031145
APA StyleBondar, A., & Mikhelson, K. (2022). Constant Potential Coulometric Measurements with Ca2+-Selective Electrode: Analysis Using Calibration Plot vs. Analysis Using the Charge Curve Fitting. Sensors, 22(3), 1145. https://doi.org/10.3390/s22031145







