Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors
Abstract
:1. Introduction
2. The Role of Nanomaterials
2.1. Metallic Nanoparticles (MNPs)
2.2. Metal Oxide Semiconductors (MOS)
2.3. Carbon Nanotubes (CNTs)
2.4. Nanowires
2.5. D-Materials
2.6. Hybrid Materials
3. Types of Nanomaterial-Based Sensors in Breath Analysis
3.1. Chemiresistors
3.1.1. Selective Applications
3.1.2. Cross-Reactive Applications
| Sensing Element | Disease | Targeted VOCs | LOD | Subjects | Classifier | Results | T | Ref. |
|---|---|---|---|---|---|---|---|---|
| In Vivo Studies—Real-World Samples | ||||||||
| Chemiresistor—arrays | ||||||||
| Molecularly capped AuNPs—14 different ligands | Lung cancer | 1-Methyl-4-(1-methyl ethyl) benzene, Toluene, 3,3-Dimethyl pentane, 2,3,4-Trimethyl hexane, Dodecane, 1,1′-1-Butenylidene)bis benzene | NA | 30 LC, 26 CC, 22 BC, 18 PC, 22 HC | PCA | Good discrimination of cancer types from HC, but not between them. No VOC overlap in abundance between HC and cancer patients. | RT | [16] |
| Colorectal cancer | 1,1′-(1-Butenylidene)bis benzene, 1,3-Dimethyl benzene, 1-Iodo nonane, (1,1-Dimethylethyl)thio acetic acid, 4-(4-Propylcyclohexyl)-4′-cyano1,1′-biphenyl-4-yl ester benzoic acid, 2-Amino-5-isopropyl-8-methyl-1-azulene carbonitrile | |||||||
| Breast cancer | 3,3-Dimethyl pentane, 2-Amino-5-isopropyl-8-methyl-1-azulene carbonitrile, 5-(2-Methylpropyl)nonane, 2,3,4-Trimethyl decane, 6-Ethyl-3-octyl ester 2-trifluoromethyl benzoic acid | |||||||
| Prostate cancer | Toluene, 2-Amino-5-isopropyl-8-methyl-1-azulene carbonitrile, 2,2-Dimethyl decane, p-Xylene | |||||||
| Molecularly capped AuNPs—7 different ligands | Prostate cancer | Toluene, 2-Amino-5-isopropyl-8-methyl-1-azulene carbonitrile, 2,2-Dimethyl decane, p-Xylene | NA | 9 PC, 10 HC | DFA | 100% specificity, 100% sensitivity | RT | [42] |
| Breast cancer | 3,3-Dimethyl pentane, 2-Amino-5-isopropyl-8-methyl-1-azulene carbonitrile, 5-(2-Methylpropyl)nonane, 2,3,4-Trimethyl decane, 6-Ethyl-3-octyl ester 2-trifluoromethyl benzoic acid | 10 BC, 11 HC | 100% sensitivity, 95% specificity | |||||
| Molecularly capped AuNPs—3 different ligands | Chronic kidney disease | healthy vs. stage 2: Isoprene, Acetone, Styrene, Toluene, 2-Butatone, 2,2,6-Trimethyl octane, 2,4-Dimethyl heptane Stage 2 vs. 3: Isoprene, Acetone, 2,2,6-Trimethyl octane, 2-Butatone, 2,4-Dimethyl heptane Stage 3 vs. 4: Acetone, Ethylene Glycol, Acetoin | 1–5 ppb | 42 CKD, 20 HC | SVM | 79% accuracy early-stage CKD vs. HC 85% accuracy CKD stage 4 vs. stage 5 | RT | [113] |
| Molecularly capped AuNPs—5 different ligands | Ovarian cancer | Styrene, Nonanal, 2-Ethylhexanol, 3-Heptanone, Decanal, Hexadecane | ppb level | 17 OV, 26 HC | DFA | 82% accuracy | RT | [44] |
| Molecularly capped AuNPs—8 different ligands | COVID-19 | NA | NA | 49 COVID-19, 33 non-COVID symptomatic, 58 HC | DFA | 76% accuracy COVID-19 vs. HC 95% accuracy COVID-19 vs. non-COVID symptomatic | RT | [24] |
| PAH-coated random SWCNTs network—4 different PAHs | Multiple sclerosis | Hexanal, 5-Methyl-undecane | NA | 37 MS, 18 HC | DFA | 80.4% accuracy | RT | [62] |
| Molecularly caped AuNPs/CDs-coated random SWCNTs network—20 different sensing films | Alzheimer’s and Parkinson’s disease | 24 VOCs | 1–5 ppb | 15 AD, 30 PD, 12 HC | DFA | 85% accuracy AD vs. HC 78% accuracy PD vs. HC 84% accuracy AD vs. PD | RT | [114] |
| Molecularly caped AuNPs/PAH-coated random SWCNTs network—20 different sensing films | 17 diseases (LC, CC, HNC, OC, BLC, PC, KC, GC, CD, UC, IBS, IPD, MS, PDISM, PH, PET, CKD) | 2-Ethylhexanol, 3-Methylhexane, 5-Ethyl-3-methyloctane, Acetone, Ethanol, Ethyl acetate, Ethyl benzene, Isononane, Isoprene, Nonanal, Styrene, Toluene, Undecane | 10 ppb | 813 any of 17 diseases, 591 HC | DFA, HCA | 86% average accuracy | RT | [115] |
| Ligand capped Au, Pt, and CuNPs—6 different sensing films | Human cutaneous leishmaniasis | 2,2,4-trimethyl pentane, 4-methyl-2-ethyl-1-pentanol, methyl vinyl ketone, nonane, 2,3,5-trimethyl hexane, hydroxy-2,4,6-trimethyl-5-(3-methyl-2 butenyl)cyclohexyl) methyl acetate, 3-ethyl-3-methyl heptane, octane, 2-methyl-6-methylene-octa-1,7-dien-3-ol | NA | 28 HCL, 32 HC | PCA, DFA | 98.2% accuracy, 96.4% sensitivity, 100% specificity | RT | [109] |
| pristine, COOH-, Hex-4T-Hex/DNA/oligomers, PTCDA/TAPC/TCTA monomers or PANI-functionalized SWCNTs | COPD | NH3, NO2, H2S, benzene, 2-propanol, acetone, ethanol, sodium hypochlorite, water | sub-ppb | 12 COPD, 9 HC | PCA | Acetone, ethanol and 2-propanol selective PANI-, TAPC- and COOH-CNTs, respectively. NO2 relevant driver of real-samples classification. Larger clinical trials needed. | RT | [107] |
| Pristine WO3, 0.008 wt % and 0.042 wt % Pt-WO3 macroporous NFs | Halitosis | H2S and Methyl mercaptan (in presence of Toluene and Acetone) | sub-ppm | 4 simulated halitosis breath samples (1 ppm), 4 HC | PCA | Successful classification | 350 °C | [96] |
| 7 different commercial MOS | Lung cancer | Ethyl benzene, 4-Methyl octane, Undecane, 2,3,4-trimethyl hexane | Down to a few ppb | 37 NSCLC (81.1% I, II), 48 HC | PCA | 75% accuracy Promising prognostic tool after LC resection surgery | 300 °C | [89] |
| 5 different commercial MOS | Lung cancer, COPD | NA | NA | 32 LC, 38 COPD, 72 HC | PCA, SVM, k-nearest neighbors | LC vs. HC: 91.3% accuracy, 84.4% sensitivity and 94.4% specificity COPD vs. HC: 90.9% accuracy, 81.6% sensitivity and 95.8% specificity | NA | [87] |
| Field Effect Transistor (FET)—arrays | ||||||||
| Molecularly modified SiNWs | Gastric cancer | 2-Propenenitril, Furfural, 6-Methyl-5-heptene-2-one | Down to a few ppb | 30 GC, 77 HC | DFA | >85% accuracy | RT | [125] |
| Molecularly modified SiNWs | Gastric cancer | 2-Propenenitril, Furfural, 6-Methyl-5-heptene-2-one | Down to a few ppb | 149 LC, 40 GC, 56 Asthma/COPD, 129 HC | DFA, ANN | >80% accuracy | RT | [43] |
| Lung cancer | Heptane, Decane, 2-Methyl pentane, 2-Ethyl-1-hexanol, Propanal, Pentanal, Acetone | |||||||
| Asthma/COPD | Pentane | |||||||
| Electrochemical sensor | ||||||||
| Commercial NO, CO sensors, carbon electrode with linear-aldehyde selective porous poly tetrafluoroethylene membrane | Diabetes | NO, CO, Formaldehyde, Acrolein, Propanal, Crotonaldehyde, Butanal, Pentanal, Hexanal, Heptanal, Octanal, Nonanal, Decanal, Acetaldehyde | Low ppb | 15 diabetic, 14 HC | LC vs. HC, diabetic vs. HC Cross-sensitivity for aldehyde sensor: Moderate for high level of ethanol and isopropanol/Weak for H2S, NO, methanol, 3-heptanone/None for NO2, propofol, isoprene, or acetone | RT | [126] | |
| Lung cancer | 3 LC, 3 smokers, 3 HC | |||||||
| Optical—Colorimetric sensor arrays | ||||||||
| 24 chemically reactive colorants | Lung cancer | NA | Low ppm | 92 LC, 137 HC | LPM | Accuracy 81.1% LC vs. HC, 82.5–89% one histology vs. HC, 86.4% ADC vs. SCC | RT | [127] |
| Optical sensors | ||||||||
| PMTFP-coated optical fiber | Vit. E deficiency | Ethane | pmol/L | 20 HC | NA | NA | RT | [128] |
| Liver diseases, Schizophrenia, Breast cancer, Rheumatoid Arthritis | Pentane | |||||||
| Lung cancer | Heptane, Octane, Decane, Benzene, Toluene, Styrene | |||||||
| Piezoelectric (SAW) sensor arrays | ||||||||
| GC-column/Polyisobutylene-coated SAW, non-coated SAW sensors | Lung cancer | Styrene, Decane, Isoprene, Benzene, Undecane, 1-Hexene, Hexanal, Propyl benzene, Heptanal, 1,2,4-Trimethyl benzene, Methyl cyclopentane | 500 ppb | 20 LC, 15 HC, 7 chronic bronchitis | ANN | 80% sensitivity and specificity | RT | [6,129] |
| Piezoelectric (QCM) sensor arrays | ||||||||
| 7 different metalloporphyrins | COPD | NA | NA | 5 COPD per GOLD stage (20), 5 HC | PLS-DA | Fair repeatability of measurements within HC and hypoxemic COPD patients (stage 4) Potential COPD severity assessment | RT | [130] |
| 8 different metalloporphyrins | Asthma | NA | NA | 27 asthma, 24 HC | PCA, FNN | 87.5% accuracy | RT | [131] |
| 8 different metalloporphyrins | Lung cancer | NA | NA | 20 LC, 10 HC | PLS-DA | 85% accuracy LC vs. HC 75% accuracy ADC vs. SCC | RT | [132] |
| 8 different metalloporphyrins | Lung cancer | NA | NA | 70 LC, 76 HC | PLS-DA | 81% sensitivity, 100% specificity | RT | [133] |
| 8 different metalloporphyrins | Tuberculosis | NA | NA | 51 TB (31/51 +HIV), 20 HC | PCA, k-nearest neighbors | 94.1% sensitivity, 90% specificity | RT | [134] |
| 7 different metalloporphyrins | Halitosis | H2S, Butyric acid, Valeric acid | 10–15 ppb | Oral malodor subjects, HC | PCA | PC1 78% of data variance | 50 °C | [135] |
| 8 different anthocyanins | Asthma | NA | NA | 15 asthma, 27 HC | Factor Analysis | 75% of total variance Repeatability similar to spirometry and eNO | RT | [136] |
| In vitro studies—Cell lines/Synthetic samples | ||||||||
| Chemiresistor arrays | ||||||||
| CNT-conductive polymer nanocomposites—5 different polymers | Lung cancer | Isopropanol, Tetrahydrofuran, Dichloromethane, Toluene, n-Heptane, Cyclohexane, Methanol, Ethanol, Water | NA | PCA | High sensitivity and selectivity for all the analytes, PC1-PC3 98% of total variance, except the two alkanes | RT | [110] | |
| Pristine rGO and rGO functionalized with 8 different amine ligands—9 elements | Cancer | Ethanol, 2-Ethylhexanol, Ethyl benzene, Nonanal | 25 ppm | NA | PCA | Successful discrimination of VOCs The LOD and the effect of humidity have to be decreased | RT | [102] |
| Pristine Pd, ZnO and polypyrrole NWs | Breast cancer | Heptanal | 8.98 ppm | NA | PCA | 73.2% PC1 variance High sensitivity and specificity | RT | [95] |
| Acetophenone | 798 ppb | |||||||
| 2-Propanol | 129.5 ppm | |||||||
| Isopropyl myristate | 134 ppm | |||||||
| Pristine In2O3 and WO3 NRs, Au, Pt, or Pd NPs-decorated In2O3 and WO3 NRs—8 elements | Diabetes | Acetone | 1.48 ppb | NA | Polar plot | Effective visual discrimination between the gases. Future PCA, DFA, HCA analysis. | 150–300 °C | [99] |
| Asthma | NO2 | 1.9 ppt | ||||||
| Halitosis | H2S | 2.47 ppb | ||||||
| WO3 NTs Pt NPs—WO3 NTs, Pd NPs—WO3 NTs | Asthma | NO | 50 ppb | NA | NA | NA | 350 °C | [56] |
| Lung cancer | Toluene | 100 ppb | 400 °C | |||||
| Pristine, 0.1% wt GO- and 0.1 wt % thin layered graphite WO3 Hemi tubes | Diabetes | Acetone | 1 ppm | NA | NA | NA | 350 °C | [57] |
| Halitosis | H2S | |||||||
| Electrochemical sensor | ||||||||
| MWCNTs/Au-Ag NPs/GCE | Gastric cancer | 3-Octanone | 0.3 ppb | MGC-803 GC and GES-1 gastric mucosa cell lines | NA | Easy cell line discrimination, high sensitivity, good VOCs selectivity in presence of CO2, acetone and ethanol | RT | [137] |
| Butanone | 0.5 ppb | |||||||
| SiNWs-rGO | Infectious diseases | Cyclohexane, Formaldehyde in presence of Methanol, Ethanol, Acetonitrile, Acetaldehyde and humidity | 1 ppm | NA | Novel electrode platform with increased sensitivity, selectivity and repeatability | [106] | ||
| Piezoelectric (SAW) sensor arrays | ||||||||
| SH-Calix4arene, AuNRs, AgNCs, Calix4arene-AuNRs, Calix4arene-AgNCs | Lung cancer | Chloroform, Toluene, Isoprene, Acetone, n-Hexane, Ethanol | 1.52–12.34 ppm for CHCl3 1.54–2.64 ppm for toluene | NA | NA | Sensitivity ↑ for all VOCs Chloroform, toluene: 6–8 times higher sensitivity than individual responses Selectivity ↑: modified AuNRs for CHCl3, modified AgNCs for Toluene | RT | [49] |
| Pristine or AuNPs-functionalized zeolitic-imidazole-framework nanocrystals (ZIF-8, ZIF-67) | Diabetes | Acetone, Ammonia, Ethanol | acetone 1.1–3.6 ppm, ethanol 0.5–3 ppm NH3 1.6–3.2 ppm | NA | PCA | Effective discrimination of diabetes biomarkers | RT | [138] |
| Piezoelectric (QCM) sensor arrays | ||||||||
| TiO2-MWCNTS and Cobalt (II) phthalocyanine-silica on Au layers | Diabetes | Acetone | 4.33 ppm | NA | NA | High sensitivity | RT | [139] |
| Asthma | NO | 5.75 ppb | ||||||
| Optical—Colorimetric arrays | ||||||||
| 36 chemically responsive dyes (porphyrin derivatives, NaFluo) | Lung cancer | p-Xylene, Styrene, Isoprene, Hexanal | 50 ppb | NA | HCA, PCA, BPNN | 100% accuracy of kind and concentration discrimination, promising for real-sample experiments | RT | [140] |
| AuNRs-modified metalloporphyrins and pH responsive dyes—36 spots | Lung cancer | Decane, Undecane, Hexanal, Heptanal, 1,2,4-Trimethylbenzene, Benzene | <1 ppm | NA | PCA, HCA | 64.2% accuracy of structurally similar VOCs, 93% photoprotection of metalloporphyrins, ↑ repeatability and long-term stability | RT | [141] |
3.2. Field-Effect Transistors (FET)
3.2.1. Selective Applications
3.2.2. Cross-Reactive Applications
3.3. Electrochemical Sensors
3.3.1. Selective Applications
3.3.2. Cross-Reactive Applications
3.4. Piezoelectric Sensors
3.4.1. Quartz Crystal Microbalance (QCM) Sensors
Selective Applications
Cross-Reactive Applications
3.4.2. Surface Acoustic Wave (SAW) Sensors
Selective Applications
Cross-Reactive Applications
3.5. Optical Sensors
3.5.1. Optical Fiber-Based Sensors
Selective Applications
Cross-Reactive Applications
3.5.2. Colorimetric
Selective and Cross-Reactive Applications
3.5.3. Localized Surface Plasmon Resonance (LSPR)-Based Sensors
3.5.4. Surface Enhanced Raman Spectroscopy (SERS)-Based Sensors
4. Differential Diagnosis and Disease Phenotyping and Staging in Breath Analysis
4.1. The Case for Lung Diseases
| Sensor | Diseases/Phenotypes/Stages | Subjects | Classifier | Results | Ref. |
|---|---|---|---|---|---|
| Differential diagnosis | |||||
| Cyranose 320 | NSCLC vs. COPD (GOLD stage I-III) | 10 NSCLC, 10 COPD | PCA, CDA | 85% acc. | [223] |
| LC vs. non-cancer (COPD, asthma, pneumonia, pulmonary embolism, BPN) | 165 LC, 91 non-cancer | SVM | 87.3% acc. | [224] | |
| LC vs. COPD vs. LC/COPD vs. HC | 63 LC, 15 COPD, 79 both, 78 HC | 77.4% acc., 100% accurate LC/COPD classification | |||
| LC vs. non-cancer (COPD, asthma, pneumonia, pulmonary embolism, bronchiectasis, BPN, TB) | 252 LC, 223 non-cancer | LRA | Sens.: 95.8% (S), 96.2% (NS) Spec.: 92.3% (S) 90.6% (NS) | [225] | |
| Asthma vs. COPD | 20 asthma, 30 COPD | PCA, CDA | 96% acc. Within/between day repeatability, reproducibility of e-Noses | [229] | |
| Fixed and classic asthma vs. COPD (GOLD stages II-III) | 21 fixed asthma, 40 COPD | PCA, CDA | 88% acc., 85% sens., 90% spec. | [230] | |
| 39 classic asthma, 40 COPD | 83% acc., 91% sens., 90% spec. | ||||
| IPF vs. COPD | 32 IPF, 33 COPD | PCA, CDA | 80% cross-validated acc., Wider patient cohorts and inclusion of more comorbidities needed | [231] | |
| COPD vs. LC vs. BC | 50 COPD, 30 LC, 50 BC | PCA, CDA, CAP | Correct classification values: LC vs. COPD 96.47%, LC vs. BC 93.05%, BC vs. COPD 100%, COPD vs. LC vs. BC 91.35% | [201] | |
| Bronchial vs. Laryngeal SCC (advanced) | 10 bronchial, 10 laryngeal | JMP Pro | 10% misclassification, 100% sens., 80% spec. | [232] | |
| AD vs. PD vs. HC | 18 AD, 16 PD, 19 HC | LDA | 76.2% sens., 45.8% spec., p < 0.0001 | [233] | |
| AeoNose | ILDs (COP, CTD) vs. COPD, ILDs subgroups (COP, HP, IPF, sarcoidosis, uILD, asbestosis, NSIP, RB-ILD, DIP) | 28 COP, 23 COPD | Athena program, t-test | AUC 0.77, 75% sens., 71% spec. | [234] |
| 25 CTD-ILD, 23 COPD | AUC 0.85, 88% sens., 71% spec. | ||||
| 174 ILDs, 23 COPD | Less accurate discrimination of ILDs subgroups (e.g., AUC IPF vs. CTD-ILD 0.86, COP vs. CTD-ILD 0.82) | ||||
| Asthma vs. CF | 20 asthma (moderate-severe), 13 CF | ANN | AUC 0.90, 89% sens., 77% spec. | [235] | |
| HNSCC vs. LC | 52 HNSCC, 32 LC | ANN | Acc., sens., spec.: 93%, 96%, 88% (best fit), 85%, 85%, 84% (cross-validation) | [236] | |
| Cancer types | 100 HNSCC, 40 bladder, 28 colon cancer | ANN | Acc., sens., spec.: 81%, 79%, 81% HNSCC vs. colon cancer, 84%, 80%, 86% HNSCC vs. bladder cancer, 84%, 88%, 79% Colon vs. bladder cancer | [237] | |
| SpiroNose | LC vs. COPD vs. asthma vs. HC | 31 LC, 31 COPD, 37 asthma, 45 HC | PCA | Cross-validation values 78–88%, repeatability ↑ | [91] |
| ILD subgroups: | 141 sarcoidosis, 85 IPF, 33 CTD-ILD, 25 HP, 11 IPAF, 10 NSIP | PLS-DA | Acc., sens., spec.: 77%, 75%, 84% IPF vs. HP, 94%, 98%, 85% IPF vs. CTD-ILD, 92%, 92%, 90% IPF vs. NSIP, 89%, 87%, 100% IPF vs. IPAF, 75%, 100%, 67% CTD-ILD vs. IPAF, 98%, 90%, 100% CTC-ILD vs. NSIP, 90%, 94%, 72% HP vs. sarcoidosis, 91%, 92%, 88% (training), 91%, 95%, 79% (validation) IPF vs. non-IPF | [207] | |
| Chemiresistor-based alkane sensor | LC vs. HC LC vs. COPD | 12 LC, 12 COPD, 13 HC | MANOVA | LC: 83.3% sens., 88% spec. Sensor acc no smoke-dependence | [108] |
| MOS, electrochemical, hot wire, and catalytic | LC vs. COPD | 48 LC, 52 COPD | 8 different | 76.9–84.75% acc., 75–81.36% sens., 78.79–88.14 spec. Highest acc. With KPCA-XGBoost | [226] |
| LC vs. COPD | 33 LC, 28 COPD | PCA-SVM, KPCA-SVM, PCA-XGBoost, KPCA-XGBoost | 82.52–96% acc., 78.33–95% sens., 85–96.67% spec. Highest acc. With KPCA-XGBoost | [227] | |
| Organically-coated AuNPs and SWCNTs based chemiresistor | LC (I/II) vs. BPN | 16 LC, 30 BPN | DFA | 87% acc., 75% sens., 93% spec. Low LC sample → careful interpretation | [228] |
| BC vs. benign | 30 HC, 15 BBT, 13 DCIS, 96 BC | DFA | Acc., sens., spec.: 88.3%, 90.6%, 83.3% BC vs. BBT/HC, 71.2–82%, 62.6–80%, 75.7–82.3% BC vs. BBT, 81.4–84.4%, 83–83.3%, 81–92% BC vs. DCIS | [238] | |
| Gca vs. OLGIM groups (0-IV) | 99 Gca, 155 OLGIM 0, 136 OLGIM I-II, 34 OLGIM III-IV, 53 PUD | DFA | Acc., sens., spec.: 92%, 73%, 98% Gca vs. 0–IV, 84%, 90%, 80% Gca vs. 0, 87%, 97%, 84% Gca vs. 0–II, 90%, 93%, 80% Gca vs. III-IV, 85%, 93%, 80% Gca vs. I–IV, 87%, 87%, 87% Gca vs. PUD | [239] | |
| Gca vs. benign gastric conditions | 37 Gca, 32 ulcers, 61 less severe conditions | DFA | 89% sens., 90% spec. 84% sens., 87% spec. | [240] | |
| ulcer vs. less severe | |||||
| AD vs. PD AD vs. PD vs. HC | 15 AD, 30 PD, 12 HC | DFA | AD vs. PD: 84% acc., 80% sens., 87% spec. Feasible overall discrimination, with large PD/HC overlap | [114] | |
| NA-NOSE | BC, benign breast conditions, normal mammographs | 11 BC, 14 benign, 7 normal mammographs | PCA/ANOVA/Student’s t-test, SVM | 94% sens., 80% spec. for benign vs. BC and negative mammography, Similar results with both methods | [116] |
| MCNPs-based chemiresistor—6 sensors-array | IBD vs. IBS | 71 IBD (35 UC, 36 CD), 26 IBS | ANN | 81/88% acc., 92/73% sens., 53/100% spec. (real/artificial) | [111] |
| CD vs. UC | 75/96% acc., 75/100% sens., 47/93% spec. (real/artificial) | ||||
| Molecularly modified SiNW FET | Gca vs. LC | 40 Gca, 149 LC, 56 asthma/COPD | DFA, ANN | 92% acc., 93% LC, and 85% Gca correct classification | [43] |
| LC vs. asthma and COPD | 89% acc., 92% sens., 80% spec. | ||||
| MOS gas sensor array | Gca vs. gastric ulcer patients | 49 Gca, 30 gastric ulcer | Back-propagation Neural network | 93% acc., 94.38% sens., 89.93% spec. Classification acc. Of malignant, benign, normal subjects: 92.54%, 93.17%, 92.49%. | [241] |
| OC vs. benign and HC | 86 OC, 51 benign, 114 HC | PCA, k-NN | Acc., sens., spec.: 85%, 6%, 84% (cross-validation/strict prediction), 87%, 89%, 86% (prediction/strict prediction), 86%, 84%, 85% (cross-validation/most probable pred.), 100%, 100%, 100% (prediction/most probable pred.) | [242] | |
| AD vs. PD vs. HC | 20 AD, 20 PD, 20 HC | PCA | Effective discrimination of AD vs. PD and HC | [243] | |
| BIONOTE | CLD vs. NC-CLD | 65 CLD, 39 NC-CLD | PLS-DA, radar plot | Successful discrimination, 16 cirrhotic patients misclassified | [244] |
| Commercial (MQ) gas sensors | CKD vs. diabetes vs. HC high creatinine, HC low creatinine | 84 CKD, 24 diabetes, 54 HC high creatinine, 54 HC low creatinine | Radar plots, PCA, SVM, PLS-regression, HCA | PCA: 96.64% of total variance expressed in PC1–3 SVM: 100% correct classification of samples | [245] |
| Disease histology/phenotyping | |||||
| Tor Vergata e-Nose | SCC vs. ADC | 10 SCC, 10 ADC | PLS-DA | 75% correct classification | [132] |
| 24 colorants | SCC vs. ADC | 22 SCC, 50 ADC | LPM | 86.4% acc. | [127] |
| SCLC vs. NSCLC | 9 SCLC, 83 NSCLC | 78.1% acc. (moderate) | |||
| UV-irradiated pristine, Au, Pt, Au/Pt, Ni, Fe-doped WO3NWs | LC vs. HC | 4 SCLC, 8 SCC, 10 ADC, 12 HC | PCA | 98.6 % acc. | [246] |
| SCLC vs. NSCLC, SCC vs. ADC | DFA | Acc.: 84.5% SCLC vs. NSCLC,77.5% SCC vs. ADC | |||
| Molecularly capped AuNPs and SWCNT based chemiresistors | LC with vs. without EGFR mutation | 19 with EGFR, 34 without EGFR | DFA | 83% acc., 79% sens., 85% spec. | [228] |
| Cyranose 320, Tor Vergata, Common In-vent, Owlstone Lonestar | Clinically stable vs. unstable episodes of asthma | 22 partly controlled persistent asthma | PCA | Correct classification: 95% baseline vs. loss of control, 86% loss of control vs. recovery Owlstone Lonestar the most prominent | [90] |
| Cyranose 320 | Asthma inflammatory phenotypes | 24 EOS., 10 NEUTR., 18 PAUC. | PCA | Acc., sens., spec.: 73%, 60%, 79% EOS. vs. NEUTR., 74%, 55%, 87% EOS vs. PAUC., 89%, 94%, 80% NEUTR. vs. PAUC. | [247] |
| Uncontrolled asthma-like symptoms | Training set: 65 cluster 1, 22 cluster 2, 34 cluster 3 | one-way ANOVA, Kruskal-Wallis | Significant differences concerning chest tightness during exercise, dyspnea and gender | [248] | |
| HC and controlled vs. partly controlled and uncontrolled asthma | 10 HC, 9 controlled, 7 partly, 12 uncontrolled | PCA, radar plot | Good predictive ability Cross-validated AUC 0.80, 79% sens., 84% spec. | [119] | |
| Organically-coated AuNPs and SWCNT-based chemiresistor | BC subtypes | 12 LuminalA, 42 LuminalB, 12 Triple Negative, 16 HER2+, 14 HER2 equivocal | DFA | Acc., sens., spec.: LuminalA vs. others 81.3–87.7%, 75–87.5%, 82.1–87.5% LuminalB vs. others78.1–86.3%, 83.3–85.3%, 74.1–87.2% HER2+ vs. others 81.3–82.4%, 81.3–91%, 80.7–81.3% Triple neg, vs. others 82.9–90.3%, 83.3–93.3%, 82.9–89.4% Luminal vs. non-Luminal 70.8–87.7%, 70.4–88.1%, 71.4–87.1% LuminalA vs. LuminalB 85.7–94%, 75–91.7%, 88.2–95.2% HER2 status/luminal 85.7–100%, 85.7–100%, 83.3–100% HER2 status/non-luminal 90.9%, 90.9%, 90.9% | [238] |
| Disease staging | |||||
| Tor Vergata e-Nose | LC Stage I vs. II/III/IV | 40 stage I, 18 stage II, 6 III/IV | PLS-DA | Sens.: stage I 90% vs. stage II-IV 57% (+ metabolic diseases), stage I 96% vs. stage II-IV 60% (LC only) | [133] |
| 24 colorants | LC Stage I/II vs. LC stage III/IV | 41 SCLC, 42 NSCLC | LPM | 79.3 % acc. (moderate) | [127] |
| 11 sensor-array (MOS, electrochemical, hot wire and catalytic) | LC Stage III vs. IV | 44 stage II, 46 stage IV | PCA-SVM, KPCA-SVM, PCA-XGBoost, KPCA-XGBoost | 70.42–82.42% acc., 45–81% sens., 79–95.5% spec. | [227] |
| Organically-coated AuNPs and SWCNTs based chemiresistor | OLGIM stages | 155 OLGIM 0, 136 OLGIM I-II, 34 OLGIM III-IV, 7 Dysplasia | DFA | Acc., sens., spec.: 0-II vs. III-IV and dysplasia 61%, 83%, 60%, 0 vs. I-II 43%, 45%, 41%, 0 vs. III-IV 66%, 90%, 61%, 0 vs. I-IV 50%, 50%, 50%, I-II vs. III-IV 64%, 80%, 60% | [239] |
| GCa I-II vs. III-IV | 17 GCa I-II, 18 GCa III-IV | DFA | 89% sens., 94% spec. | [240] | |
| Molecularly modified SiNW FET | LC staging (I-II vs. III-IV) | 34 early stage, 110 advanced stage | DFA, ANN | 81% acc., 34.5%sens.,95% spec. | [43] |
| GCa staging (I-II vs. III-IV) | 86.5% correct classification, 84.6 early stage, 87.5 advanced | ||||
| Cyranose 320 | Bronchial/Laryngeal in situ vs. advanced | bronchial: 10 in situ, 10 advanced, laryngeal: 12 in situ, 10 advanced | JMP Pro | 21% misclassification rate, 82% sens., 75% spec. | [232] |
| BIONOTE | Liver cirrhosis (A, B, C Child–Pugh) | NA | PLS-DA | Successful discrimination | [244] |
4.2. Cancers
4.3. Liver, Renal, and Intestinal Diseases
4.4. Neurodegenerative Diseases
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Liu, T.; Li, T.; Zhao, H.; Chen, Q. Exhaled breath analysis in disease detection. Clin. Chim. Acta 2021, 515, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Gashimova, E.; Temerdashev, A.; Porkhanov, V.; Polyakov, I.; Perunov, D.; Azaryan, A.; Dmitrieva, E. Investigation of different approaches for exhaled breath and tumor tissue analyses to identify lung cancer biomarkers. Heliyon 2020, 6, e04224. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile biomarkers in the breath of women with breast cancer. J. Breath Res. 2010, 4, 026003. [Google Scholar] [CrossRef] [PubMed]
- Oakley-Girvan, I.; Davis, S.W. Breath based volatile organic compounds in the detection of breast, lung, and colorectal cancers: A systematic review. Cancer Biomarkers 2017, 21, 29–39. [Google Scholar] [CrossRef]
- Pite, H.; Morais-Almeida, M.; Rocha, S.M. Metabolomics in asthma: Where do we stand? Curr. Opin. Pulm. Med. 2018, 24, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Vishinkin, R.; Haick, H. Nanoscale Sensor Technologies for Disease Detection via Volatolomics. Small 2015, 11, 6142–6164. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Vishinkin, R.; Barash, O.; Nakhleh, M.K.; Haick, H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. [Google Scholar] [CrossRef]
- Tisch, U.; Haick, H. Chemical sensors for breath gas analysis: The latest developments at the Breath Analysis Summit 2013. J. Breath Res. 2014, 8, 027103. [Google Scholar] [CrossRef]
- Song, G.; Qin, T.; Liu, H.; Xu, G.B.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; Sun, G.P.; Chen, Z.D. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer 2010, 67, 227–231. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Chen, J.; Wang, H.; Cheng, T.; Chen, K.; Xu, S. Analysis of human breath samples of lung cancer patients and healthy controls with solid-phase microextraction (SPME) and flow-modulated comprehensive two-dimensional gas chromatography (GC × GC). Anal. Methods 2014, 6, 6841–6849. [Google Scholar] [CrossRef]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.E. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Bos, L.D.; Sterk, P.J.; Fowler, S.J. Breathomics in the setting of asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Broza, Y.Y.; Haick, H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; van der Schee, M.P.; Brinkman, P.; Sterk, P.J. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin. Exp. Allergy 2013, 43, 705–715. [Google Scholar] [CrossRef]
- Wang, M.; Sheng, J.; Wu, Q.; Zou, Y.; Hu, Y.; Ying, K.; Wan, H.; Wang, P. Confounding effect of benign pulmonary diseases in selecting volatile organic compounds as markers of lung cancer. J. Breath Res. 2018, 12, 046013. [Google Scholar] [CrossRef]
- Hashoul, D.; Haick, H. Sensors for detecting pulmonary diseases from exhaled breath. Eur. Respir. Rev. 2019, 28, 190011. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Dallinga, J.W.; Nuss, S.J.; Moonen, E.J.C.; van Berkel, J.J.B.N.; Akdis, C.; van Schooten, F.J.; Menz, G. Defining adult asthma endotypes by clinical features and patterns of volatile organic compounds in exhaled air. Respir. Res. 2014, 15, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corradi, M.; Poli, D.; Bouza, M. Observation of nonanoic acid and aldehydes in exhaled breath of patients with lung cancer Observation of nonanoic acid and aldehydes in exhaled breath of patients with lung cancer. J. Breath Res. 2017, 11, 26004. [Google Scholar] [CrossRef]
- Boots, A.W.; Van Berkel, J.J.B.N.; Dallinga, J.W.; Smolinska, A.; Wouters, E.F.; Van Schooten, F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 2012, 6, 027108. [Google Scholar] [CrossRef]
- Haick, H.; Cohen-Kaminsky, S. Detecting lung infections in breathprints: Empty promise or next generation diagnosis of infections. Eur. Respir. J. 2015, 45, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, B.; Broza, Y.Y.; Li, W.; Wang, Y.; Wu, S.; Liu, Z.; Wang, J.; Gui, S.; Wang, L.; Zhang, Z.; et al. Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath. ACS Nano 2020, 14, 12125–12132. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, G.; Haick, H.; Garoli, D. Detecting COVID-19 from Breath: A Game Changer for a Big Challenge. ACS Sens. 2021, 6, 1408–1417. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Roquencourt, C.; Moine, P.; Saffroy, G.; Carn, S.; Heming, N.; Fleuriet, J.; Salvator, H.; Naline, E.; Couderc, L.J.; et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study. EBioMedicine 2021, 63, 103154. [Google Scholar] [CrossRef]
- BreFenceTM Go COVID-19 Breath Test System Rapid Breath Test for COVID-19 Detection. Available online: https://secureservercdn.net/160.153.138.177/8g1.9f8.myftpupload.com/wp-content/uploads/2021/05/Breathonix-BreFence-Go-Breath-Test-System.pdf (accessed on 25 November 2021).
- HSA Grants Provisional Authorisation for ‘BreFence Go COVID-19 Breath Test System’ and ‘Traciex Breathalyser’ for Detection of COVID-19 Infection. Available online: https://www.hsa.gov.sg/announcements/regulatory-updates/hsa-grants-provisional-authorisation-for-brefence-go-covid-19-breath-test-system-and-traciex-breathalyser-for-detection-of-covid-19-infection (accessed on 25 November 2021).
- Provisional Authorisation for COVID-19 Tests—Breathonix Pte Ltd. Available online: https://www.hsa.gov.sg/docs/hprg-mdb/breathonix_brefence-go-covid-19-breath-test-system_provisional-authorisation-for-covid-19-tests_19052021.pdf (accessed on 25 November 2021).
- Wang, C.; Sun, B.; Guo, L.; Wang, X.; Ke, C.; Liu, S.; Zhao, W.; Luo, S.; Guo, Z.; Zhang, Y.; et al. Volatile organic metabolites identify patients with breast cancer, cyclomastopathy, and mammary gland fibroma. Sci. Rep. 2014, 4, 5383. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [Green Version]
- Gaida, A.; Holz, O.; Nell, C.; Schuchardt, S.; Lavae-Mokhtari, B.; Kruse, L.; Boas, U.; Langejuergen, J.; Allers, M.; Zimmermann, S.; et al. A dual center study to compare breath volatile organic compounds from smokers and non-smokers with and without COPD. J. Breath Res. 2016, 10, 026006. [Google Scholar] [CrossRef]
- Neerincx, A.H.; Vijverberg, S.J.H.; Bos, L.D.J.; Brinkman, P.; van der Schee, M.P.; de Vries, R.; Sterk, P.J.; Maitland-van der Zee, A.H. Breathomics from exhaled volatile organic compounds in pediatric asthma. Pediatr. Pulmonol. 2017, 52, 1616–1627. [Google Scholar] [CrossRef]
- Amal, H.; Haick, H. Point of care breath analysis systems. In Advanced Nanomaterials for Inexpensive Gas Microsensors: Synthesis, Integration and Applications, 1st ed.; Llobet, E., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2019; pp. 315–334. [Google Scholar]
- Basanta, M.; Jarvis, R.M.; Xu, Y.; Blackburn, G.; Tal-Singer, R.; Woodcock, A.; Singh, D.; Goodacre, R.; Paul Thomas, C.L.; Fowler, S.J. Non-invasive metabolomic analysis of breath using differential mobility spectrometry in patients with chronic obstructive pulmonary disease and healthy smokers. Analyst 2010, 135, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Haick, H. Nanomaterials for cross-reactive sensor arrays. MRS Bull. 2010, 35, 797–803. [Google Scholar] [CrossRef]
- Van Der Schee, M.P.; Fens, N.; Brinkman, P.; Bos, L.D.J.; Angelo, M.D.; Nijsen, T.M.E.; Raabe, R.; Knobel, H.H.; Vink, T.J.; Sterk, P.J. Effect of transportation and storage using sorbent tubes of exhaled breath samples on diagnostic accuracy of electronic nose analysis. J. Breath Res. 2013, 7, 016002. [Google Scholar] [CrossRef]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 24001. [Google Scholar] [CrossRef]
- Madianos, L.; Skotadis, E.; Patsiouras, L.; Filippidou, M.K.; Chatzandroulis, S.; Tsoukalas, D. Nanoparticle based gas-sensing array for pesticide detection. J. Environ. Chem. Eng. 2018, 6, 6641–6646. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Effect of humidity on nanoparticle-based chemiresistors: A comparison between synthetic and real-world samples. ACS Appl. Mater. Interfaces 2012, 4, 317–325. [Google Scholar] [CrossRef]
- Shehada, N.; Cancilla, J.C.; Torrecilla, J.S.; Pariente, E.S.; Brönstrup, G.; Christiansen, S.; Johnson, D.W.; Leja, M.; Davies, M.P.A.; Liran, O.; et al. Silicon Nanowire Sensors Enable Diagnosis of Patients via Exhaled Breath. ACS Nano 2016, 10, 7047–7057. [Google Scholar] [CrossRef] [Green Version]
- Kahn, N.; Lavie, O.; Paz, M.; Segev, Y.; Haick, H. Dynamic Nanoparticle-Based Flexible Sensors: Diagnosis of Ovarian Carcinoma from Exhaled Breath. Nano Lett. 2015, 15, 7023–7028. [Google Scholar] [CrossRef]
- Phillips, C.O.; Syed, Y.; Parthaláin, N.M.; Zwiggelaar, R.; Claypole, T.C.; Lewis, K.E. Machine learning methods on exhaled volatile organic compounds for distinguishing COPD patients from healthy controls. J. Breath Res. 2012, 6, 036003. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.; Curto, V.F.; Benito-Lopez, F.; Florea, L.; Diamond, D. Wearable Bio and Chemical Sensors. In Wearable Sensors: Fundamentals, Implementation and Applications, 1st ed.; Sazonov, E., Neuman, M.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 65–83. [Google Scholar]
- Skotadis, E.; Tang, J.; Tsouti, V.; Tsoukalas, D. Chemiresistive sensor fabricated by the sequential ink-jet printing deposition of a gold nanoparticle and polymer layer. Microelectron. Eng. 2010, 87, 2258–2263. [Google Scholar] [CrossRef]
- Skotadis, E.; Tanner, J.L.; Stathopoulos, S.; Tsouti, V.; Tsoukalas, D. Chemical sensing based on double layer PHEMA polymer and platinum nanoparticle films. Sens. Actuators B Chem. 2012, 175, 85–91. [Google Scholar] [CrossRef]
- Kus, F.; Altinkok, C.; Zayim, E.; Erdemir, S.; Tasaltin, C.; Gurol, I. Surface acoustic wave (SAW) sensor for volatile organic compounds (VOCs) detection with calix[4]arene functionalized Gold nanorods (AuNRs) and silver nanocubes (AgNCs). Sens. Actuators B Chem. 2021, 330, 129402. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Myung, N.V.; Deshusses, M.A. Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19, 332001. [Google Scholar] [CrossRef]
- Haick, H. Chemical sensors based on molecularly modified metallic nanoparticles. J. Phys. D. Appl. Phys. 2007, 40, 7173–7186. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Characteristics of Surface Acoustic Wave Sensors with Nanoparticles Embedded in Polymer Sensitive Layers for VOC Detection. Sensors 2018, 18, 2401. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.W.; Lee, J.H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab. Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef]
- Vasilescu, A.; Hrinczenko, B.; Swain, G.M.; Peteu, S.F. Exhaled breath biomarker sensing. Biosens. Bioelectron. 2021, 182, 113193. [Google Scholar] [CrossRef]
- Moon, H.G.; Choi, Y.R.; Shim, Y.S.; Choi, K.I.; Lee, J.H.; Kim, J.S.; Yoon, S.J.; Park, H.H.; Kang, C.Y.; Jang, H.W. Extremely sensitive and selective NO probe based on villi-like WO3 nanostructures for application to exhaled breath analyzers. ACS Appl. Mater. Interfaces 2013, 5, 10591–10596. [Google Scholar] [CrossRef]
- Koo, W.T.; Choi, S.J.; Kim, N.H.; Jang, J.S.; Kim, I.D. Catalyst-decorated hollow WO3 nanotubes using layer-by-layer self-assembly on polymeric nanofiber templates and their application in exhaled breath sensor. Sens. Actuators B Chem. 2016, 223, 301–310. [Google Scholar] [CrossRef]
- Choi, S.J.; Fuchs, F.; Demadrille, R.; Grévin, B.; Jang, B.H.; Lee, S.J.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Fast responding exhaled-breath sensors using WO3 hemitubes functionalized by graphene-based electronic sensitizers for diagnosis of diseases. ACS Appl. Mater. Interfaces 2014, 6, 9061–9070. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.J.; Lee, I.; Youn, D.Y.; Park, C.O.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Masuda, Y.; Itoh, T.; Shin, W.; Kato, K. SnO2 nanosheet/nanoparticle detector for the sensing of 1-nonanal gas produced by lung cancer. Sci. Rep. 2015, 5, 10122. [Google Scholar] [CrossRef] [PubMed]
- Giebelhaus, I.; Varechkina, E.; Fischer, T.; Rumyantseva, M.; Ivanov, V.; Gaskov, A.; Morante, J.R.; Arbiol, J.; Tyrra, W.; Mathur, S. One-dimensional CuO-SnO2 p-n heterojunctions for enhanced detection of H2S. J. Mater. Chem. A 2013, 1, 11261–11268. [Google Scholar] [CrossRef]
- Wei, C.; Dai, L.; Roy, A.; Tolle, T.B. Multifunctional chemical vapor sensors of aligned carbon nanotube and polymer composites. J. Am. Chem. Soc. 2006, 128, 1412–1413. [Google Scholar] [CrossRef]
- Ionescu, R.; Broza, Y.; Shaltieli, H.; Sadeh, D.; Zilberman, Y.; Feng, X.; Glass-Marmor, L.; Lejbkowicz, I.; Müllen, K.; Miller, A.; et al. Detection of multiple sclerosis from exhaled breath using bilayers of polycyclic aromatic hydrocarbons and single-wall carbon nanotubes. ACS Chem. Neurosci. 2011, 2, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari-Saatlu, M.; Procek, M.; Mattsson, C.; Thungström, G.; Nilsson, H.E.; Xiong, W.; Xu, B.; Li, Y.; Radamson, H.H. Silicon nanowires for gas sensing: A review. Nanomaterials 2020, 10, 2215. [Google Scholar] [CrossRef]
- Wang, B.; Haick, H. Effect of chain length on the sensing of volatile organic compounds by means of silicon nanowires. ACS Appl. Mater. Interfaces 2013, 5, 5748–5756. [Google Scholar] [CrossRef]
- Ermanok, R.; Assad, O.; Zigelboim, K.; Wang, B.; Haick, H. Discriminative power of chemically sensitive silicon nanowire field effect transistors to volatile organic compounds. ACS Appl. Mater. Interfaces 2013, 5, 11172–11183. [Google Scholar] [CrossRef]
- Wang, B.; Cancilla, J.C.; Torrecilla, J.S.; Haick, H. Artificial sensing intelligence with silicon nanowires for ultraselective detection in the gas phase. Nano Lett. 2014, 14, 933–938. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, I.D. Recent Developments in 2D Nanomaterials for Chemiresistive-Type Gas Sensors. Electron. Mater. Lett. 2018, 14, 221–260. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J.; Lee, J. Rational Design of Semiconductor-Based Chemiresistors and their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Yen, C.C.; Xue, S.; Wang, H.; Stanciu, L.A. Surface Functionalization of Layered Molybdenum Disulfide for the Selective Detection of Volatile Organic Compounds at Room Temperature. ACS Appl. Mater. Interfaces 2019, 11, 34135–34143. [Google Scholar] [CrossRef]
- Chu, X.; Hu, R.; Wang, J.; Dong, Y.; Zhang, W.; Bai, L.; Sun, W. Preparation and gas sensing properties of graphene-Zn2SnO4 composite materials. Sens. Actuators B Chem. 2017, 251, 120–126. [Google Scholar] [CrossRef]
- Tang, J.; Skotadis, E.; Stathopoulos, S.; Roussi, V.; Tsouti, V.; Tsoukalas, D. PHEMA functionalization of gold nanoparticles for vapor sensing: Chemi-resistance, chemi-capacitance and chemi-impedance. Sens. Actuators B Chem. 2012, 170, 129–136. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wyszynski, B.; Yatabe, R.; Hayashi, K.; Toko, K. Molecularly imprinted sol-gel-based QCM sensor arrays for the detection and recognition of volatile aldehydes. Sensors 2017, 17, 382. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Wang, Z.; Gu, S.; Wang, J. Chemical Detection of hexanal in humid circumstances using hydrophobic molecularly imprinted polymers composite. Sens. Actuators B. Chem. 2019, 291, 141–147. [Google Scholar] [CrossRef]
- Amor, R.E.; Nakhleh, M.K.; Barash, O.; Haick, H. Breath analysis of cancer in the present and the future. Eur. Respir. Rev. 2019, 28, 190002. [Google Scholar] [CrossRef]
- Güntner, A.T.; Koren, V.; Chikkadi, K.; Righettoni, M.; Pratsinis, S.E. E-Nose Sensing of Low-ppb Formaldehyde in Gas Mixtures at High Relative Humidity for Breath Screening of Lung Cancer? ACS Sens. 2016, 1, 528–535. [Google Scholar] [CrossRef]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P.; Chouksey, A.; Lal, M.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sens. Actuators B Chem. 2017, 240, 1134–1140. [Google Scholar] [CrossRef]
- Young, S.J.; Lin, Z.D. Ethanol gas sensors based on multi-wall carbon nanotubes on oxidized Si substrate. Microsyst. Technol. 2018, 24, 55–58. [Google Scholar] [CrossRef]
- Hanh, N.H.; van Duy, L.; Hung, C.M.; Xuan, C.T.; van Duy, N.; Hoa, N.D. High-performance acetone gas sensor based on Pt–Zn2SnO4 hollow octahedra for diabetic diagnosis. J. Alloys Compd. 2021, 886, 161284. [Google Scholar] [CrossRef]
- Panes-Ruiz, L.A.; Riemenschneider, L.; Al Chawa, M.M.; Löffler, M.; Rellinghaus, B.; Tetzlaff, R.; Bezugly, V.; Ibarlucea, B.; Cuniberti, G. Selective and self-validating breath-level detection of hydrogen sulfide in humid air by gold nanoparticle-functionalized nanotube arrays. Nano Res. 2021. [Google Scholar] [CrossRef]
- Choi, S.J.; Jang, B.H.; Lee, S.J.; Min, B.K.; Rothschild, A.; Kim, I.D. Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C.; Liu, A.; Shi, G. Electrosynthesis of graphene oxide/polypyrene composite films and their applications for sensing organic vapors. J. Mater. Chem. 2012, 22, 8438–8443. [Google Scholar] [CrossRef]
- Lee, J.S.; Jeong, D.W.; Byun, Y.T. Porphyrin nanofiber/single-walled carbon nanotube nanocomposite-based sensors for monitoring hydrogen peroxide vapor. Sens. Actuators B Chem. 2020, 306, 127518. [Google Scholar] [CrossRef]
- Bachhav, S.G.; Patil, D.R. Study of Polypyrrole-Coated MWCNT Nanocomposites for Ammonia Sensing at Room Temperature. J. Mater. Sci. Chem. Eng. 2015, 3, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Binson, V.A.; Subramoniam, M. Design and development of an e-nose system for the diagnosis of pulmonary diseases. Acta Bioeng. Biomech. 2021, 23, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Binson, V.A.; Subramoniam, M.; Mathew, L. Discrimination of COPD and lung cancer from controls through breath analysis using a self-developed e-nose. J. Breath Res. 2021, 15, 46003. [Google Scholar] [CrossRef]
- Marzorati, D.; Mainardi, L.; Sedda, G.; Gasparri, R.; Spaggiari, L.; Cerveri, P. MOS sensors array for the discrimination of lung cancer and at-risk subjects with exhaled breath analysis. Chemosensors 2021, 9, 209. [Google Scholar] [CrossRef]
- Chang, J.; Lee, D.; Ban, S.; Oh, J.; Youn, M.; Kim, S.; Park, S.; Persaud, K.; Jheon, S. Chemical Analysis of volatile organic compounds in exhaled breath for lung cancer diagnosis using a sensor system. Sens. Actuators B. Chem. 2018, 255, 800–807. [Google Scholar] [CrossRef]
- Brinkman, P.; van de Pol, M.A.; Gerritsen, M.G.; Bos, L.D.; Dekker, T.; Smids, B.S.; Sinha, A.; Majoor, C.J.; Sneeboer, M.M.; Knobel, H.H.; et al. Exhaled breath profiles in the monitoring of loss of control and clinical recovery in asthma. Clin. Exp. Allergy 2017, 47, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.; Brinkman, P.; Van Der Schee, M.P.; Fens, N.; Dijkers, E.; Bootsma, S.K.; De Jongh, F.H.C.; Sterk, P.J. Integration of electronic nose technology with spirometry: Validation of a new approach for exhaled breath analysis. J. Breath Res. 2015, 9, 046001. [Google Scholar] [CrossRef]
- Krauss, E.; Haberer, J.; Barreto, G.; Degen, M.; Seeger, W.; Guenther, A. Recognition of breathprints of lung cancer and chronic obstructive pulmonary disease using the Aeonose® electronic nose. J. Breath Res. 2020, 14, 046004. [Google Scholar] [CrossRef]
- Wang, X.R.; Lizier, J.T.; Berna, A.Z.; Bravo, F.G.; Trowell, S.C. Human breath-print identification by E-nose, using information-theoretic feature selection prior to classification. Sens. Actuators B Chem. 2015, 217, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, P.; Mudhalwadkar, R.P. Pellet sensor based asthma detection system using exhaled breath analysis. In Proceedings of the 2015 International Conference on Industrial Instrumentation and Control (ICIC), Pune, India, 28–30 May 2015; pp. 139–142. [Google Scholar] [CrossRef]
- Xu, Y.; Lee, H.; Hu, Y.; Huang, J.; Kim, S.; Yun, M. Detection and identification of breast cancer volatile organic compounds biomarkers using highly-sensitive single nanowire array on a chip. J. Biomed. Nanotechnol. 2013, 9, 1164–1172. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; Ku, K.H.; Kim, B.J.; Kim, I.D. Novel Templating Route Using Pt Infiltrated Block Copolymer Microparticles for Catalytic Pt Functionalized Macroporous WO3 Nanofibers and Its Application in Breath Pattern Recognition. ACS Sens. 2016, 1, 1124–1131. [Google Scholar] [CrossRef]
- Hong, D.; Hoon, T.; Sohn, W.; Min, J.; Shim, Y. Chemical Au decoration of vertical hematite nanotube arrays for further selective detection of acetone in exhaled breath. Sens. Actuators B. Chem. 2018, 274, 587–594. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, I.; Jang, B.H.; Youn, D.Y.; Ryu, W.H.; Park, C.O.; Kim, I.D. Selective diagnosis of diabetes using Pt-functionalized WO3 hemitube networks as a sensing layer of acetone in exhaled breath. Anal. Chem. 2013, 85, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.S.; Park, Y.; Shin, J.; Song, Y.G.; Kang, C.Y. Metal oxide nanorods-based sensor array for selective detection of biomarker gases. Sensors 2021, 21, 1922. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.G.; Jung, Y.; Han, S.D.; Shim, Y.S.; Shin, B.; Lee, T.; Kim, J.S.; Lee, S.; Jun, S.C.; Park, H.H.; et al. Chemiresistive Electronic Nose toward Detection of Biomarkers in Exhaled Breath. ACS Appl. Mater. Interfaces 2016, 8, 20969–20976. [Google Scholar] [CrossRef] [PubMed]
- Carlos, S.; Santos, P.; Sayago, I. Graphene-Doped Tin Oxide Nanofibers and Nanoribbons as Gas Sensors to Detect Biomarkers of Different Diseases through the Breath. Sensors 2020, 20, 7223. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Kam, K.W.; Cheung, W.F.; Zhao, N.; Zheng, B. Functionalized graphene-based chemiresistive electronic nose for discrimination of disease-related volatile organic compounds. Biosens. Bioelectron. X 2019, 1, 100016. [Google Scholar] [CrossRef]
- Sarkar, T.; Srinives, S.; Rodriquez, A.; Mulchandani, A. Single-walled Carbon Nanotube-Calixarene Based Chemiresistor for Volatile Organic Compounds. Electroanalysis 2018, 30, 2077–2084. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Winkler, M.; Loghin, F.; Zeiser, C.; Lugli, P.; Abdellah, A. Highly sensitive and selective carbon nanotube-based gas sensor arrays functionalized with different metallic nanoparticles. Sens. Actuators B Chem. 2015, 220, 1288–1296. [Google Scholar] [CrossRef]
- Gao, Z.; Lou, Z.; Chen, S.; Li, L.; Jiang, K.; Fu, Z.; Han, W.; Shen, G. Fiber gas sensor-integrated smart face mask for room-temperature distinguishing of target gases. Nano Res. 2018, 11, 511–519. [Google Scholar] [CrossRef]
- Gautam, V.; Kumar, A.; Kumar, R.; Jain, V.K.; Nagpal, S. Silicon nanowires/reduced graphene oxide nanocomposite based novel sensor platform for detection of cyclohexane and formaldehyde. Mater. Sci. Semicond. Process. 2021, 123, 105571. [Google Scholar] [CrossRef]
- Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Drera, G.; Pagliara, S.; Kopylova, D.S.; Chiesa, M.; Santini, G.; Mores, N.; Moscato, U.; et al. Development of a Sensing Array for Human Breath Analysis Based on SWCNT Layers Functionalized with Semiconductor Organic Molecules. Adv. Healthc. Mater. 2020, 9, 2000377. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Yong, Z.X.; Liam, C.K. Using a chemiresistor-based alkane sensor to distinguish exhaled breaths of lung cancer patients from subjects with no lung cancer. J. Thorac. Dis. 2016, 8, 2772–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welearegay, T.G.; Diouani, M.F.; Österlund, L.; Ionescu, F.; Belgacem, K.; Smadhi, H.; Khaled, S.; Kidar, A.; Cindemir, U.; Laouini, D.; et al. Ligand-Capped Ultrapure Metal Nanoparticle Sensors for the Detection of Cutaneous Leishmaniasis Disease in Exhaled Breath. ACS Sens. 2018, 3, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Kumar, B.; Feller, J.F.; Haddi, Z.; Amari, A.; Bouchikhi, B. Chemical Novel e-nose for the discrimination of volatile organic biomarkers with an array of carbon nanotubes (CNT) conductive polymer nanocomposites (CPC) sensors. Sens. Actuators B. Chem. 2011, 159, 213–219. [Google Scholar] [CrossRef]
- Karban, A.; Nakhleh, M.K.; Cancilla, J.C.; Vishinkin, R.; Rainis, T.; Koifman, E.; Jeries, R.; Ivgi, H.; Torrecilla, J.S.; Haick, H. Programmed Nanoparticles for Tailoring the Detection of Inflammatory Bowel Diseases and Irritable Bowel Syndrome Disease via Breathprint. Adv. Healthc. Mater. 2016, 5, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Khatib, S.; Gharra, A.; Krilaviciute, A.; Amal, H.; Polaka, I.; Parshutin, S.; Kikuste, I.; Gasenko, E.; Skapars, R.; et al. Screening for gastric cancer using exhaled breath samples. Br. J. Surg. 2019, 106, 1122–1125. [Google Scholar] [CrossRef]
- Marom, O.; Nakhoul, F.; Tisch, U.; Shiban, A.; Abassi, Z.; Haick, H. Gold nanoparticle sensors for detecting chronic kidney disease and disease progression. Nanomedicine 2012, 7, 639–650. [Google Scholar] [CrossRef]
- Tisch, U.; Schlesinger, I.; Ionescu, R.; Nassar, M.; Axelrod, N.; Robertman, D.; Tessler, Y.; Azar, F.; Marmur, A.; Aharon-Peretz, J.; et al. Detection of Alzheimer’s and Parkinson’s disease from exhaled breath using nanomaterial-based sensors. Nanomedicine 2013, 8, 43–56. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Shuster, G.; Gallimidi, Z.; Reiss, A.H.; Dovgolevsky, E.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Engel, A.; Shiban, A.; Tisch, U.; et al. Classification of breast cancer precursors through exhaled breath. Breast Cancer Res. Treat. 2011, 126, 791–796. [Google Scholar] [CrossRef]
- Licht, J.C.; Grasemann, H. Potential of the electronic nose for the detection of respiratory diseases with and without infection. Int. J. Mol. Sci. 2020, 21, 9416. [Google Scholar] [CrossRef]
- Bikov, A.; Lázár, Z.; Horvath, I. Established methodological issues in electronic nose research: How far are we from using these instruments in clinical settings of breath analysis? J. Breath Res. 2015, 9, 034001. [Google Scholar] [CrossRef]
- Tenero, L.; Sandri, M.; Piazza, M.; Paiola, G.; Zaffanello, M.; Piacentini, G. Electronic nose in discrimination of children with uncontrolled asthma. J. Breath Res. 2020, 14, 046003. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; De Nijs, S.B.; Peters, S.; Dekker, T.; Knobel, H.H.; Vink, T.J.; Willard, N.P.; Zwinderman, A.H.; Krouwels, F.H.; Lutter, R.; et al. Exhaled air molecular profiling in relation to inflammatory subtype and activity in COPD. Eur. Respir. J. 2011, 38, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- De León-Martínez, L.D.; Rodríguez-Aguilar, M.; Gorocica-Rosete, P.; Domínguez-Reyes, C.A.; Martínez-Bustos, V.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Cruz-Ramos, J.A.; Balderas-Segura, B.; Flores-Ramírez, R. Identification of profiles of volatile organic compounds in exhaled breath by means of an electronic nose as a proposal for a screening method for breast cancer: A case-control study. J. Breath Res. 2020, 14, 046009. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, W.C.; Yang, H.Y. Diagnosis of ventilator-associated pneumonia using electronic nose sensor array signals: Solutions to improve the application of machine learning in respiratory research. Respir. Res. 2020, 21, 45. [Google Scholar] [CrossRef]
- Skotadis, E.; Mousadakos, D.; Katsabrokou, K.; Stathopoulos, S.; Tsoukalas, D. Flexible polyimide chemical sensors using platinum nanoparticles. Sens. Actuators B Chem. 2013, 189, 106–112. [Google Scholar] [CrossRef]
- Skotadis, E.; Kanaris, A.; Aslanidis, E.; Kalatzis, N.; Chatzipapadopoulos, F.; Marianos, N.; Tsoukalas, D. Identification of two commercial pesticides by a nanoparticle gas-sensing array. Sensors 2021, 21, 5803. [Google Scholar] [CrossRef] [PubMed]
- Shehada, N.; Brönstrup, G.; Funka, K.; Christiansen, S.; Leja, M.; Haick, H. Ultrasensitive silicon nanowire for real-world gas sensing: Noninvasive diagnosis of cancer from breath volatolome. Nano Lett. 2015, 15, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, J.; Trefz, P.; Wex, K.; Sabel, B.; Schubert, J.K.; Miekisch, W. Electrochemical sensor system for breath analysis of aldehydes, CO and NO. J. Breath Res. 2015, 9, 016008. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Wang, X.; Xu, Y. Exhaled Breath Analysis with a Colorimetric Sensor Array for the Identification and Characterization of Lung Cancer. J. Thorac. Oncol. 2012, 7, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.I.B.; Freitas, A.C.; Rocha-Santos, T.A.P.; Pereira, M.E.; Duarte, A.C. Breath analysis by optical fiber sensor for the determination of exhaled organic compounds with a view to diagnostics. Talanta 2011, 83, 1586–1594. [Google Scholar] [CrossRef]
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A study of an electronic nose for detection of lung cancer based on a virtual SAW gas sensors array and imaging recognition method. Meas. Sci. Technol. 2005, 16, 1535–1546. [Google Scholar] [CrossRef]
- Incalzi, R.A.; Pennazza, G.; Scarlata, S.; Santonico, M.; Petriaggi, M.; Chiurco, D.; Pedone, C.; D’Amico, A. Reproducibility and Respiratory Function Correlates of Exhaled Breath Fingerprint in Chronic Obstructive Pulmonary Disease. PLoS ONE 2012, 7, e45396. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Santonico, M.; Mondino, C. Diagnostic Performance of an Electronic Nose, Fractional Exhaled Nitric Oxide, and Lung Function Testing in Asthma. Chest 2010, 137, 790–796. [Google Scholar] [CrossRef]
- Santonico, M.; Lucantoni, G.; Pennazza, G.; Capuano, R.; Galluccio, G.; Roscioni, C.; La Delfa, G.; Consoli, D.; Martinelli, E.; Paolesse, R.; et al. In situ detection of lung cancer volatile fingerprints using bronchoscopic air-sampling. Lung Cancer 2012, 77, 46–50. [Google Scholar] [CrossRef]
- Gasparri, R.; Santonico, M.; Valentini, C.; Sedda, G.; Borri, A.; Petrella, F.; Maisonneuve, P.; Pennazza, G.; D’Amico, A.; Di Natale, C.; et al. Volatile signature for the early diagnosis of lung cancer. J. Breath Res. 2016, 10, 016007. [Google Scholar] [CrossRef] [PubMed]
- Zetola, N.M.; Modongo, C.; Matsiri, O.; Tamuhla, T.; Mbongwe, B.; Matlhagela, K.; Sepako, E.; Catini, A.; Sirugo, G.; Martinelli, E.; et al. Diagnosis of pulmonary tuberculosis and assessment of treatment response through analyses of volatile compound patterns in exhaled breath samples. J. Infect. 2017, 74, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennazza, G.; Marchetti, E.; Santonico, M.; Mantini, G.; Mummolo, S.; Marzo, G.; Paolesse, R.; D’Amico, A.; Di Natale, C. Application of a quartz microbalance based gas sensor array for the study of halitosis. J. Breath Res. 2008, 2, 017009. [Google Scholar] [CrossRef]
- Fasola, S.; Ferrante, G.; Sabatini, A.; Santonico, M.; Zompanti, A.; Grasso, S.; Antonelli Incalzi, R.; La Grutta, S. Repeatability of exhaled breath fingerprint collected by a modern sampling system in asthmatic and healthy children. J. Breath Res. 2019, 13, 036007. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, G.; Liu, H.; Fu, H.; Fan, J.; Wang, K.; Chen, Y.; Li, B.; Zhang, C.; Zhi, X.; et al. Identification of volatile biomarkers of gastric cancer cells and ultrasensitive electrochemical detection based on sensing interface of Au-Ag alloy coated MWCNTs. Theranostics 2014, 4, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Bahos, F.A.; Sainz-Vidal, A.; Sánchez-Pérez, C.; Saniger, J.M.; Gràcia, I.; Saniger-Alba, M.M.; Matatagui, D. ZIF nanocrystal-based surface acousticwave (SAW) electronic nose to detect diabetes in human breath. Biosensors 2019, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Lin, P.; Ai, Y.; Wang, H.; Ke, S.; Zeng, X. Multichannel quartz crystal microbalance array: Fabrication, evaluation, application in biomarker detection. Anal. Biochem. 2016, 494, 85–92. [Google Scholar] [CrossRef]
- Zhao, S.; Lei, J.; Huo, D.; Hou, C.; Luo, X.; Wu, H.; Fa, H.; Yang, M. Chemical A colorimetric detector for lung cancer related volatile organic compounds based on cross-response mechanism. Sens. Actuators B. Chem. 2018, 256, 543–552. [Google Scholar] [CrossRef]
- Huo, D.; Xu, Y.; Hou, C.; Yang, M.; Fa, H. A novel optical chemical sensor based AuNR-MTPP and dyes for lung cancer biomarkers in exhaled breath identification. Sens. Actuators B Chem. 2014, 199, 446–456. [Google Scholar] [CrossRef]
- Feng, P.; Shao, F.; Shi, Y.; Wan, Q. Gas sensors based on semiconducting nanowire field-effect transistors. Sensors 2014, 14, 17406. [Google Scholar] [CrossRef] [Green Version]
- Fraden, J. Chemical sensors. In Handbook of Modern Sensors: Physics, Designs, and Applications, 4th ed.; Springer: New York, NY, USA, 2010; pp. 569–606. [Google Scholar]
- Paska, Y.; Stelzner, T.; Assad, O.; Tisch, U.; Christiansen, S.; Haick, H. Molecular gating of silicon nanowire field-effect transistors with nonpolar analytes. ACS Nano 2012, 6, 335–345. [Google Scholar] [CrossRef]
- Chen, G.; Paronyan, T.M.; Pigos, E.M.; Harutyunyan, A.R. Enhanced gas sensing in pristine carbon nanotubes under continuous ultraviolet light illumination. Sci. Rep. 2012, 2, 343. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, J.; Ye, J.; Tange, M.; Zhang, X.; Xu, W.; Zhang, K.; Okazaki, T.; Cui, Z. Printed thin-film transistors and NO2 gas sensors based on sorted semiconducting carbon nanotubes by isoindigo-based copolymer. Carbon N. Y. 2016, 108, 372–380. [Google Scholar] [CrossRef]
- Chang, Y.W.; Oh, J.S.; Yoo, S.H.; Choi, H.H.; Yoo, K.H. Electrically refreshable carbon-nanotube-based gas sensors. Nanotechnology 2007, 18, 435504. [Google Scholar] [CrossRef]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.S.; Xia, J.B.; Li, J. Photoresponsive and Gas Sensing Field-Effect Transistors based on Multilayer WS2 Nanoflakes. Sci. Rep. 2014, 4, 5209. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Huang, Y.K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing behavior of atomically thin-layered MoS2 transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, H.; Li, X.; Lu, C.; Ding, Y.; Lu, B. Fabrication of a graphene field effect transistor array on microchannels for ethanol sensing. Appl. Surf. Sci. 2012, 258, 1971–1975. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Mattson, E.C.; Wen, Z.; Chang, J.; Hou, Y.; Hirschmugl, C.J.; Chen, J. Ultrasensitive chemical sensing through facile tuning defects and functional groups in reduced graphene oxide. Anal. Chem. 2014, 86, 7516–7522. [Google Scholar] [CrossRef]
- Mortazavi Zanjani, S.M.; Sadeghi, M.M.; Holt, M.; Chowdhury, S.F.; Tao, L.; Akinwande, D. Enhanced sensitivity of graphene ammonia gas sensors using molecular doping. Appl. Phys. Lett. 2016, 108, 033106. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.H.; Park, S.G.; Kwon, J.D.; Lee, Y.J.; Lee, K.H.; Lee, B.H.; et al. Chemical Sensing of 2D Graphene/MoS2 Heterostructure device. ACS Appl. Mater. Interfaces 2015, 7, 16775–16780. [Google Scholar] [CrossRef]
- Van Hieu, N.; Dung, N.Q.; Tam, P.D.; Trung, T.; Chien, N.D. Thin film polypyrrole/SWCNTs nanocomposites-based NH3 sensor operated at room temperature. Sens. Actuators B Chem. 2009, 140, 500–507. [Google Scholar] [CrossRef]
- Mubeen, S.; Lim, J.H.; Srirangarajan, A.; Mulchandani, A.; Deshusses, M.A.; Myung, N.V. Gas sensing mechanism of gold nanoparticles decorated single-walled carbon nanotubes. Electroanalysis 2011, 23, 2687–2692. [Google Scholar] [CrossRef]
- Kaiser, A.; Ceja, E.T.; Liu, Y.; Huber, F.; Müller, R.; Herr, U.; Thonke, K. H2S sensing for breath analysis with Au functionalized ZnO nanowires. Nanotechnology 2021, 32, 205505. [Google Scholar] [CrossRef]
- Niskanen, A.O.; Colli, A.; White, R.; Li, H.W.; Spigone, E.; Kivioja, J.M. Silicon nanowire arrays as learning chemical vapour classifiers. Nanotechnology 2011, 22, 295502. [Google Scholar] [CrossRef] [PubMed]
- Dattoli, E.N.; Davydov, A.V.; Benkstein, K.D. Tin oxide nanowire sensor with integrated temperature and gate control for multi-gas recognition. Nanoscale 2012, 4, 1760–1769. [Google Scholar] [CrossRef]
- Li, D.; Hu, J.; Wu, R.; Lu, J.G. Conductometric chemical sensor based on individual CuO nanowires. Nanotechnology 2010, 21, 485502. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, N.; Ben-Cohen, A.; Vaknin, Y.; Henning, A.; Hayon, J.; Shimanovich, K.; Greenspan, H.; Rosenwaks, Y. Electrostatic Selectivity of Volatile Organic Compounds Using Electrostatically Formed Nanowire Sensor. ACS Sens. 2018, 3, 709–715. [Google Scholar] [CrossRef]
- Chen, P.C.; Ishikawa, F.N.; Chang, H.K.; Ryu, K.; Zhou, C. A nanoelectronic nose: A hybrid nanowire/carbon nanotube sensor array with integrated micromachined hotplates for sensitive gas discrimination. Nanotechnology 2009, 20, 125503. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tisch, U.; Haick, H. Detection of nonpolar molecules by means of carrier scattering in random networks of carbon nanotubes: Toward diagnosis of diseases via breath samples. Nano Lett. 2009, 9, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Maier, D.; Laubender, E.; Basavanna, A.; Schumann, S.; Güder, F.; Urban, G.A.; Dincer, C. Toward Continuous Monitoring of Breath Biochemistry: A Paper-Based Wearable Sensor for Real-Time Hydrogen Peroxide Measurement in Simulated Breath. ACS Sens. 2019, 4, 2945–2951. [Google Scholar] [CrossRef]
- Chuang, M.Y.; Chen, C.C.; Zan, H.W.; Meng, H.F.; Lu, C.J. Organic Gas Sensor with an Improved Lifetime for Detecting Breath Ammonia in Hemodialysis Patients. ACS Sens. 2017, 2, 1788–1795. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, G.; Goledzinowski, M.; Comeau, F.J.E.; Li, K.; Cumberland, T.; Lenos, J.; Xu, P.; Li, M.; Yu, A.; et al. Green Solid Electrolyte with Cofunctionalized Nanocellulose/Graphene Oxide Interpenetrating Network for Electrochemical Gas Sensors. Small Methods 2017, 1, 1700237. [Google Scholar] [CrossRef]
- Queralto, N.; Berliner, A.N.; Goldsmith, B.; Martino, R.; Rhodes, P.; Lim, S.H. Detecting cancer by breath volatile organic compound analysis: A review of array-based sensors. J. Breath Res. 2014, 8, 027112. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Zhu, Z. Ammonia sensing characteristics of ZnO nanowires studied by quartz crystal microbalance. Appl. Surf. Sci. 2006, 252, 2404–2411. [Google Scholar] [CrossRef]
- Van Quy, N.; Minh, V.A.; Van Luan, N.; Hung, V.N.; Van Hieu, N. Gas sensing properties at room temperature of a quartz crystal microbalance coated with ZnO nanorods. Sens. Actuators B Chem. 2011, 153, 188–193. [Google Scholar] [CrossRef]
- Ding, B.; Kim, J.; Miyazaki, Y.; Shiratori, S. Electrospun nanofibrous membranes coated quartz crystal microbalance as gas sensor for NH3 detection. Sens. Actuators B Chem. 2004, 101, 373–380. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Sun, M.; Yu, J.; Sun, G. Nanofibrous polyethyleneimine membranes as sensitive coatings for quartz crystal microbalance-based formaldehyde sensors. Sens. Actuators B Chem. 2010, 144, 11–17. [Google Scholar] [CrossRef]
- Lukman Hekiem, N.L.; Md Ralib, A.A.; bt Mat Hattar, M.A.; Ahmad, F.B.; Nordin, A.N.; Rahim, R.A.; Za’bah, N.F. Advanced vapour sensing materials: Existing and latent to acoustic wave sensors for VOCs detection as the potential exhaled breath biomarkers for lung cancer. Sens. Actuators A Phys. 2021, 329, 112792. [Google Scholar] [CrossRef]
- Si, P.; Mortensen, J.; Komolov, A.; Denborg, J.; Møller, P.J. Polymer coated quartz crystal microbalance sensors for detection of volatile organic compounds in gas mixtures. Anal. Chim. Acta 2007, 597, 223–230. [Google Scholar] [CrossRef]
- Koshets, I.A.; Kazantseva, Z.I.; Shirshov, Y.M.; Cherenok, S.A.; Kalchenko, V.I. Calixarene films as sensitive coatings for QCM-based gas sensors. Sens. Actuators B Chem. 2005, 106, 177–181. [Google Scholar] [CrossRef]
- D’Amico, A.; Pennazza, G.; Santonico, M.; Martinelli, E.; Roscioni, C.; Galluccio, G.; Paolesse, R.; Di Natale, C. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer 2010, 68, 170–176. [Google Scholar] [CrossRef]
- Santonico, M.; Pennazza, G.; Grasso, S.; D’Amico, A.; Bizzarri, M. Design and test of a Biosensor-based multisensorial system: A proof of concept study. Sensors 2013, 13, 16625–16640. [Google Scholar] [CrossRef]
- Ippolito, S.J.; Ponzoni, A.; Kalantar-Zadeh, K.; Wlodarski, W.; Comini, E.; Faglia, G.; Sberveglieri, G. Layered WO3/ZnO/36° LiTaO3 SAW gas sensor sensitive towards ethanol vapour and humidity. Sens. Actuators B Chem. 2006, 117, 442–450. [Google Scholar] [CrossRef]
- Tang, Y.L.; Li, Z.J.; Ma, J.Y.; Guo, Y.J.; Fu, Y.Q.; Zu, X.T. Ammonia gas sensors based on ZnO/SiO2 bi-layer nanofilms on ST-cut quartz surface acoustic wave devices. Sens. Actuators B Chem. 2014, 201, 114–121. [Google Scholar] [CrossRef]
- Tang, Y.; Ao, D.; Li, W.; Zu, X.; Li, S.; Fu, Y.Q. NH3 sensing property and mechanisms of quartz surface acoustic wave sensors deposited with SiO2, TiO2, and SiO2-TiO2 composite films. Sens. Actuators B Chem. 2018, 254, 1165–1173. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Beatty, J.D.; Cataneo, R.N.; Huston, J.; Kaplan, P.D.; Lalisang, R.I.; Lambin, P.; Lobbes, M.B.I.; Mundada, M.; Pappas, N.; et al. Rapid point-of-care breath test for biomarkers of breast cancer and abnormal mammograms. PLoS ONE 2014, 9, e90226. [Google Scholar] [CrossRef]
- Phillips, M.; Basa-Dalay, V.; Blais, J.; Bothamley, G.; Chaturvedi, A.; Modi, K.D.; Pandya, M.; Natividad, M.P.R.; Patel, U.; Ramraje, N.N.; et al. Point-of-care breath test for biomarkers of active pulmonary tuberculosis. Tuberculosis 2012, 92, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Stahl, U.; Voigt, A.; Irschka, M.; Barié, N.; Richter, C.; Waldbaur, A.; Gruhl, F.J.; Rapp, B.E.; Rapp, M.; Länge, K. Long-term stability of polymer-coated surface transversewave sensors for the detection of organic solvent vapors. Sensors 2017, 17, 2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Yadava, R. Feature Extraction by Wavelet Decomposition of Surface. Def. Sci. J. 2010, 60, 377–386. [Google Scholar] [CrossRef]
- Penza, M.; Antolini, F.; Vittori-Antisari, M. Carbon nanotubes-based surface acoustic waves oscillating sensor for vapour detection. Thin Solid Films 2005, 472, 246–252. [Google Scholar] [CrossRef]
- Sayago, I.; Fernández, M.J.; Fontecha, J.L.; Horrillo, M.C.; Vera, C.; Obieta, I.; Bustero, I. New sensitive layers for surface acoustic wave gas sensors based on polymer and carbon nanotube composites. Sens. Actuators B Chem. 2012, 175, 67–72. [Google Scholar] [CrossRef]
- David, M.; Arab, M.; Martino, C.; Delmas, L.; Guinneton, F.; Gavarri, J.R. Carbon nanotubes/ceria composite layers deposited on surface acoustic wave devices for gas detection at room temperature. Thin Solid Films 2012, 520, 4786–4791. [Google Scholar] [CrossRef]
- Matatagui, D.; Kolokoltsev, O.; Saniger, J.M.; Gràcia, I.; Fernández, M.J.; Fontecha, J.L.; Horrillo, M.D.C. Acoustic sensors based on amino-functionalized nanoparticles to detect volatile organic solvents. Sensors 2017, 17, 2624. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Wu, Y.; Liu, X.; Fu, F.; Gong, Y.; Rao, Y.J.; Chen, Y. Miniature fiber-optic NH3 gas sensor based on Pt nanoparticle-incorporated graphene oxide. Sens. Actuators B Chem. 2017, 244, 107–113. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Manivannan, S. Uniform decoration of silver nanoparticle on exfoliated graphene oxide sheets and its ammonia gas detection. Ceram. Int. 2016, 42, 1769–1776. [Google Scholar] [CrossRef]
- Okuda, H.; Wang, T.; Lee, S.W. Selective Methanol Gas Detection Using a U-Bent Optical Fiber Modified with a Silica Nanoparticle Multilayer. Electron. Commun. Jpn. 2017, 100, 43–49. [Google Scholar] [CrossRef]
- Manjula, M.; Karthikeyan, B.; Sastikumar, D. Sensing characteristics of nanocrystalline bismuth oxide clad-modified fiber optic gas sensor. Opt. Lasers Eng. 2017, 95, 78–82. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, D.H.; Choi, S.J.; Koo, W.T.; Kim, I.D. Sub-Parts-per-Million Hydrogen Sulfide Colorimetric Sensor: Lead Acetate Anchored Nanofibers toward Halitosis Diagnosis. Anal. Chem. 2018, 90, 8769–8775. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, D.; Du, W.; Yan, M.; Wang, Y.; Huo, D.; Hou, C. Rapid recognition of volatile organic compounds with colorimetric sensor arrays for lung cancer screening. Anal. Bioanal. Chem. 2018, 410, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Hammel, J.; Dweik, R.; Na, J.; Czich, C.; Laskowski, D.; Mekhail, T. Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 2007, 62, 565–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.S.; Chen, Y.Q.; Lu, C.J. Organic vapour sensing using localized surface plasmon resonance spectrum of metallic nanoparticles self assemble monolayer. Talanta 2007, 73, 358–365. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Ge, L.; Hayashi, K. Localized surface plasmon resonance gas sensor of Au nano-islands coated with molecularly imprinted polymer: Influence of polymer thickness on sensitivity and selectivity. Sens. Actuators B Chem. 2016, 231, 787–792. [Google Scholar] [CrossRef]
- Cui, J.; Hu, K.; Sun, J.J.; Qu, L.L.; Li, D.W. SERS nanoprobes for the monitoring of endogenous nitric oxide in living cells. Biosens. Bioelectron. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- Qiao, X.; Su, B.; Liu, C.; Song, Q.; Luo, D.; Mo, G.; Wang, T. Selective Surface Enhanced Raman Scattering for Quantitative Detection of Lung Cancer Biomarkers in Superparticle@MOF Structure. Adv. Mater. 2018, 30, 1702275. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, W.; Wang, J.; Luo, D.; Qiao, X.; Qin, X.; Wang, T. Ultrasensitive Surface-Enhanced Raman Scattering Sensor of Gaseous Aldehydes as Biomarkers of Lung Cancer on Dendritic Ag Nanocrystals. Anal. Chem. 2017, 89, 1416–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target analysis of volatile organic compounds in exhaled breath for lung cancer discrimination from other pulmonary diseases and healthy persons. Metabolites 2020, 10, 317. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Díaz de León-Martínez, L.; Gorocica-Rosete, P.; Pérez-Padilla, R.; Domínguez-Reyes, C.A.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Mehta, G.; Zamora-Mendoza, B.N.; Flores-Ramírez, R. Application of chemoresistive gas sensors and chemometric analysis to differentiate the fingerprints of global volatile organic compounds from diseases. Preliminary results of COPD, lung cancer and breast cancer. Clin. Chim. Acta 2021, 518, 83–92. [Google Scholar] [CrossRef] [PubMed]
- van der Vliet, A.; Janssen-Heininger, Y.M.W.; Anathy, V. Oxidative stress in chronic lung disease: From mitochondrial dysfunction to dysregulated redox signaling. Mol. Aspects Med. 2018, 63, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Aguilar, M.; Ramírez-García, S.; Ilizaliturri-Hernández, C.; Gómez-Gómez, A.; Van-Brussel, E.; Díaz-Barriga, F.; Medellín-Garibay, S.; Flores-Ramírez, R. Ultrafast gas chromatography coupled to electronic nose to identify volatile biomarkers in exhaled breath from chronic obstructive pulmonary disease patients: A pilot study. Biomed. Chromatogr. 2019, 33, e4684. [Google Scholar] [CrossRef]
- Chen, X.; Muhammad, K.G.; Madeeha, C.; Fu, W.; Xu, L.; Hu, Y.; Liu, J.; Ying, K.; Chen, L.; Yurievna, G.O. Calculated indices of volatile organic compounds (VOCs) in exhalation for lung cancer screening and early detection. Lung Cancer 2021, 154, 197–205. [Google Scholar] [CrossRef]
- Schleich, F.N.; Zanella, D.; Stefanuto, P.; Bessonov, K.; Smolinska, A.; Dallinga, J.W.; Henket, M.; Paulus, V.; Guissard, F.; Graff, S.; et al. Exhaled Volatile Organic Compounds Are Able to Discriminate between Neutrophilic and Eosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 444–453. [Google Scholar] [CrossRef]
- Stefanuto, P.H.; Zanella, D.; Vercammen, J.; Henket, M.; Schleich, F.; Louis, R.; Focant, J.F. Multimodal combination of GC×GC-HRTOFMS and SIFT-MS for asthma phenotyping using exhaled breath. Sci. Rep. 2020, 10, 16159. [Google Scholar] [CrossRef]
- Moor, C.C.; Oppenheimer, J.C.; Nakshbandi, G.; Aerts, J.G.J.V.; Brinkman, P.; Maitland-Van Der Zee, A.H.; Wijsenbeek, M.S. Exhaled breath analysis by use of eNose technology: A novel diagnostic tool for interstitial lung disease. Eur. Respir. J. 2021, 57, 2002042. [Google Scholar] [CrossRef]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Lucas, M.Á.; Jareño-Esteban, J.; Gutiérrez-Ortega, C.; López-Guijarro, P.; Collado-Yurrita, L.; Quintana-Díaz, M.; Callol-Sánchez, L. Influence of Chronic Obstructive Pulmonary Disease on Volatile Organic Compounds in Patients with Non-Small Cell Lung Cancer. Arch. Bronconeumol. 2020, 56, 801–805. [Google Scholar] [CrossRef]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: Cross sectional and nested short-term follow-up study. Respir. Res. 2005, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koureas, M.; Kalompatsios, D.; Amoutzias, G.D.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Comparison of targeted and untargeted approaches in breath analysis for the discrimination of lung cancer from benign pulmonary diseases and healthy persons. Molecules 2021, 26, 2609. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, X.; Chen, X.; Hu, Y.; Ying, K.; Wang, P. Optimization of volatile markers of lung cancer to exclude interferences of non-malignant disease. Cancer Biomarkers 2014, 14, 371–379. [Google Scholar] [CrossRef]
- Fu, X.-A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181. [Google Scholar] [CrossRef]
- Ii, M.B.; Schumer, E.; Li, M.; Knipp, R.J.; Nantz, M.H.; Van Berkel, V.; Fu, X. Quantitative analysis of exhaled carbonyl compounds distinguishes benign from malignant pulmonary disease. J. Thorac. Cardiovasc. Surg. 2014, 148, 1074–1081. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yang, D.; Brock, G.; Knipp, R.J.; Bousamra, M.; Nantz, M.H.; Fu, X.A. Breath carbonyl compounds as biomarkers of lung cancer. Lung Cancer 2015, 90, 92–97. [Google Scholar] [CrossRef]
- Ibrahim, B.; Basanta, M.; Cadden, P.; Singh, D.; Douce, D.; Woodcock, A.; Fowler, S.J. Non-invasive phenotyping using exhaled volatile organic compounds in asthma. Thorax 2011, 66, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Basanta, M.; Ibrahim, B.; Dockry, R.; Douce, D.; Morris, M.; Singh, D.; Woodcock, A.; Fowler, S.J. Exhaled volatile organic compounds for phenotyping chronic obstructive pulmonary disease: A cross-sectional study. Respir. Res. 2012, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, D.; Smolinska, A.; Jöbsis, Q.; Rosias, P.P.R.; Muris, J.W.M.; Dallinga, J.W.; Van Schooten, F.J.; Dompeling, E. Association between exhaled inflammatory markers and asthma control in children. J. Breath Res. 2016, 10, 016014. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, D.; Smolinska, A.; Jöbsis, Q.; Rosias, P.; Muris, J.; Dallinga, J.; Dompeling, E.; Van Schooten, F.J. Can exhaled volatile organic compounds predict asthma exacerbations in children? J. Breath Res. 2017, 11, 016016. [Google Scholar] [CrossRef] [PubMed]
- Robroeks, C.M.; Van Berkel, J.J.; Jöbsis, Q.; Van Schooten, F.J.; Dallinga, J.W.; Wouters, E.F.; Dompeling, E. Exhaled volatile organic compounds predict exacerbations of childhood asthma in a 1-year prospective study. Eur. Respir. J. 2013, 42, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzini, A.; Filipiak, W.; Wille, J.; Ager, C.; Wiesenhofer, H.; Kubinec, R.; Blaško, J.; Tschurtschenthaler, C.; Mayhew, C.A.; Weiss, G.; et al. Analysis of volatile organic compounds in the breath of patients with stable or acute exacerbation of chronic obstructive pulmonary disease. J. Breath Res. 2018, 12, 036002. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; Annema, J.T.; Schot, R.; Van Der Schee, M.P.C.; Spanevello, A.; Carratú, P.; Resta, O.; Rabe, K.F.; Sterk, P.J. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 2009, 64, 166–170. [Google Scholar] [CrossRef]
- Tirzite, M.; Bukovskis, M.; Strazda, G.; Jurka, N.; Taivans, I. Detection of lung cancer in exhaled breath with an electronic nose using support vector machine analysis. J. Breath Res. 2017, 11, 036009. [Google Scholar] [CrossRef] [PubMed]
- Tirzïte, M.; Bukovskis, M.; Strazda, G.; Jurka, N.; Taivans, I. Detection of lung cancer with electronic nose and logistic regression analysis. J. Breath Res. 2019, 13, 016006. [Google Scholar] [CrossRef] [Green Version]
- Binson, V.A.; Subramoniam, M.; Sunny, Y.; Mathew, L. Prediction of Pulmonary Diseases with Electronic Nose Using SVM and XGBoost. IEEE Sens. J. 2021, 21, 20886–20895. [Google Scholar] [CrossRef]
- Chen, K.; Liu, L.; Nie, B.; Lu, B.; Fu, L.; He, Z.; Li, W.; Pi, X.; Liu, H. Recognizing lung cancer and stages using a self-developed electronic nose system. Comput. Biol. Med. 2021, 131, 104294. [Google Scholar] [CrossRef]
- Shlomi, D.; Abud, M.; Liran, O.; Bar, J.; Gai-Mor, N.; Ilouze, M.; Onn, A.; Ben-Nun, A.; Haick, H.; Peled, N. Detection of Lung Cancer and EGFR Mutation by Electronic Nose System. J. Thorac. Oncol. 2017, 12, 1544–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fens, N.; Zwinderman, A.H.; Van Der Schee, M.P.; De Nijs, S.B.; Dijkers, E.; Roldaan, A.C.; Cheung, D.; Bel, E.H.; Sterk, P.J. Exhaled Breath Profiling Enables Discrimination of Chronic Obstructive Pulmonary Disease and Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; Roldaan, A.C.; van der Schee, M.P.; Boksem, R.J.; Zwinderman, A.H.; Bel, E.H.; Sterk, P.J. External validation of exhaled breath profiling using an electronic nose in the discrimination of asthma with fixed airways obstruction and chronic obstructive pulmonary disease. Clin. Exp. Allergy 2011, 41, 1371–1378. [Google Scholar] [CrossRef]
- Dragonieri, S.; Scioscia, G.; Quaranta, V.N.; Carratu, P.; Venuti, M.P.; Falcone, M.; Carpagnano, G.E.; Foschino Barbaro, M.P.; Resta, O.; Lacedonia, D. Exhaled volatile organic compounds analysis by e-nose can detect idiopathic pulmonary fibrosis. J. Breath Res. 2020, 14, 047101. [Google Scholar] [CrossRef]
- Fielding, D.; Hartel, G.; Pass, D.; Davis, M.; Brown, M.; Dent, A.; Agnew, J.; Dickie, G.; Ware, R.S.; Hodge, R. Volatile organic compound breath testing detects in-situ squamous cell carcinoma of bronchial and laryngeal regions and shows distinct profiles of each tumour. J. Breath Res. 2020, 14, 046013. [Google Scholar] [CrossRef]
- Bach, J.P.; Gold, M.; Mengel, D.; Hattesohl, A.; Lubbe, D.; Schmid, S.; Tackenberg, B.; Rieke, J.; Maddula, S.; Baumbach, J.I.; et al. Measuring compounds in exhaled air to detect Alzheimer’s disease and Parkinson’s disease. PLoS ONE 2015, 10, e0132227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss, E.; Haberer, J.; Maurer, O.; Barreto, G.; Drakopanagiotakis, F.; Degen, M.; Seeger, W.; Guenther, A. Exploring the ability of electronic nose technology to recognize interstitial lung diseases (ILD) by non-invasive breath screening of exhaled volatile compounds (VOC): A pilot study from the european ipf registry (eurIPFreg) and biobank. J. Clin. Med. 2019, 8, 1698. [Google Scholar] [CrossRef]
- Bannier, M.A.G.E.; Van De Kant, K.D.G.; Jöbsis, Q.; Dompeling, E. Feasibility and diagnostic accuracy of an electronic nose in children with asthma and cystic fibrosis. J. Breath Res. 2019, 13, 36009. [Google Scholar] [CrossRef]
- van Hooren, M.R.A.; Leunis, N.; Brandsma, D.S.; Dingemans, A.M.C.; Kremer, B.; Kross, K.W. Differentiating head and neck carcinoma from lung carcinoma with an electronic nose: A proof of concept study. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3897–3903. [Google Scholar] [CrossRef]
- van de Goor, R.M.G.E.; Leunis, N.; van Hooren, M.R.A.; Francisca, E.; Masclee, A.; Kremer, B.; Kross, K.W. Feasibility of electronic nose technology for discriminating between head and neck, bladder, and colon carcinomas. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Barash, O.; Zhang, W.; Halpern, J.M.; Hua, Q.L.; Pan, Y.Y.; Kayal, H.; Khoury, K.; Liu, H.; Davies, M.P.A.; Haick, H. Differentiation between genetic mutations of breast cancer by breath volatolomics. Oncotarget 2015, 6, 44864–44876. [Google Scholar] [CrossRef] [Green Version]
- Amal, H.; Leja, M.; Funka, K.; Skapars, R.; Sivins, A.; Ancans, G.; Liepniece-karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016, 65, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.; Xiong, F.; Gu, K. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br. J. Cancer 2013, 108, 941–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, D.; Thangavel, K. Breathomics for gastric cancer classification using back-propagation neural network. J. Med. Signals Sens. 2016, 6, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Raspagliesi, F.; Bogani, G.; Benedetti, S.; Grassi, S.; Ferla, S.; Buratti, S. Detection of ovarian cancer through exhaled breath by electronic nose: A prospective study. Cancers 2020, 12, 2408. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.C.; Yu, J.B.; Lee, H.W.; Huh, J.S.; Lim, J.O. Investigation of exhaled breath samples from patients with Alzheimer’s disease using gas chromatography-mass spectrometry and an exhaled breath sensor system. Sensors 2017, 17, 1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennazza, G.; Santonico, M.; Vernile, C.; Antonelli Incalzi, R.; De Vincentis, A.; Picardi, A. Breathprinting of liver diseases. In Proceedings of the 2015 18th AISEM Annual Conference, Trento, Italy, 3–5 February 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Saidi, T.; Zaim, O.; Moufid, M.; El Bari, N.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B Chem. 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Saidi, T.; Moufid, M.; de Jesus Beleño-Saenz, K.; Welearegay, T.G.; El Bari, N.; Lisset Jaimes-Mogollon, A.; Ionescu, R.; Bourkadi, J.E.; Benamor, J.; El Ftouh, M.; et al. Non-invasive prediction of lung cancer histological types through exhaled breath analysis by UV-irradiated electronic nose and GC/QTOF/MS. Sens. Actuators B Chem. 2020, 311, 127932. [Google Scholar] [CrossRef]
- Plaza, V.; Crespo, A.; Giner, J.; Merino, J.L.; Ramos-Barbón, D.; Mateus, E.F.; Torrego, A.; Cosio, B.G.; Agustí, A.; Sibila, O. Inflammatory asthma phenotype discrimination using an electronic nose breath analyzer. J. Investig. Allergol. Clin. Immunol. 2015, 25, 431–437. [Google Scholar]
- Farraia, M.; Rufo, J.C.; Paciência, I.; Castro, F.; Ana, M.; Rama, T.; Rocha, S.M.; Delgado, L.; Brinkman, P.; Moreira, A. Human volatilome analysis using eNose to assess uncontrolled asthma in a clinical setting. Allergy 2020, 75, 1630–1639. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.S.; Tietje, O.; Wong, C. Prediction of breast cancer using volatile biomarkers in the breath. Breast Cancer Res. Treat. 2006, 99, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Tiele, A.; Daulton, E.; Ifeachor, E.; Eyre, V.; Clarke, S. Breath-based non-invasive diagnosis of Alzheimer ’ s disease : A pilot study Breath-based non-invasive diagnosis of Alzheimer’ s disease: A pilot study. J. Breath Res. 2020, 14, 026003. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Review-Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Sakumura, Y.; Koyama, Y.; Tokutake, H.; Hida, T.; Sato, K.; Itoh, T.; Akamatsu, T.; Shin, W. Diagnosis by volatile organic compounds in exhaled breath from lung cancer patients using support vector machine algorithm. Sensors 2017, 17, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
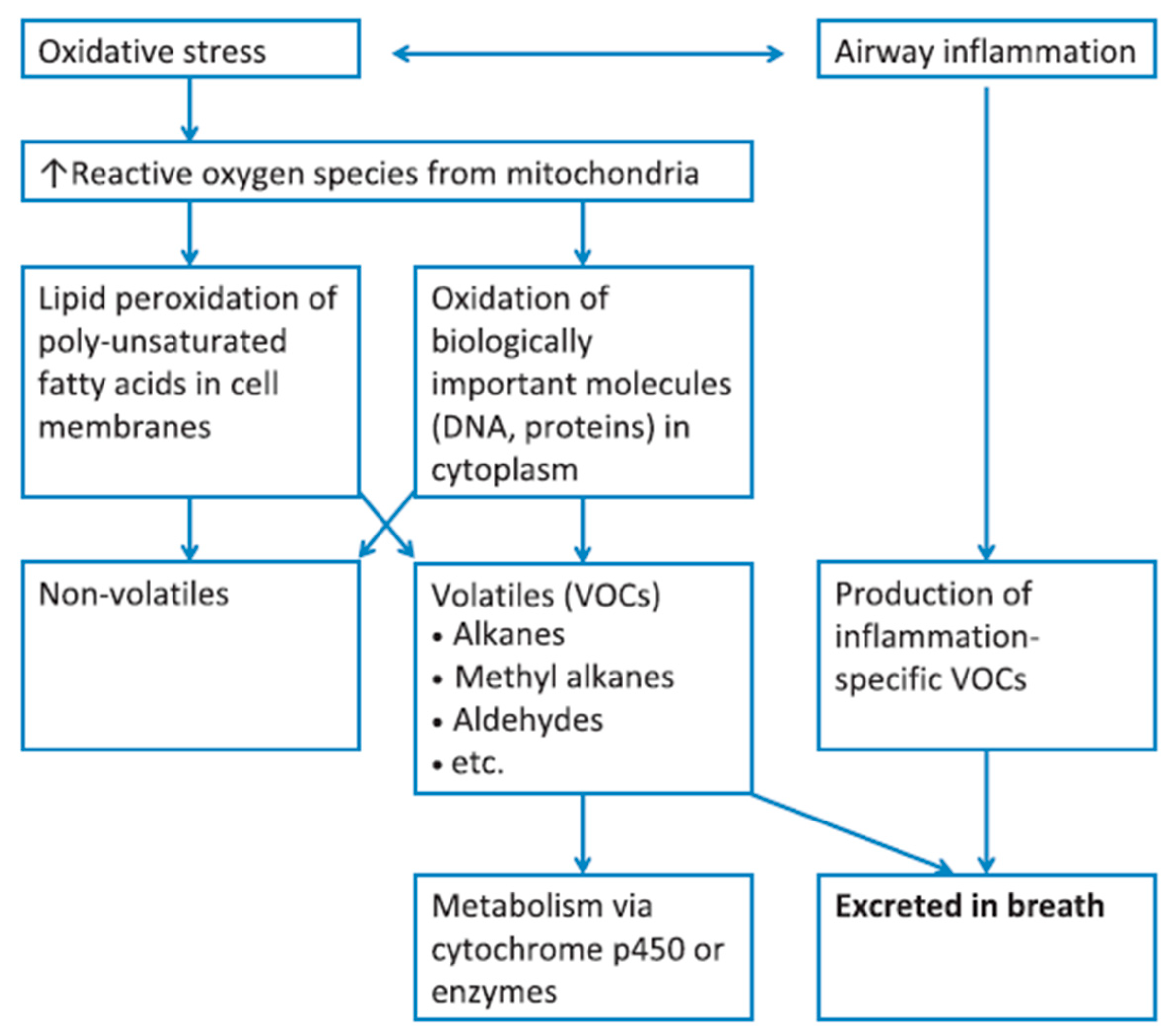
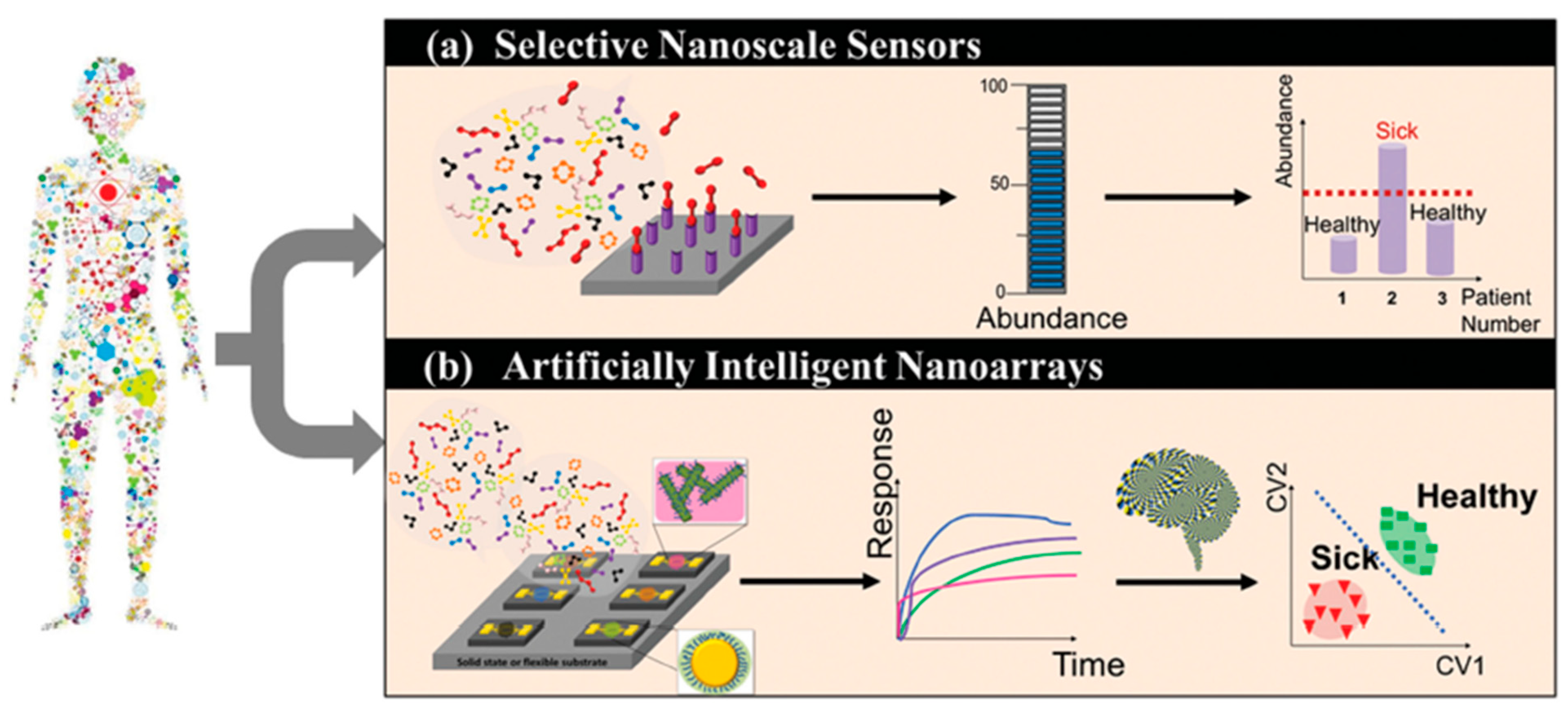
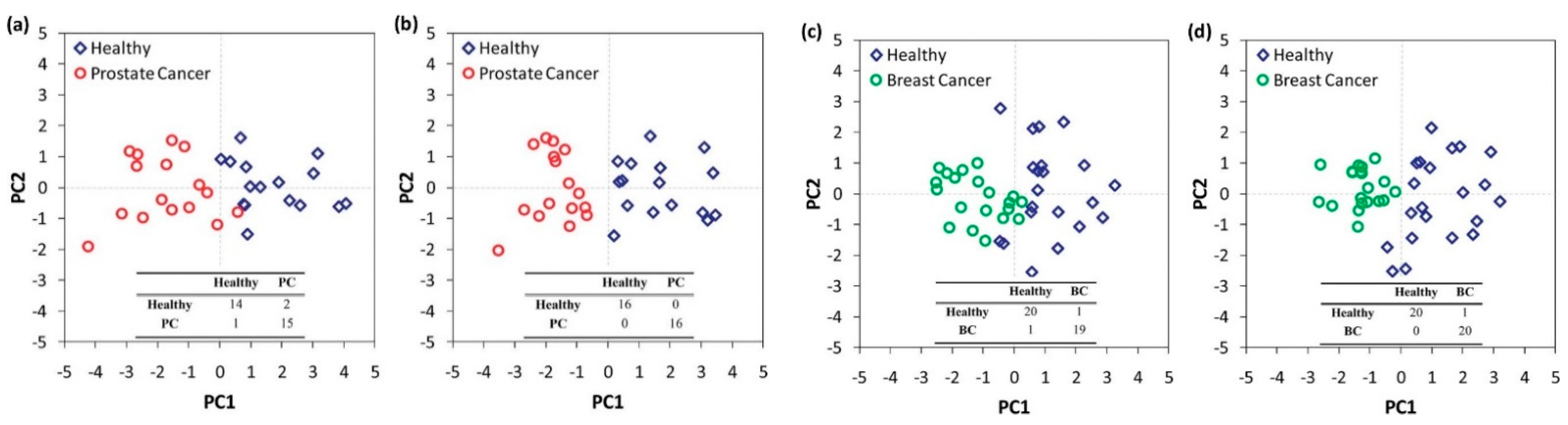
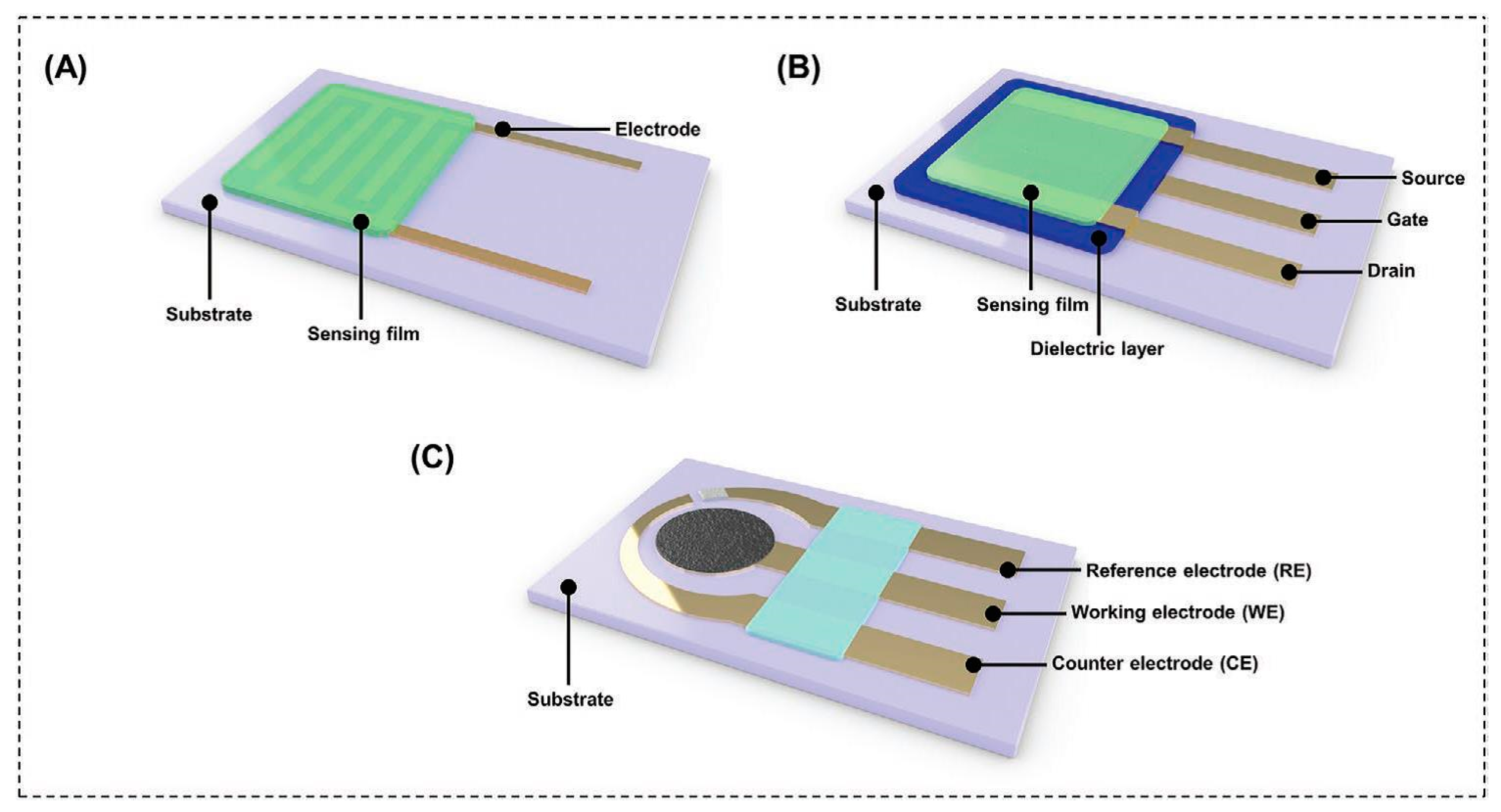





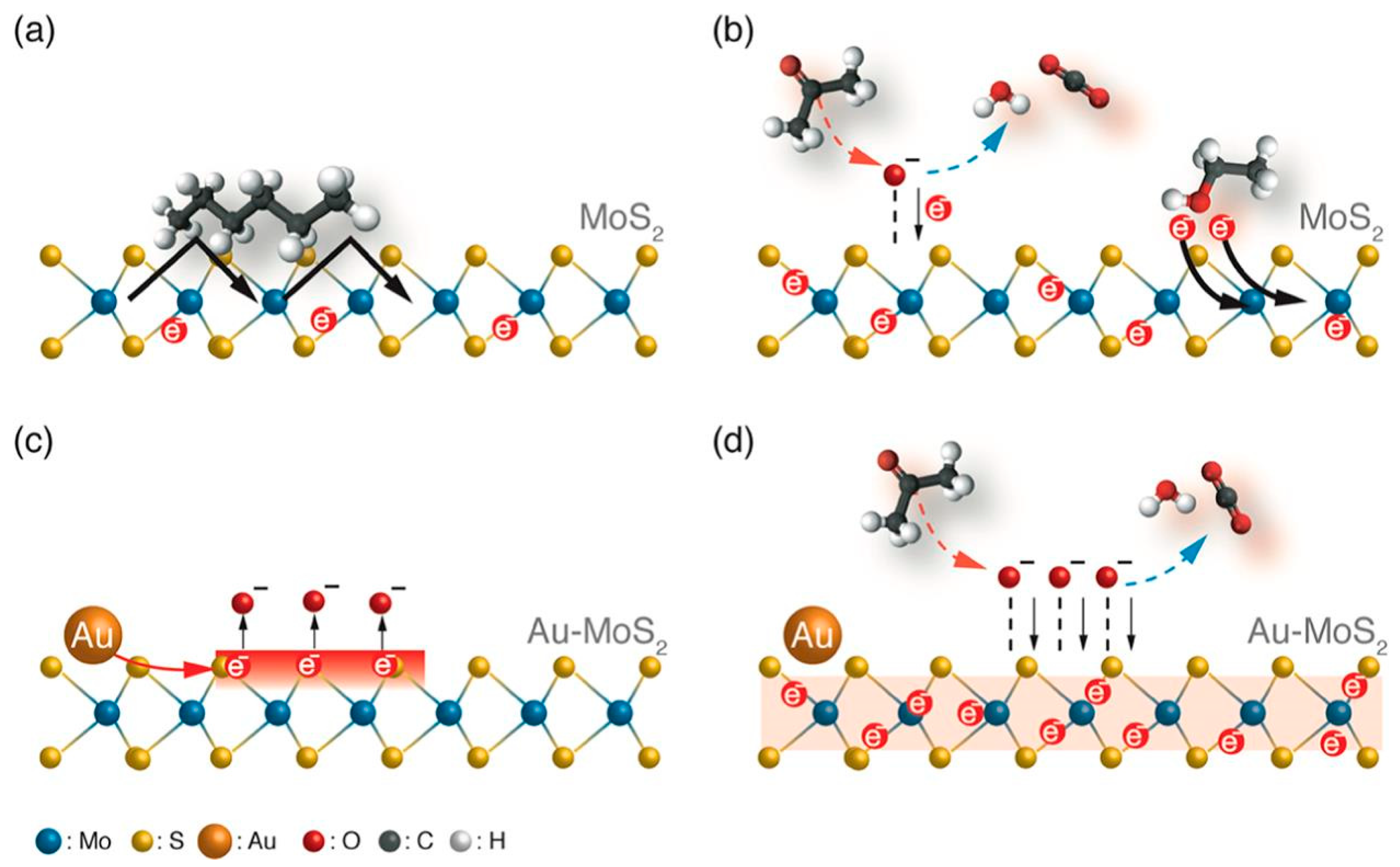

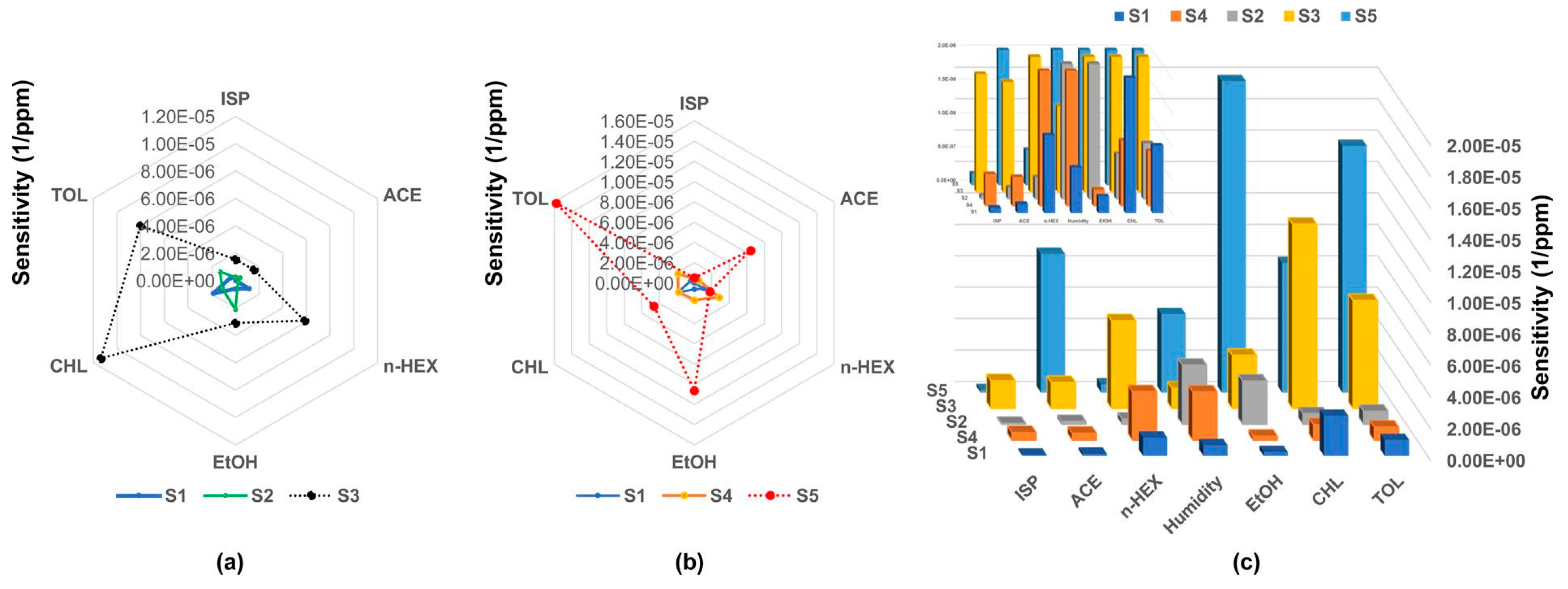
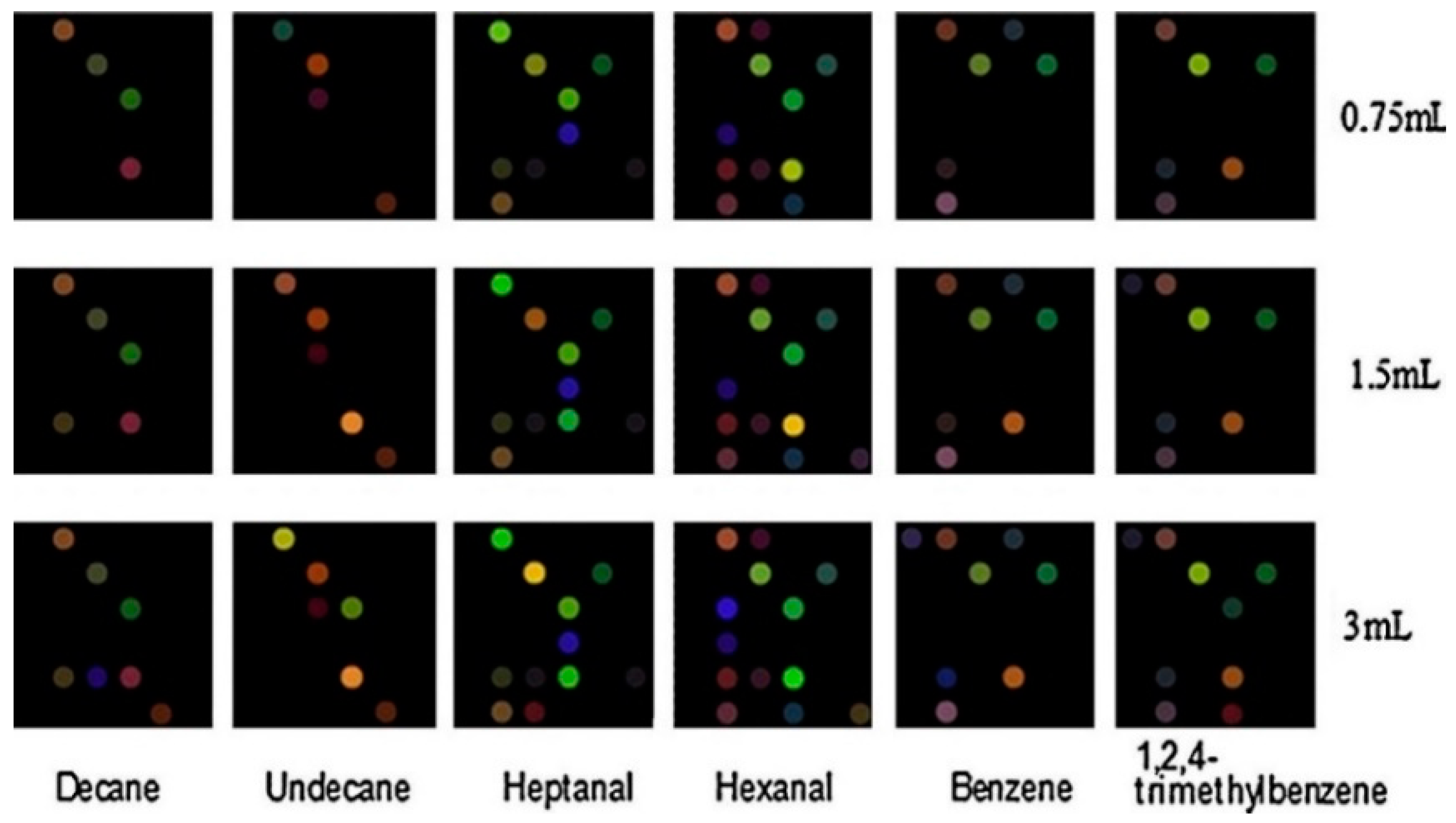
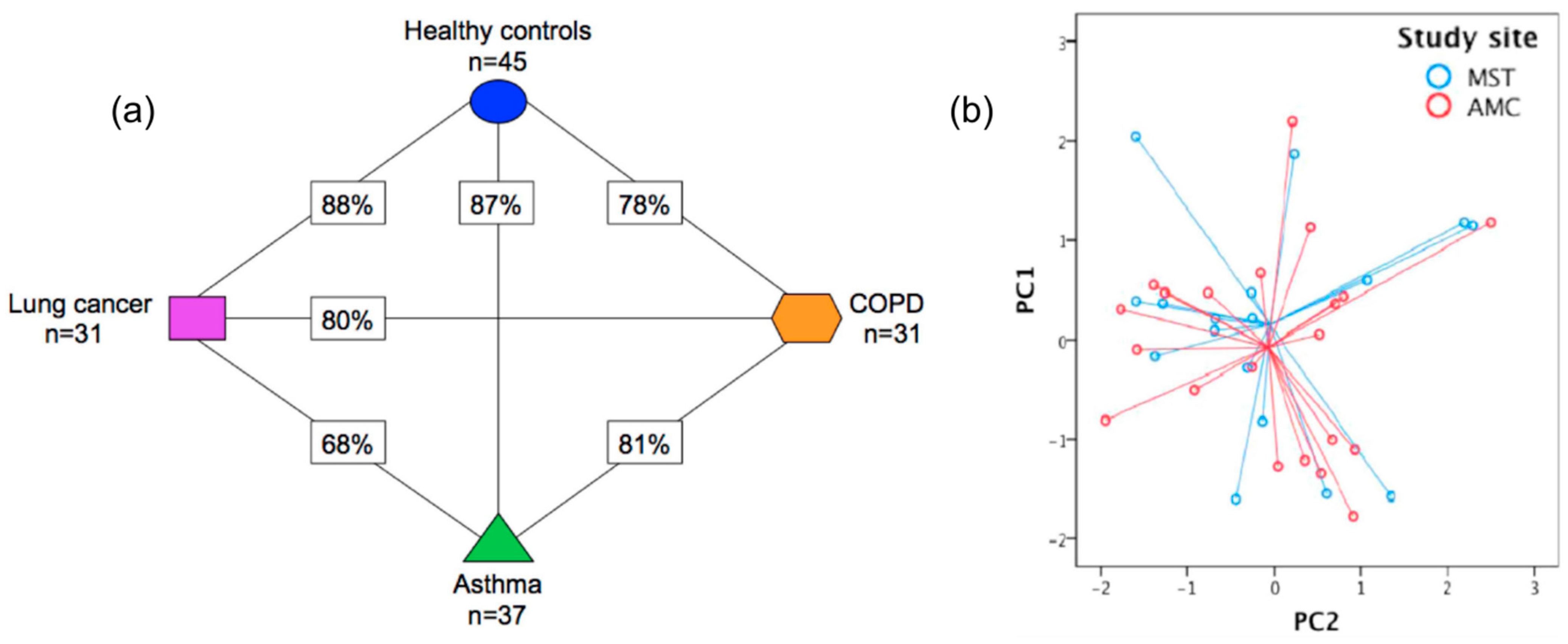

| Disease Type | Diseases | Ref. |
|---|---|---|
| Respiratory | Asthma, COPD, obstructive sleep apnea syndrome, pulmonary arterial hypertension, cystic fibrosis | [19] |
| Malignant | Lung, gastric, head and neck, breast, colon, prostate cancer | [15] |
| Neurodegenerative | Alzheimer’s disease, Parkinson’s diseases, multiple sclerosis | [15] |
| Metabolic | Diabetes, hyperglycemia | [12,31] |
| Bacterial infections | Upper respiratory tract infection, Mycobacterium tuberculosis, Pseudomonas, Helicobacter pylori infection | [32,22] |
| Viral infections | SARS-CoV-2 | [24,25,26] |
| Electronic Nose | Technology Used | Ref. |
|---|---|---|
| Cyranose 320 | 32 carbon black-polymer composite chemiresistors | [117,118] |
| Common Invent e-Nose | MOS sensors | [90,117] |
| SpiroNose | 5 sensor arrays, each composed of 4 MOS sensors | [91,117] |
| AeoNose | Micro hotplate MOS | [92,117] |
| DiagNose | 12 doped MOS | [93,118] |
| BIONOTE e-Nose | 8 QCM sensors using anthocyanin-coated gold electrodes | [117,136] |
| Tor Vergata e-Nose | QCM covered with metalloporphyrins | [117] |
| Owlstone Lonestar e-Nose | Field asymmetric ion mobility spectrometry | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 2022, 22, 1238. https://doi.org/10.3390/s22031238
Kaloumenou M, Skotadis E, Lagopati N, Efstathopoulos E, Tsoukalas D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors. 2022; 22(3):1238. https://doi.org/10.3390/s22031238
Chicago/Turabian StyleKaloumenou, Maria, Evangelos Skotadis, Nefeli Lagopati, Efstathios Efstathopoulos, and Dimitris Tsoukalas. 2022. "Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors" Sensors 22, no. 3: 1238. https://doi.org/10.3390/s22031238
APA StyleKaloumenou, M., Skotadis, E., Lagopati, N., Efstathopoulos, E., & Tsoukalas, D. (2022). Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors, 22(3), 1238. https://doi.org/10.3390/s22031238









