Progress on Infrared Imaging Technology in Animal Production: A Review
Abstract
:1. Introduction
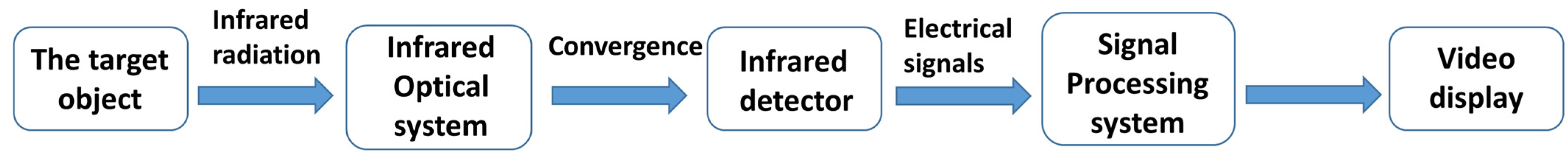
2. Overview of IRT Imaging Technology
2.1. Principles of IRT Imaging Technology
2.2. Parameters Affecting IRT Imaging
2.2.1. Emissivity
2.2.2. Reflected Temperature
2.2.3. Ambient Temperature, Humidity, and Measurement Distance
3. Applications of IRT Imaging Technology in Animal Production
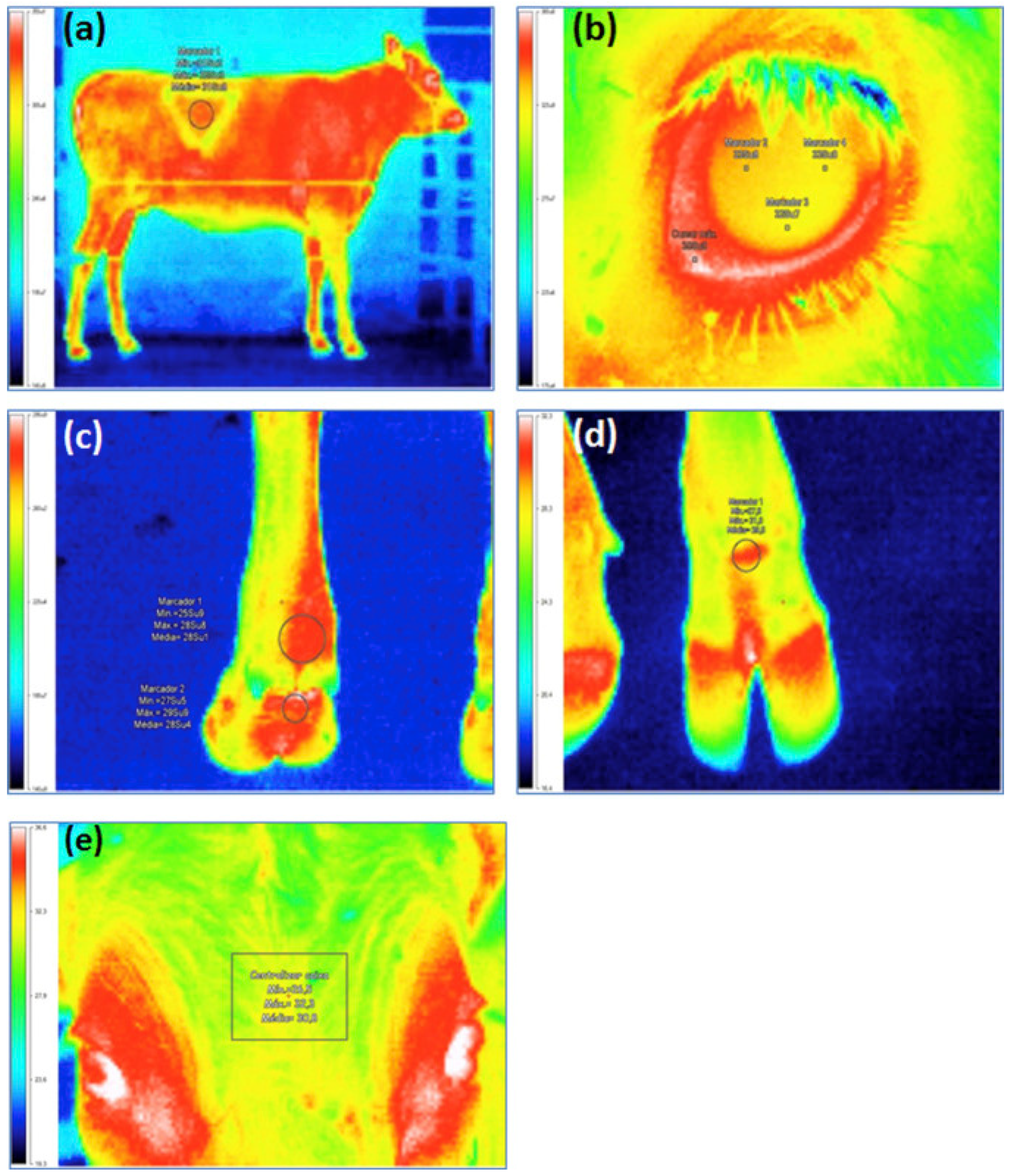
3.1. Detection of Animal Surfaces and Core Anatomical Areas’ Temperature Fluctuation
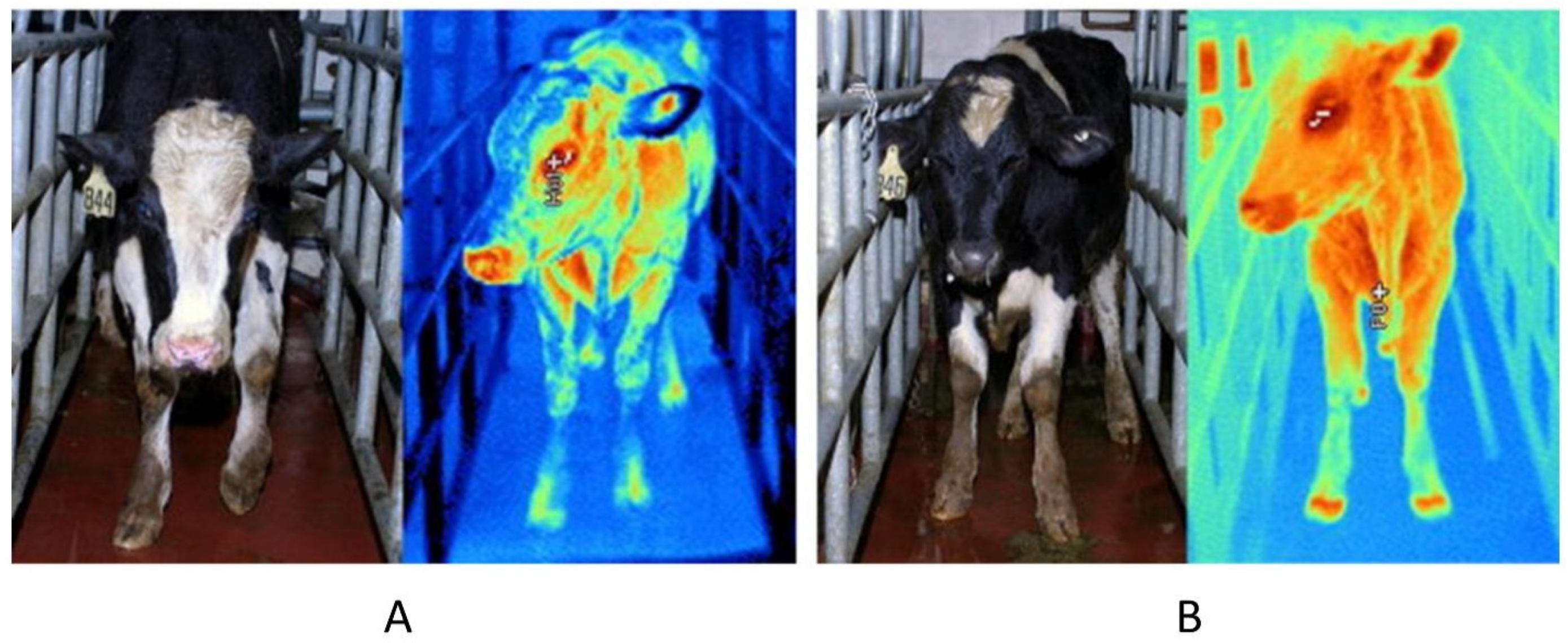
3.2. Early Diagnosis of Animal Diseases and Inflammation
3.3. Detection of Animal Stress Level
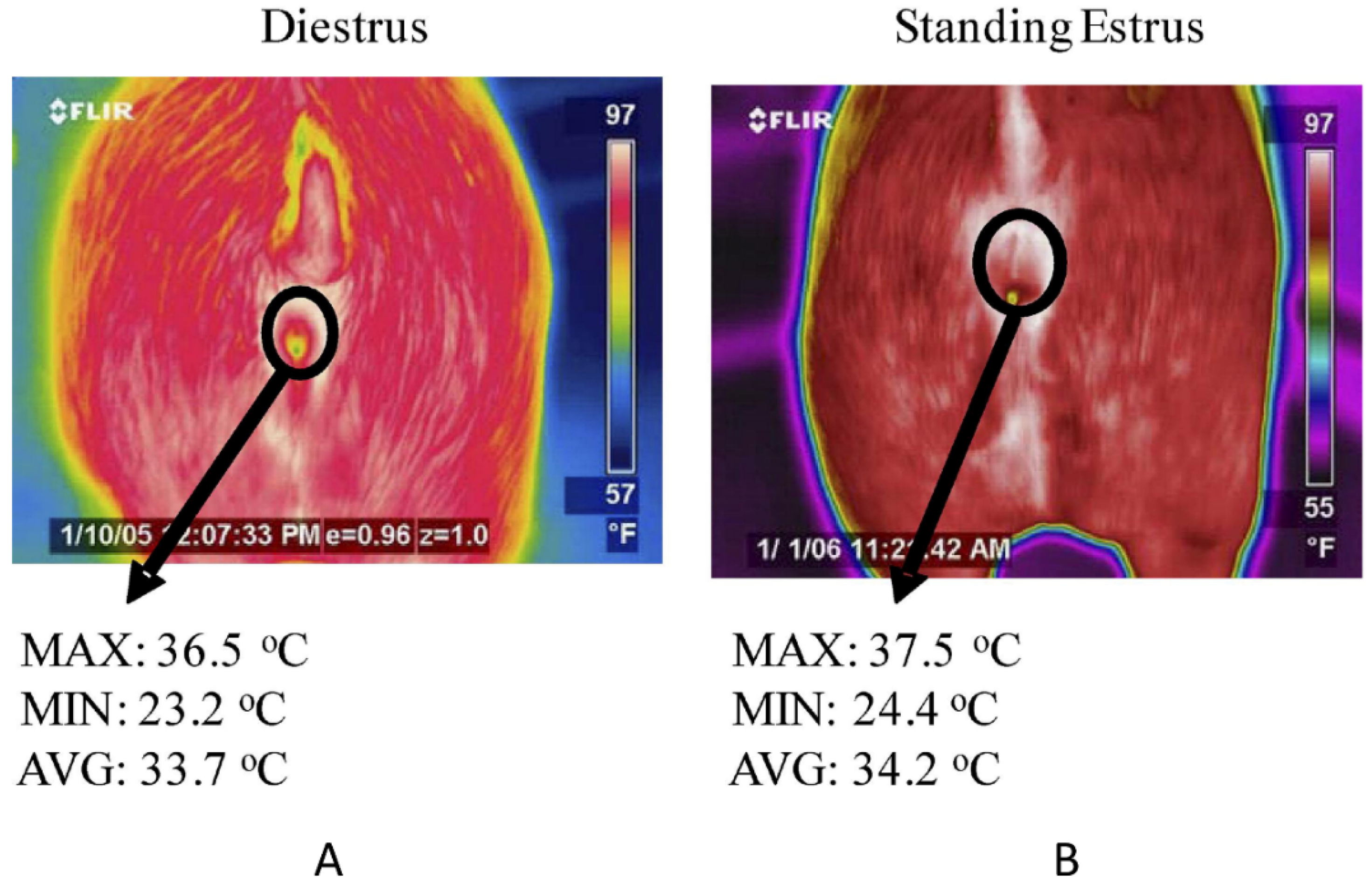
3.4. Early Identification of Estrus and Ovulation in Animals
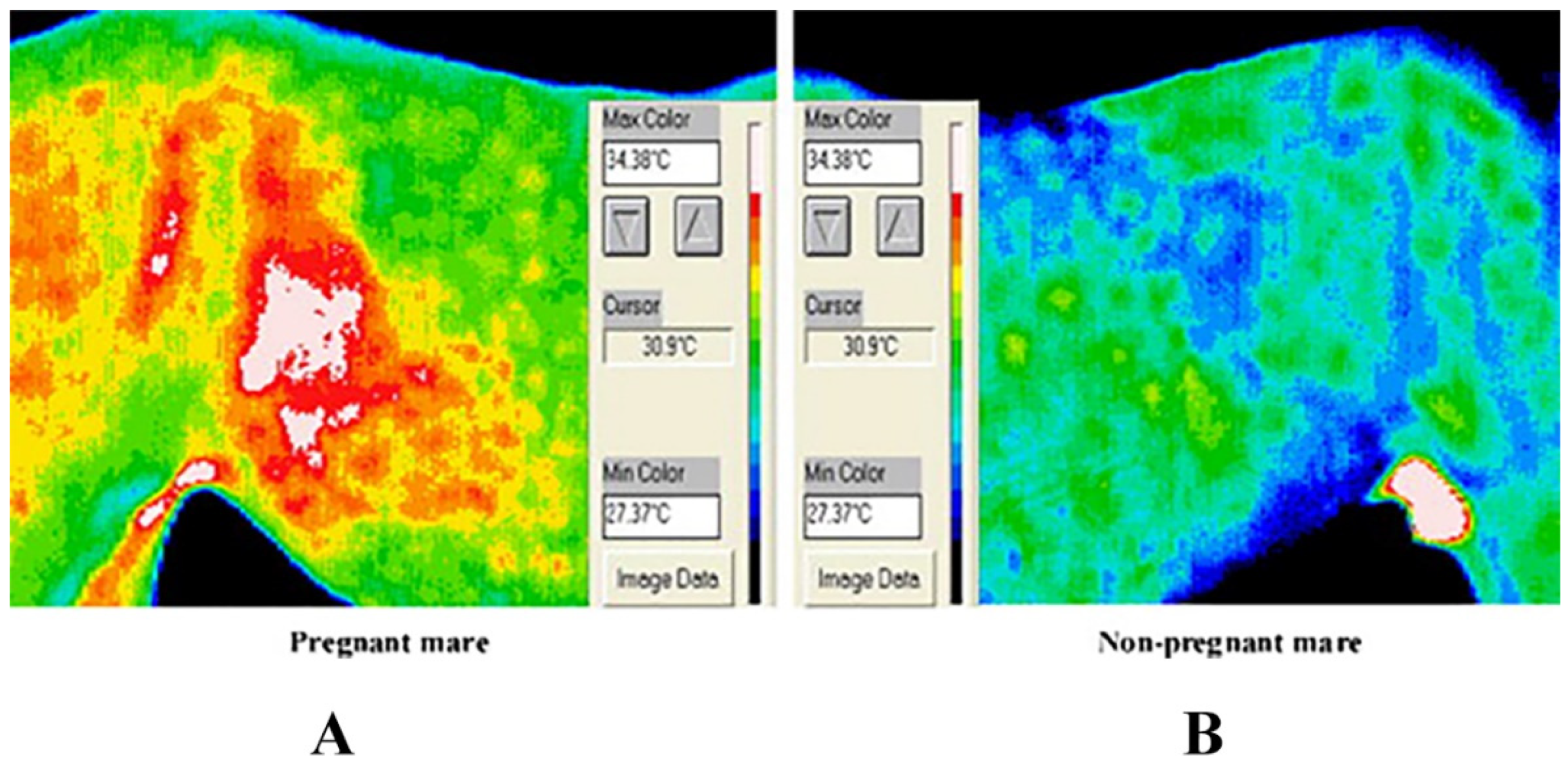
3.5. Pregnancy Diagnosis of Animals
3.6. Animals Welfare
4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, B.; Gonzalez-Garcia, E.; Thomas, M.; Fortun-Lamothe, L.; Ducrot, C.; Dourmad, J.Y.; Tichit, M. Forty research issues for the redesign of animal production systems in the 21st century. Animal 2014, 8, 1382–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neethirajan, S.; Kemp, B. Digital twins in livestock farming. Animals 2021, 11, 1382–1393. [Google Scholar]
- Hanigan, M.D.; Daley, V.L. Use of mechanistic nutrition models to identify sustainable food animal production. Annu. Rev. Anim. Biosci. 2020, 8, 355–376. [Google Scholar]
- Nayeri, S.; Sargolzaei, M.; Tulpan, D. A review of traditional and machine learning methods applied to animal breeding. Anim. Health Res. Rev. 2019, 20, 31–46. [Google Scholar] [CrossRef]
- King, A. Technology: The future of agriculture. Nature 2017, 544, S21–S23. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, G.A.; Smith, L.N.; Smith, M.L.; Reynolds, C.K.; Humphries, D.J.; Moorby, J.M.; Leemans, D.K.; Kingston-Smith, A.H. A computer vision approach to improving cattle digestive health by the monitoring of faecal samples. Sci. Rep. 2020, 10, 17557. [Google Scholar] [CrossRef]
- Du, X.; Carpentier, L.; Teng, G.; Liu, M.; Wang, C.; Norton, T. Assessment of laying hens′ thermal comfort using sound technology. Sensors 2020, 20, 473. [Google Scholar]
- Morrison, S.F.; Nakamura, K. Central mechanisms for thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Burfeind, O.; Suthar, V.S.; Heuwieser, W. Effect of heat stress on body temperature in healthy early postpartum dairy cows. Theriogenology 2012, 78, 2031–2038. [Google Scholar] [CrossRef]
- Carneiro, M.W.; Brancato, L.; Wylleman, B.; van Zwol, E.; Conings, L.; Vueghs, P.; Gorbaslieva, I.; Van den Bossche, J.; Rudenko, O.; Janicot, M.; et al. Safety evaluation of long-term temperature controlled whole-body thermal treatment in female Aachen minipig. Int. J. Hyperth. 2021, 38, 165–175. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernandez-Avalos, I.; Mora-Medina, P.; Olmos-Hernandez, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martinez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef]
- Bruins, A.A.; Kistemaker, K.R.J.; Boom, A.; Klaessens, J.; Verdaasdonk, R.M.; Boer, C. Thermographic skin temperature measurement compared with cold sensation in predicting the efficacy and distribution of epidural anesthesia. J. Clin. Monit. Comput. 2018, 32, 335–341. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Olmos-Hernandez, A.; Verduzco-Mendoza, A.; Lecona-Butron, H.; Martinez-Burnes, J.; Mora-Medina, P.; Gomez-Prado, J.; Orihuela, A. Infrared thermal imaging associated with pain in laboratory animals. Exp. Anim. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar]
- Farrar, K.L.; Field, A.E.; Norris, S.L.; Jacobsen, K.O. Comparison of rectal and infrared thermometry temperatures in anesthetized swine (Sus scrofa). J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 221–225. [Google Scholar] [CrossRef]
- Omobowale, T.O.; Ogunro, B.N.; Odigie, E.A.; Otuh, P.I.; Olugasa, B.O. A comparison of surface infrared with rectal thermometry in dogs. Niger. J. Physiol. Sci. 2017, 32, 123–127. [Google Scholar]
- Qu, Z.; Jiang, P.; Zhang, W. Development and application of infrared thermography non-destructive testing techniques. Sensors 2020, 20, 3851. [Google Scholar]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
- Harrap, M.J.M.; Hempel de Ibarra, N.; Whitney, H.M.; Rands, S.A. Reporting of thermography parameters in biology: A systematic review of thermal imaging literature. R. Soc. Open. Sci. 2018, 5, 181281. [Google Scholar] [CrossRef] [Green Version]
- Perpetuini, D.; Filippini, C.; Cardone, D.; Merla, A. An overview of thermal infrared imaging-based screenings during pandemic emergencies. Int. J. Environ. Res. Public Health 2021, 18, 3286. [Google Scholar] [CrossRef]
- Khaksari, K.; Nguyen, T.; Hill, B.; Quang, T.; Perreault, J.; Gorti, V.; Malpani, R.; Blick, E.; Gonzalez Cano, T.; Shadgan, B.; et al. Review of the efficacy of infrared thermography for screening infectious diseases with applications to COVID-19. J. Med. Imaging 2021, 8, 010901. [Google Scholar] [CrossRef]
- Brzezinski, R.Y.; Rabin, N.; Lewis, N.; Peled, R.; Kerpel, A.; Tsur, A.M.; Gendelman, O.; Naftali-Shani, N.; Gringauz, I.; Amital, H. Automated processing of thermal imaging to detect COVID-19. Sci. Rep. 2021, 11, 17489. [Google Scholar] [CrossRef]
- ElMasry, G.; ElGamal, R.; Mandour, N.; Gou, P.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Emerging thermal imaging techniques for seed quality evaluation: Principles and applications. Food Res. Int. 2020, 131, 109025. [Google Scholar] [CrossRef]
- Prashar, A.; Jones, H.G. Assessing drought responses using thermal infrared imaging. Methods Mol. Biol. 2016, 1398, 209–219. [Google Scholar]
- Romero-Bravo, S.; Mendez-Espinoza, A.M.; Garriga, M.; Estrada, F.; Escobar, A.; Gonzalez-Martinez, L.; Poblete-Echeverria, C.; Sepulveda, D.; Matus, I.; Castillo, D. Thermal imaging reliability for estimating grain yield and carbon isotope discrimination in wheat genotypes: Importance of the environmental conditions. Sensors 2019, 19, 2676. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Ren, Z.; Li, D.; Liu, X. Phenotypic techniques and applications in fruit trees: A review. Plant Methods 2020, 16, 107. [Google Scholar] [CrossRef]
- Vadivambal, R.; Jayas, D. Applications of thermal imaging in agriculture and food Industry—A review. Food Bioprocess Technol. 2011, 4, 186–199. [Google Scholar] [CrossRef]
- Ghazal, M.; Basmaji, T.; Yaghi, M.; Alkhedher, M.; Mahmoud, M.; El-Baz, A.S. Cloud-based monitoring of thermal anomalies in industrial environments using AI and the internet of robotic things. Sensors 2020, 20, 6348. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, S.A. Infrared techniques in buildings and structures: Operation and maintenance. Infrared Methodol. Technol. 1994, 7, 211–252. [Google Scholar]
- Du, B.; He, Y.; He, Y.; Zhang, C. Progress and trends in fault diagnosis for renewable and sustainable energy system based on infrared thermography: A review. Infrared Phys. Techn. 2020, 109, 103383. [Google Scholar] [CrossRef]
- Ciampa, F.; Mahmoodi, P.; Pinto, F.; Meo, M. Recent advances in active infrared thermography for non-destructive testing of aerospace components. Sensors 2018, 18, 609. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Hu, Q.P.; Qiu, J.X.; Pei, C.X.; Li, X.Y.; Zhou, H.B.; Xia, R.C.; Liu, J. Image enhancement method for laser infrared thermography defect detection in aviation composites. Opt. Eng. 2019, 58, 103104. [Google Scholar] [CrossRef]
- Menegassi, S.R.O.; Pereira, G.R.; Dias, E.A.; Rocha, M.K.; Carvalho, H.R.; Koetz, C., Jr.; Oberst, E.R.; Barcellos, J.O.J. Infrared thermography as a noninvasive method to assess scrotal insulation on sperm production in beef bulls. Andrologia 2018, 50, e12904. [Google Scholar] [CrossRef]
- Weimer, S.L.; Wideman, R.F.; Scanes, C.G.; Mauromoustakos, A.; Christensen, K.D.; Vizzier-Thaxton, Y. Broiler stress responses to light intensity, flooring type, and leg weakness as assessed by heterophil-to-lymphocyte ratios, serum corticosterone, infrared thermography, and latency to lie. Poult. Sci. 2020, 99, 3301–3311. [Google Scholar] [CrossRef]
- Isola, J.V.V.; Menegazzi, G.; Busanello, M.; Dos Santos, S.B.; Agner, H.S.S.; Sarubbi, J. Differences in body temperature between black-and-white and red-and-white Holstein cows reared on a hot climate using infrared thermography. J. Therm. Biol. 2020, 94, 102775. [Google Scholar] [CrossRef]
- Radigonda, V.L.; Pereira, G.R.; da Cruz Favaro, P.; Barca Junior, F.A.; Borges, M.H.F.; Galdioli, V.H.G.; Junior, C.K. Infrared thermography relationship between the temperature of the vulvar skin, ovarian activity, and pregnancy rates in Braford cows. Trop. Anim. Health Prod. 2017, 49, 1787–1791. [Google Scholar] [CrossRef]
- Schmitt, O.; O′Driscoll, K. Use of infrared thermography to noninvasively assess neonatal piglet temperature. Transl. Anim. Sci. 2021, 5, 208. [Google Scholar] [CrossRef]
- Vollmer, M.; Möllmann, K.P. Infrared thermal imaging: Fundamentals, research and applications. Infrared Therm. Imaging 2010, 32, 5. [Google Scholar] [CrossRef]
- Usamentiaga, R.; Pablo, V.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F. Infrared thermography for temperature measurement and non-destructive testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef] [Green Version]
- Minkina, W. How infrared radiation was discovered—Range of this discovery and detailed, unknown information. Appl. Sci. 2021, 11, 9824. [Google Scholar] [CrossRef]
- Tang, K.; Dong, K.; Nicolai, C.J.; Li, Y.; Li, J.; Lou, S.; Qiu, C.W.; Raulet, D.H.; Yao, J.; Wu, J. Millikelvin-resolved ambient thermography. Sci. Adv. 2020, 6, eabd8688. [Google Scholar] [CrossRef]
- Chevalier, S.; Tourvieille, J.N.; Sommier, A.; Batsale, J.C.; Beccard, B.; Pradere, C. Thermal camera-based fourier transform infrared thermospectroscopic imager. Appl. Spectrosc. 2021, 75, 462–474. [Google Scholar] [CrossRef]
- Kastberger, G.; Stachl, R. Infrared imaging technology and biological applications. Behav. Res. Methods Instrum. Comput. 2003, 35, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Ring, E.F.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef]
- Nääs, I.; Romanini, C.; Neves, D.; Nascimento, G.; Vercellino, R. Broiler surface temperature distribution of 42 day old chickens. Sci. Agric. 2010, 67, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Moe, R.O.; Stubsjøen, S.M.; Bohlin, J.; Flø, A.; Bakken, M. Peripheral temperature drop in response to anticipation and consumption of a signaled palatable reward in laying hens (Gallus domesticus). Physiol. Behav. 2012, 106, 527–533. [Google Scholar] [CrossRef]
- Hristov, N.I.; Betke, M.; Kunz, T.H. Applications of thermal infrared imaging for research in aeroecology. Integr. Comp. Biol. 2008, 48, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, R.; Krebs, C.J.; Boutin, S.; Eadie, J.M. Finding mammals using far-infrared thermal imaging. J. Mammal. 1994, 75, 1063–1068. [Google Scholar] [CrossRef]
- Bowers, S.; Gandy, S.; Anderson, B.; Ryan, P.; Willard, S. Assessment of pregnancy in the late-gestation mare using digital infrared thermography. Theriogenology 2009, 72, 372–377. [Google Scholar] [CrossRef]
- Ermatinger, F.A.; Brugger, R.K.; Burkart, J.M. The use of infrared thermography to investigate emotions in common marmosets. Physiol. Behav. 2019, 211, 112672. [Google Scholar] [CrossRef] [PubMed]
- Peric, D.; Livada, B.; Peric, M.; Vujic, S. Thermal imager range: Predictions, expectations, and reality. Sensors 2019, 19, 3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strojnik, M.; Scholl, M.K.; Garcia-Torales, G. Black-body radiation, emissivity, and absorptivity. Proc. SPIE 2016, 9973, 997310. [Google Scholar] [CrossRef]
- Rubio, E.; Caselles, V.; Badenas, C. Emissivity measurements of several soils and vegetation types in the 8–14, μm Wave band: Analysis of two field methods. Remote Sens. Environ. 1997, 59, 490–521. [Google Scholar] [CrossRef]
- Zhang, K.; Jiao, L.; Zhao, X.; Dong, D. An instantaneous approach for determining the infrared emissivity of swine surface and the influencing factors. J. Therm. Biol. 2016, 57, 78–83. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, E.J.; Cho, Y.H.; Kang, J.W.; Jo, J.H. A field study on application of infrared thermography for estimating mean radiant temperatures in large stadiums. Energ. Build. 2019, 202, 109360. [Google Scholar] [CrossRef]
- Rocha, J.H.A.; Povoas, Y.V.; Santos, C.F. Detection of delaminations in sunlight-unexposed concrete elements of bridges using infrared thermography. J. Nondestruct. Eval. 2019, 38, 8. [Google Scholar] [CrossRef]
- Lowe, G.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Infrared thermography-a non-invasive method of measuring respiration rate in Calves. Animals 2019, 9, 535. [Google Scholar] [CrossRef] [Green Version]
- Andrews, R. IR image parametric up-conversion. IEEE J. Quantum Elect. 1970, 6, 68–80. [Google Scholar] [CrossRef]
- Karvatte, N.J.; Miyagi, E.S.; de Oliveira, C.C.; Barreto, C.D.; Mastelaro, A.P.; Bungenstab, D.J.; Alves, F.V. Infrared thermography for microclimate assessment in agroforestry systems. Sci. Total Environ. 2020, 731, 139252. [Google Scholar] [CrossRef]
- Barreto, C.D.; Alves, F.V.; de Oliveira Ramos, C.E.C.; de Paula Leite, M.C.; Leite, L.C.; Junior, N.K. Infrared thermography for evaluation of the environmental thermal comfort for livestock. Int. J. Biometeorol. 2020, 64, 881–888. [Google Scholar] [CrossRef]
- Sellier, N.; Guettier, E.; Staub, C. A review of methods to measure animal body temperature in precision farming. J. Agric. Sci. Tech-Iran. 2014, 2, 74–99. [Google Scholar] [CrossRef]
- Machado, N.A.F.; Da Costa, L.B.S.; Barbosa-Filho, J.A.D.; De Oliveira, K.P.L.; De Sampaio, L.C.; Peixoto, M.S.M.; Damasceno, F.A. Using infrared thermography to detect subclinical mastitis in dairy cows in compost barn systems. J. Therm. Biol. 2021, 97, 102881. [Google Scholar] [CrossRef]
- Moe, R.O.; Bohlin, J.; Flo, A.; Vasdal, G.; Erlandsen, H.; Guneriussen, E.; Sjokvist, E.C.; Stubsjoen, S.M. Effects of subclinical footpad dermatitis and emotional arousal on surface foot temperature recorded with infrared thermography in turkey toms (Meleagris gallopavo). Poult. Sci. 2018, 97, 2249–2257. [Google Scholar] [CrossRef]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First evaluation of infrared thermography as a tool for the monitoring of udder health status in farms of dairy cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef] [Green Version]
- Tabuaciri, P.; Bunter, K.; Graser, H.-U. Thermal imaging as a potential tool for identifying piglets at risk. AGBU Pig Genet. Workshop 2016, 5, 23–30. [Google Scholar]
- Vicente Pérez, R.; Avendaño-Reyes, L.; Mejía-Vázquez, Á.; Álvarez-Valenzuela, F.; Correa-Calderón, A.; Mellado, M.; Meza-Herrera, C.A.; Guerra-Liera, J.; Robinson, P.H.; Macías-Cruz, U. Prediction of rectal temperature using non-invasive physiologic variable measurements in hair pregnant ewes subjected to natural conditions of heat stress. J. Therm. Biol. 2016, 55, 1–6. [Google Scholar] [CrossRef]
- George, W.D.; Godfrey, R.; Ketring, R.C.; Vinson, M.C.; Willard, S.T. Relationship among eye and muzzle temperatures measured using digital infrared thermal imaging and vaginal and rectal temperatures in hair sheep and cattle. J. Anim. Sci. 2014, 92, 4949–4955. [Google Scholar] [CrossRef] [Green Version]
- Church, J.; Hegadoren, P.; Paetkau, M.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K. Influence of environmental factors on infrared eye temperature measurement in cattle. Res. Vet. Sci. 2013, 96, 220–226. [Google Scholar] [CrossRef]
- Peng, D.; Chen, S.; Li, G.; Chen, J.; Wang, J.; Gu, X. Infrared thermography measured body surface temperature and its relationship with rectal temperature in dairy cows under different temperature-humidity indexes. Int. J. Biometeorol. 2019, 63, 327–336. [Google Scholar] [CrossRef]
- Vicente-Perez, R.; Avendano-Reyes, L.; Correa-Calderon, A.; Mellado, M.; Meza-Herrera, C.A.; Montanez-Valdez, O.D.; Macias-Cruz, U. Relationships of body surface thermography with core temperature, birth weight and climatic variables in neonatal lambs born during early spring in an arid region. J. Therm. Biol. 2019, 82, 142–149. [Google Scholar] [CrossRef]
- Berry, R.J.; Kennedy, A.D.; Tracey, S.; Kyle, B.L.; Schaefer, A.L. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: Potential for mastitis detection. Can. J. Anim. Sci. 2003, 83, 687–693. [Google Scholar] [CrossRef]
- Metzner, M.; Sauter-Louis, C.; Seemueller, A.; Petzl, W.; Klee, W. Infrared thermography of the udder surface of dairy cattle: Characteristics, methods, and correlation with rectal temperature. Vet. J. 2013, 199, 57–62. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Cook, N.; Tessaro, S.; Deregt, D.; Desroches, G.; Dubeski, P.; Tong, A.K.W.; Godson, D. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Rainwater-Lovett, K.; Pacheco, J.; Packer, C.; Rodriguez, L. Detection of foot-and-mouth disease virus infected cattle using infrared thermography. Vet. J. 2008, 180, 317–324. [Google Scholar] [CrossRef]
- Eddy, A.; Hoogmoed, L.M.; Snyder, J.R. The role of thermography in the management of equine lameness. Vet. J. 2001, 162, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Siewert, C.; Dänicke, S.; Kersten, S.; Brosig, B.; Rohweder, D.; Beyerbach, M.; Seifert, H. Difference method for analysing infrared images in pigs with elevated body temperatures. Z. Med. Phys. 2014, 24, 6–15. [Google Scholar] [CrossRef]
- Montanholi, Y.; Odongo, N.; Swanson, K.; Schenkel, F.; McBride, B.; Miller, S. Application of infrared thermography as an indicator of heat and methane production and its use in the study of skin temperature in response to physiological events in dairy cattle (Bos taurus). J. Therm. Biol. 2008, 33, 468–475. [Google Scholar] [CrossRef]
- Soerensen, D.; Clausen, S.; Mercer, J.; Pedersen, L. Determining the emissivity of pig skin for accurate infrared thermography. Comput. Electron. Agric. 2014, 109, 52–58. [Google Scholar] [CrossRef]
- Tangorra, F.M.; Redaelli, V.; Luzi, F.; Zaninelli, M. The use of infrared thermography for the monitoring of udder teat stress caused by milking machines. Animals 2019, 9, 384. [Google Scholar] [CrossRef] [Green Version]
- Paim, T.; Borges, B.; de Mello Tavares Lima, P.; Dallago, B.; Louvandini, H.; McManus, C. Relation between thermographic temperatures of lambs and thermal comfort indices. Int. J. Appl. Anim. Sci. 2012, 1, 108–115. [Google Scholar]
- Valera, M.; Bartolomé, E.; Sánchez Guerrero, M.; Molina Alcalá, A.; Cook, N.; Schaefer, A. Changes in eye temperature and stress assessment in horses during show jumping competitions. J. Equine Vet. Sci. 2012, 32, 827–830. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Samara, E.M.; Hussein, E.O.S.; Al-Ghadi, M.a.Q.; Al-Atiyat, R.M. Impacts of stocking density on the performance and welfare of broiler chickens. Ital. J. Anim. Sci. 2013, 12, e11. [Google Scholar] [CrossRef] [Green Version]
- Narayan, E.; Perakis, A.; Meikle, W. Using thermal imaging to monitor body temperature of Koalas (phascolarctos cinereus) in a zoo setting. Animals 2019, 9, 1094. [Google Scholar] [CrossRef] [Green Version]
- Jerem, P.; Herborn, K.; McCafferty, D.; McKeegan, D.; Nager, R. Thermal imaging to study stress non-invasively in unrestrained birds. JoVE 2015, 105, e53184. [Google Scholar] [CrossRef] [Green Version]
- Perez Marquez, H.J.; Ambrose, D.J.; Schaefer, A.L.; Cook, N.J.; Bench, C.J. Evaluation of infrared thermography combined with behavioral biometrics for estrus detection in naturally cycling dairy cows. Animal 2021, 15, 100205. [Google Scholar] [CrossRef]
- Saranika, T.; Thomson, P.; Kerrisk, K.; Clark, C.; Celi, P. Evaluation of infrared thermography (IRT) body temperature and collar mounted accelerometer and acoustic technology for predicting time of ovulation of cows in a pasture-based system. Theriogenology 2015, 83, 739–748. [Google Scholar]
- Freitas, A.; Ortiz Vega, W.; Quirino, C.; Bartholazzi Junior, A.; David, C.; Geraldo, A.; Rua, M.; Rojas, L.; Filho, J.; Dias, A. Surface temperature of ewes during estrous cycle measured by infrared thermography. Theriogenology 2018, 119, 245–251. [Google Scholar] [CrossRef]
- Scolari, S.C.; Clark, S.G.; Knox, R.V.; Tamassia, M.A. Vulvar skin temperature changes significantly during estrus in swine as determined by digital infrared thermography. J. Swine Health Prod. 2011, 19, 151–155. [Google Scholar]
- Sykes, D.J.; Couvillion, J.S.; Cromiak, A.; Bowers, S.; Schenck, E.; Crenshaw, M.; Ryan, P. The use of digital infrared thermal imaging to detect estrus in gilts. Theriogenology 2012, 78, 147–152. [Google Scholar] [CrossRef]
- Simões, V.; Lyazrhi, F.; Picard-Hagen, N.; Gayrard, V.; Martineau, G.-P.; Waret-Szkuta, A. Variations in the vulvar temperature of sows during proestrus and estrus as determined by infrared thermography and its relation to ovulation. Theriogenology 2014, 82, 1080–1085. [Google Scholar] [CrossRef]
- Durrant, B.S.; Ravida, N.; Spady, T.; Cheng, A. New technologies for the study of carnivore reproduction. Theriogenology 2006, 66, 1729–1736. [Google Scholar] [CrossRef]
- Masko, M.; Witkowska-Pilaszewicz, O.; Jasinski, T.; Domino, M. Thermal features, ambient temperature and hair coat lengths: Limitations of infrared imaging in pregnant primitive breed mares within a year. Reprod. Domest. Anim. 2021, 56, 1315–1328. [Google Scholar] [CrossRef]
- Salles, M.S.V.; da Silva, S.C.; Salles, F.A.; Roma, L.C.; El Faro, L.; Bustos Mac Lean, P.A.; Lins de Oliveira, C.E.; Martello, L.S. Mapping the body surface temperature of cattle by infrared thermography. J. Therm. Biol. 2016, 62, 63–69. [Google Scholar] [CrossRef]
- Vande Pol, K.D.; Tolosa, A.F.; Bautista, R.O.; Willard, N.C.; Gates, R.S.; Shull, C.M.; Brown, C.B.; Alencar, S.A.S.; Lents, C.A.; Ellis, M. Effects of drying and providing supplemental oxygen to piglets at birth on rectal temperature over the first 24 h after birth. Transl. Anim. Sci. 2021, 5, 095. [Google Scholar] [CrossRef]
- Knížková, I.; Kunc, P.; Gürdil, G.; Pinar, Y.; Selvi, K. Applications of infrared thermography in animal production. J. Fac. Agric. 2007, 22, 329–336. [Google Scholar]
- Soerensen, D.; Pedersen, L. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef] [Green Version]
- Traulsen, I.; Kathrin, N.; Karin, M.; Krieter, J. Application of infrared thermography to measure body temperature of sows. Zuchtungskunde 2010, 82, 437–446. [Google Scholar]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary applications of infrared thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.; Barden, M.; Tulloch, J.; Williams, K.; Griffiths, B.; Bedford, C.; Rudd, M.; Psifidi, A.; Banos, G.; Oikonomou, G. A study on the use of thermal imaging as a diagnostic tool for the detection of digital dermatitis in dairy cattle. J. Dairy Sci. 2021, 104, 10194–10202. [Google Scholar] [CrossRef]
- Timsit, E.; Assié, S.; Quiniou, R.; Seegers, H.; Bareille, N. Improved detection of bovine respiratory disease in young bulls using rumen temperature boluses. Vet. J. 2010, 190, 136–142. [Google Scholar] [CrossRef]
- Amezcua, M.; Walsh, S.; Luimes, P.; Friendship, R. Infrared thermography to evaluate lameness in pregnant sows. Can. Vet. J. 2014, 55, 268–272. [Google Scholar]
- Rashamol, V.P.; Sejian, V.; Pragna, P.; Lees, A.M.; Bagath, M.; Krishnan, G.; Gaughan, J.B. Prediction models, assessment methodologies and biotechnological tools to quantify heat stress response in ruminant livestock. Int. J. Biometeorol. 2019, 63, 1265–1281. [Google Scholar] [CrossRef] [Green Version]
- Weschenfelder, A.; Maldague, X.; Rocha, L.; Schaefer, A.; Faucitano, L. The use of infrared thermography for pork quality prediction. Meat Sci. 2014, 96, 120. [Google Scholar] [CrossRef]
- Safar-Hermann, N.; Ismail, M.; Choi, H.; Möstl, E.; Bamberg, E. Pregnancy diagnosis in zoo animals by estrogen determination in feces. Zoo Biol. 1987, 6, 189–193. [Google Scholar] [CrossRef]
- Benesch, A.R.; Hilsberg, S. Infrared thermographic study of surface temperature in zebras. Zool. Gart. 2003, 73, 74–82. [Google Scholar]
- Jones, M.; Denson, A.; Williams, E.; Santos, A.; Graves, K.; Kouba, A.; Willard, S. Assessing pregnancy status using digital infrared thermal imaging in Holstein heifers. J. Dairy Sci. 2005, 88, 40–41. [Google Scholar]
- Hampton, J.O.; Baker, S.E.; Beausoleil, N.J.; Cattet, M.; Forsyth, D.M.; McMahon, C.R.; Proulx, G.; Warburton, B. Animal welfare science aids conservation. Science 2020, 370, 180–181. [Google Scholar] [CrossRef]
- Redaelli, V.; Ludwig, N.; Nanni Costa, L.; Crosta, L.; Riva, J.; Luzi, F. Potential application of thermography (IRT) in animal production and for animal welfare. A case report of working dogs. Ann. Ist. Super. Sanita 2014, 50, 147–152. [Google Scholar] [PubMed]

| Animals | Type of IR Camera | Emissivity | Distance | Environment Temperature | Humidity | Application | References |
|---|---|---|---|---|---|---|---|
| Piglet | FLIR | 0.98 | Detecting animal surfaces and core anatomical areas temperature fluctuation | [66] | |||
| Ewe | Fluke Ti10 | 25.7–42.0 °C | 42% | [67] | |||
| Ewe | FLIR P65HS | 30 cm | 25.6–34.0 °C | 81.3% ± 9.4% | [68] | ||
| Cattle | FLIR I40 and E60 | 0.95 | 1.0 m | [69] | |||
| Dairy cow | InfraTec | 1.5 m | [70] | ||||
| neonatal lamb | Fluke Ti10 | 1.0 m | 10.4–34 °C | 7.9–100% | [71] | ||
| Dairy cow | FLIR 760 | 2.0–2.5 m | 11.1–27.4 °C | Diagnosing animal early diseases and inflammation | [72] | ||
| Dairy cattle | FLIR B320 | 0.96 | 1.8 m | [73] | |||
| Heifer | FLIR 760 | 1–3 m | 14 °C | 28% | [74] | ||
| Cattle | FLIR EX320 | 1.5–2.0 m | [75] | ||||
| Sow | FLIR T300 | 0.5–0.8 m | [76] | ||||
| Pig | FLUKE R2 | 0.97 | 30–50 cm | 21.1–22.6 °C | [77] | ||
| Dairy cattle | FLIR SC2000 | 0.98 | 1.5 m | 11.93–29.04 °C | 26.09–79.13% | Detecting animal stress level | [78] |
| Pig | FLIR SC660 | 0.98 | 50 cm | 16.2–24.3 °C | [79] | ||
| Cow | GEAR-G120 | 0.98 | 0.5 m | 15–25 °C | 67–78% | [80] | |
| Lamb | FLIR series | 0.95 | [81] | ||||
| Horse | FLIR I700 | 1 m | 16–31 °C | 56–68% | [82] | ||
| Broiler chicken | VisIR-Ti200 | 0.97 | 24.73–25.18 °C | 23.04–34.43% | [83] | ||
| Koala | FLIR 530 | 1 m | [84] | ||||
| Bird | 0.97 | 40–500 mm | [85] | ||||
| Dairy cow | FLIR A310 | 0.98 | 1 m | 14.05 ± 3.06 °C | 68.86% ± 6.94% | Diagnosing animal estrus and ovulation | [86] |
| Cow | FLIR 620 | 0.98 | 1 m | [87] | |||
| Ewe | FLIR I50 | 0.98 | 1 m | 26.77–31.01 °C | 64.74–83.62% | [88] | |
| Swine | Fluke | 0.61 m | [89] | ||||
| Gilt | FLIR S60 | 1 | 121.9–152.4 cm | 1.5–25.8 °C | [90] | ||
| Sow | Fluke 9 HZ | 1 m | [91] | ||||
| Giant panda | FLIR PM545 | 0.6–0.9 m | Diagnosing animal pregnancy | [92] | |||
| Mare | Compix vet2000 | 0.96 | 147.3–157.5 cm | 4.2–28.9 °C | [50] | ||
| Mare | FLIR E60 | 0.99 | 2.0 m | 10–40 °C | [93] | ||
| Piglet | FLIR T420 | 0.98 | 1 m | animals’ welfare | [38] | ||
| Cattle | Fluke Ti20TM | 0.98 | 20 cm–2 m | 17.7–27.3 °C | 59.3–90.9% | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Zhou, C.; Jiang, X.; Huang, J.; Xu, D. Progress on Infrared Imaging Technology in Animal Production: A Review. Sensors 2022, 22, 705. https://doi.org/10.3390/s22030705
Zheng S, Zhou C, Jiang X, Huang J, Xu D. Progress on Infrared Imaging Technology in Animal Production: A Review. Sensors. 2022; 22(3):705. https://doi.org/10.3390/s22030705
Chicago/Turabian StyleZheng, Shuailong, Changfan Zhou, Xunping Jiang, Jingshu Huang, and Dequan Xu. 2022. "Progress on Infrared Imaging Technology in Animal Production: A Review" Sensors 22, no. 3: 705. https://doi.org/10.3390/s22030705
APA StyleZheng, S., Zhou, C., Jiang, X., Huang, J., & Xu, D. (2022). Progress on Infrared Imaging Technology in Animal Production: A Review. Sensors, 22(3), 705. https://doi.org/10.3390/s22030705






