1. Introduction
The pressure of the circulating blood against the walls of the arteries is called blood pressure (BP). The heart pumps blood through the circulatory system, which is the primary cause of the pressure. BP, along with the respiratory rate, heart rate, oxygen saturation, and body temperature, constitute the vital signs that medical doctors use to assess patient health. Therefore, monitoring BP for maintenance within the normal range is very important for planning a healthy life. BP is generally expressed as a fraction, where the numerator represents the systolic blood pressure (SBP) and the denominator represents the diastolic blood pressure (DBP). According to the American Heart Association [
1], BP less than 120/80 mmHg is considered normal. The BP ranges for various health conditions are shown in
Table 1.
Photoplethysmography (PPG) is an optical method of detecting changes in the blood volume in the microvascular layer of tissues. PPG is frequently used for noninvasive measurements from the skin surface. PPG sensing is a low-cost, easy-to-use technology that requires only a few electronic components for acquiring signals. A light source is used to illuminate the skin surface, and a detector is used to detect the light from the source. Depending on the placement of the light source and detector, PPG signals can be divided into two types: transmission and reflection [
2]. If the light source and detector are placed on the same side of the measured area, it is considered as reflection-type PPG, and if they are placed on opposite sides, it is considered as transmission-type PPG.
Over the last few years, PPG has been used to monitor and evaluate various physiological parameters, such as blood glucose, heart rate variability, BP, arterial oxygen saturation (SpO
2), and glycated hemoglobin (HbA1c) [
3,
4,
5,
6,
7]. In [
3], the authors developed a gray-box model to estimate HbA1c using a digital pulse waveform. Alqaraawi et al. [
4] estimated heart rate variability with PPG signals using a Bayesian learning algorithm. The authors of [
5] developed a machine-learning-based model to estimate blood glucose levels from PPG signals; they reported an all-purpose system that collects PPG signals to estimate blood glucose levels with two machine-learning models: namely, random forest and XGBoost. BP measurement is a popular application of PPG signals, and several studies have reported various algorithms and models for estimating BP using PPG signals. Mousavi et al. [
6] described an algorithm for estimating BP using a whole-based method, which is a feature formation technique in which the time-domain features from PPG and ECG or only from PPG are extracted at specific intervals; they also reported a method of distinguishing between appropriate and inappropriate PPG signals to estimate BP. Machine-learning and deep-learning techniques have also been used for BP measurements. The authors of [
7] extracted time- and frequency-domain features from PPG signals and used them to estimate BP with a machine-learning approach; they also used a feature selection algorithm to reduce the computational complexity of the model, and their study reported that the Gaussian process regression (GPR) performs best for estimating SBP and DBP. Athaya et al. [
8] proposed a method of estimating arterial blood pressure (ABP) waveforms using PPG for continuous BP measurements. In [
8], a U-net architecture-based approach was described to estimate the ABP waveforms.
Monte Carlo simulation (MCS) is an adaptive computational technique that uses a random sampling procedure from a probability distribution; it is a widely used technique for simulating photon propagations in biological tissues. The basic principle of MCS in tissue results in a random walk process for transmitting light through biological tissues [
9]. Each photon packet in the simulation has an initial weight, which is assumed to be 1. Then, the absorption and scattering coefficients of the tissue model are used to illustrate the probability of absorption and scattering for a unit path length [
10]. The anisotropy factor
, which is the normal cosine of the scattering angle, determines the probability distribution of the scattering angle. The change in the refractive index n determines the angle of refraction, which changes between two regions of the tissue model or at the air–tissue interface. The fraction of the photon packet leaving on the same side of the medium is recorded as the reflected photon intensity (weight), and the fraction leaving on the opposite side of the medium is considered as the transmitted photon intensity [
11]. Photon transport in biological tissues has become one of the popular methods in biomedical applications for estimating health parameters, such as BP and blood glucose concentration. MCS for light propagation in tissues is an important tool for understanding how light interacts with biological tissues [
12], and this understanding can be used to noninvasively estimate the health parameters.
Cuffless measurement of BP is a new research trend that reduces the drawbacks of conventional BP monitoring. The conventional BP measurements have several problems, such as invasive monitoring and cuff-based or manual operating procedures. Kachuee et al. [
13] proposed a cuffless BP estimation algorithm based on the pulse arrival times. According to the British Hypertension Society (BHS) standard, the results of their estimations achieve grade A for DBP and grade B for the mean arterial pressure (MAP). The authors of [
14] developed a hybrid deep-learning-based model to predict BP from PPG and ECG signals. They implemented an automatic feature extraction layer in a deep-learning model to extract the optimal features from PPG signals for predicting BP. Atomi et al. [
15] designed a BP estimation model using wrist-type PPG sensor signals and machine-learning models; they also developed a cloud system to store the medical information of patients and provide them appropriate advice. Esmaelpoor et al. [
16] proposed a multistage deep-neural-network-based model for estimating the SBP and DBP; their model consists of two successive stages, where the first stage involves two convolutional neural networks to extract features from PPG signals and the second stage captures temporal dependencies using long short-term memory (LSTM). Then, their model incorporates these two stages and estimates the SBP and DBP. A real-time BP measurement algorithm was proposed in [
17], whose random forest model outperforms all other machine-learning models based on feature vectors. However, machine-learning or deep-learning-based models for estimating BP are generally very complex and rely mostly on the signal measurement sites.
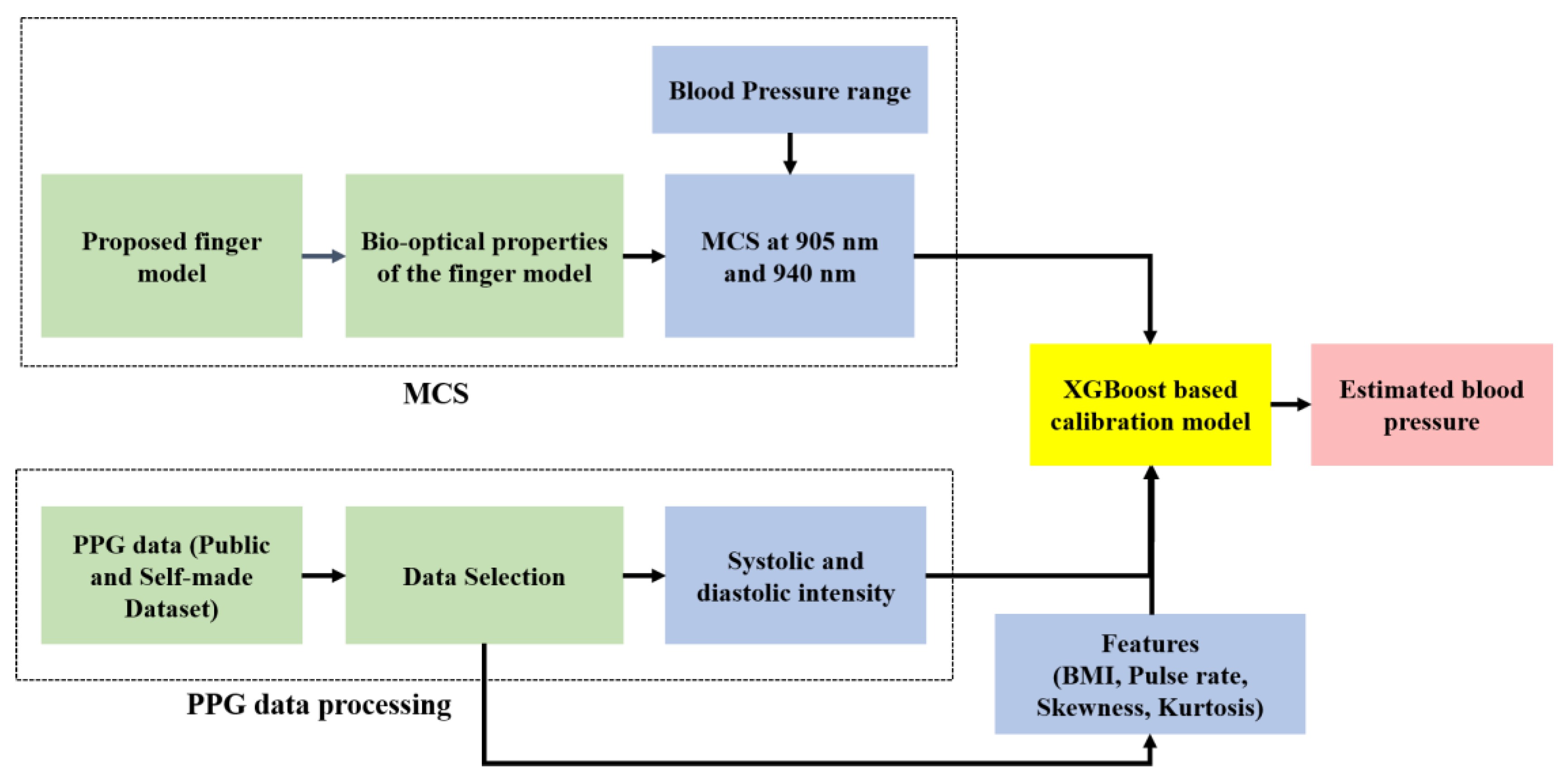
In our study, we propose the MCS-based model for estimating BP using fingertip PPG signals. We executed the MCS for both transmission and reflection types with wavelengths of 905 nm and 940 nm. For the MCS, we propose a finger model with the bio-optical properties of the layers for wavelengths of 905 nm and 940 nm. To evaluate the proposed model, we consider two datasets: a publicly available dataset and a dataset created by us. The publicly available dataset was used to evaluate the transmission-type MCS at 905 nm, and our self-made dataset was used for both transmission- and reflection-type MCSs at 940 nm.
The remainder of this study is organized as follows.
Section 2 describes the materials and methods of this study, including the proposed finger description, MCS procedure, dataset description, and calibration model.
Section 3 describes the model evaluation results. Finally,
Section 4 present the conclusions of the study.
3. Results
In this study, we evaluated the performance of the proposed model on both publicly available and home-made datasets. We also compared the results obtained from these two datasets. In the public dataset, the PPG signal was collected at a wavelength of 905 nm, and in the self-made dataset, the PPG signal was collected at a wavelength of 940 nm. In the proposed hardware, the IR wavelength is 940 nm, and this device is well established and used in our previous studies [
5,
43]. It is difficult to change the IR LED in the hardware to match the wavelength of the public dataset. However, the authors of [
44] categorized 905 nm and 940 nm into the same IR wavelength group (IR-A); it can be seen that there are no significant differences in the penetration depths in skin tissue between 905 and 940 nm [
19]. Furthermore, if there are no significant differences in the penetration depths of the two wavelengths in skin tissue for a given measurement site, the collected PPG signals can be said to be identical based on the PPG signal generation principle. Thus, we compared the estimated results of the two datasets used in our study.
Herein, we present the evaluation of the proposed BP estimation model on both the publicly available and self-made datasets. We also present the fluence rate plots of the MCS in the proposed voxel-based finger model and its calibration accuracy. To evaluate the proposed model, we considered five metrics: mean absolute error (MAE), root mean-squared error (RMSE), standard deviation (SD), Pearson’s correlation coefficient (Pearson’s r), and coefficient of determination (R
2). To visualize the accuracies of the estimated BP and estimation errors, we also present the error grid analysis (EGA) for MAP [
45] and Bland–Altman analyses for the SBP and DBP. There are five regions in the EGA plot: A, B, C, D, and E. The points in region A indicate that there are no clinical differences between the reference and estimated values. Points in region B would probably result in benign findings or no treatments. Points in regions C, D, and E may eventually result in unnecessary treatments with moderate non-life-threatening, severe non-life-threatening, and life-threatening consequences for the patient, respectively. The MAP can be calculated from the SBP and DBP as in Equation (12) [
46].
3.1. MCS and Calibration Accuracy
In our study, the voxel-based MCS algorithm was used to propagate photons in the proposed finger model for estimating BP. The Monte Carlo photon propagation was performed on a 10.04 mm thick slab designed for the whole finger. The transmission type used wavelengths of 905 nm and 940 nm, and the reflection type used a wavelength of 940 nm. In the case of reflection-type simulation, the optical source and detector were separated by 0.4 mm, while for the transmission-type simulation, the detector was placed 10.06 mm away from the opposite side of the model. Simulations were performed on a 64-bit operating system (Windows OS) with 20 GB RAM and an Intel Core i7 CPU. The scattering events at the specific wavelengths are depicted by the photon fluence rate plot in a voxel-based finger model.
Figure 9 and
Figure 10 show the fluence rates throughout the voxel-based finger model in 3D and XY plane views, respectively. The voxel unit (1 unit = 0.02 mm) is represented by the dots on the axes, and the color bars depict the scattering events from minimum to maximum. The maximum number of scattering events was found near the artery, that is, between the upper blood net dermis and reticular dermis, as shown in
Figure 9 and
Figure 10. Since the diameter of the artery and absorption coefficient change as the BP varies, the maximum scattering events occur near the artery. As previously stated, there are no significant differences between 905 and 940 nm in terms of the penetration depths in skin tissues. At both wavelengths, the maximum scattering events occur near the artery, which is another reason for comparing the evaluation results between 905 and 940 nm.
To determine the accuracy of the calibration model (XGBoost), we performed a repeated k-fold cross-validation, which is a technique for improving the estimation performance of machine-learning models. It is also a standard method of estimating model performance. In our study, we used the k-fold cross-validation to test the accuracy of the XGBoost-based calibration model. A 10-fold cross-validation process was repeated three times to estimate the model performance. The average R
2 value was found to be 0.984 for the public dataset and 0.979 for the self-made dataset. The dataset sizes for training and testing to perform the calibration were 90% and 10%, respectively.
Figure 11 shows the fitted curves of the calibration performed on the publicly available dataset and MC photon intensity. The parameters of the XGBoost calibration model and feature importance plots of the input features during calibration are presented in the
Supplementary Document (see Section S2 of the supplementary document).
3.2. Blood Pressure Estimation (Public Dataset)
The following results were obtained after interpolating the calibrated intensity of the PPG signal with the data table of photon intensities (see
Table S1 of the Supplementary Document).
Figure 12 and
Figure 13 show the fitted scatter plots and Bland–Altman analyses of the estimated SBP and DBP, respectively.
Figure 12b indicates that the estimated SBP provides a bias of 0.04 ± 6.04 with limits of agreement (95%, 1.96 SD) ranging from −11.78 to 11.87.
Figure 13b shows that the DBP estimation provides a bias of −0.15 ± 2.64 with limits of agreement (95%, 1.96 SD) ranging from −5.32 to 5.03.
Figure 14 shows the EGA plot of the MAP. As can be seen from the figure, there are a negligible number of points in region B. The values of the evaluation metrics for the SBP, DBP, and MAP estimations are listed in
Table 9.
3.3. Blood Pressure Estimation (Self-Made Dataset)
3.3.1. Transmission-Type MCS
The following results were found from our dataset for the transmission-type MCS.
Figure 15 and
Figure 16 depict the fitted scatter plots and Bland–Altman analyses of the estimated SBP and DBP, respectively.
Figure 15b indicates that the estimated SBP provides a bias of −0.81 ± 4.23 with limits of agreement (95%, 1.96 SD) ranging from −9.13 to 7.5.
Figure 16b shows that the DBP estimation provides a bias of −0.65 ± 2.82 with limits of agreement (95%, 1.96 SD) ranging from −6.17 to 4.88.
In
Figure 17, the EGA plot of the MAP is shown, and it is seen from the figure that there are no points in the C, D, and E regions. The values of the evaluation metrics for the SBP, DBP, and MAP estimations are listed in
Table 10.
3.3.2. Reflection-Type MCS
The following results were obtained from our dataset for the reflection-type MCS.
Figure 18 and
Figure 19 depict the fitted scatter plots and Bland–Altman analyses of the estimated SBP and DBP, respectively.
Figure 18b indicates that the estimated SBP provides a bias of 0.8 ± 5.06 with limits of agreement (95%, 1.96 SD) ranging from −9.13 to 10.73.
Figure 19b shows that the DBP estimation provides a bias of −0.56 ± 2.82 with limits of agreement (95%, 1.96 SD) ranging from −6.1 to 4.98.
In
Figure 20, the EGA plot of the MAP is shown; it can be seen from the figure that there are no points in the B, C, D, and E regions. The values of the evaluation metrics for the SBP, DBP, and MAP estimations are listed in
Table 11.
3.4. Compliance with Standards
The obtained results were compared with the Association for the Advancement of Medical Instrumentation (AAMI) error range. According to the AAMI standard, the mean difference and standard deviation must be less than or equal to 5 ± 8 mmHg [
47]. The estimations using our model for both the public and self-made datasets are fully acceptable for the SBP and DBP values. The comparison with the AAMI standard is shown in
Table 12. From this table, it is seen that the BP estimation with our model satisfies the AAMI standard as the MAE value is within 5 ± 8 mmHg.
The accuracy of the model was also checked from the point of view of the British Hypertension Society (BHS) grading standard [
48]. The BHS grading standard and cumulative error percentage of our data are shown in
Table 13. From the results, it is seen that the SBP and DBP estimations using our model fall within “Grade A” for both the public and self-made datasets
We compared the performances of our model for the two datasets. As previously stated, there are no significant differences between the 905 nm and 940 nm wavelengths in terms of PPG signal acquisition, because the penetration depths of these two wavelengths in skin tissue are almost identical. Therefore, we compared the results of the transmission-type MCS between the public and self-made datasets. The MAEs for SBP, DBP, and MAP in the public dataset for the transmission-type MCS are 3.32 ± 6.03 mmHg, 2.02 ± 2.64 mmHg, and 1.76 ± 2.8 mmHg, respectively. The self-made dataset was used for both transmission- and reflection-type MCS. In the transmission mode, the MAEs are 2.54 ± 4.24 mmHg for SBP, 1.49 ± 2.82 mmHg for DBP, and 1.51 ± 2.41 mmHg for MAP. In the reflection type, the MAEs are 3.35 ± 5.06 mmHg for SBP, 2.07 ± 2.83 mmHg for DBP, and 2.12 ± 2.83 mmHg for MAP. It can be seen that in the transmission type for the self-made dataset, the estimation results have less errors than the reflection type for the self-made dataset; this is because the performance of the estimation model depends on the MCS, so more accurate photon intensities are recorded during the transmission-type MCS. The artery is considered to be located between the upper blood net dermis and reticular dermis in our proposed voxel-based MCS model, so that while propagating photons into the model, the photodetector can record the intensities of the photons transmitted through the finger model. These photon intensities are more accurate than those recorded during the reflection type. With relatively more accurate photon intensities, the XGBoost-based calibration model performs well on the systolic and diastolic intensities of the PPG signals, thus resulting in slightly better performance.
3.5. Comparisons with Related Works
In general, similar studies in this field are difficult to evaluate owing to various evaluation criteria and insufficient datasets. However, we compared the results of our proposed model with previous works that have used PPG signals for estimating the SBP and DBP. For comparing performances, we considered the MAE, SD, and Pearson’s r of the estimation models.
Table 14 shows the performance comparisons of these studies. In the table, the performance evaluation metrics are listed for both datasets used in our model. The Pearson’s r values for the SBP, DBP, and MAP estimations also prove the estimation accuracy of our model. Although the Pearson’s r value is slightly lower for the self-made dataset, it satisfies the AAMI and BHS standards with small MAE and SD values.
While reviewing other relevant studies, Chowdhury et al. [
7] used a dataset similar to that used to validate our model for the public dataset; they used a Gaussian process regression (GPR) to estimate the SBP and DBP. They also used feature selection techniques in their study to provide inputs to machine-learning models. However, in our study, only four features were used to calibrate the model and obtain lower errors. Furthermore, as shown in
Table 10 and
Table 12, SBP estimations with their approach do not meet the AAMI requirements. In [
8], the authors proposed a U-net deep-learning architecture to estimate the ABP waveforms and calculate the SBP, DBP, and MAP from these waveforms. Since the deep-learning-based model has to estimate the ABP waveforms for estimating BP, it can provide erroneous results when there is a lack of stable PPG signals. In
Table 14, it is seen that our proposed model outperforms the model of [
8] in terms of MAE and SD values, which are important parameters for validating the estimation results compared to the AAMI and BHS standards. Xie et al. [
17] used the random forest model to estimate the SBP and DBP. In terms of the MAE and SD, our proposed model outperforms the random forest model on both the public and self-made datasets. Furthermore, our proposed model outperforms the model in [
16] for all evaluation parameters.
Compared with the above models, our MCS-based model obtains lower MAE and SD, with relatively similar accuracy for estimating the SBP and DBP values. Therefore, our proposed model can be said to be more accurate for estimating SBP and DBP according to the AAMI and BHS standards.
4. Conclusions
Cuffless and comfortable BP measurements are currently an active area of research. In this study, we proposed a cuffless BP estimation model based on MCS. After MCS for a specific range of blood pressure, photon intensities were recorded to generate a data table. PPG signals from both a publicly available dataset (number of subjects = 100) and self-made dataset (number of subjects = 30) have been used to evaluate the proposed model. The systolic and diastolic photon intensities detected in real PPG signals were calibrated using a supervised machine learning based calibration model. Both transmission- and reflection-type MCSs were performed for estimating the SBP and DBP, from which we also calculated the MAP. We present the EGA plots for MAP and Bland–Altman analyses for the SBP and DBP to evaluate our model performance. The EGA plot for MAP proves the accuracy of our estimations for use in regular BP measurements, and the Bland–Altman analysis shows the errors of the SBP and DBP estimation results. From the Bland–Altman plot, it can also be concluded that the performance of the proposed model is within the margin of error, as most of the estimated values are within ±1.96 SD. Therefore, our proposed model satisfies the standards of the AAMI and complies with the ‘Grade A’ category according to the BHS.
Previous studies in the field of blood pressure (BP) estimation [
7,
8,
16,
17] focused on using machine learning or deep learning models for BP estimation, which often create obstacles in estimating the desired parameters accurately. Therefore, to reduce the possibility of inaccurate estimation of BP, we have proposed a finger model to execute MCS for recording the precise intensities during the systolic and diastolic phases of the PPG signal. In addition, in this study, a relationship between BP and artery diameter is also deduced. The heterogeneous finger model proposed in this study provides a more precise design of the model and more accurate results within the range that can provide a chance to optimize the model. Since earlier studies in this field focused on machine or deep learning models for BP estimation, there is little scope for optimizing the model as well as model complexity.
In our study, the thickness of the finger model is considered as a constant for all simulations but may vary from person to person in real scenarios. Therefore, to evaluate the model performance more accurately, it will be necessary to create a self-made dataset from more subjects. Despite the limitations of the estimation model, the proposed method can be used for BP monitoring without the use of a cuff. We expect that our proposed model will provide an easy cuffless BP estimation method to help people plan healthy lifestyles.