Wearable Electrochemical Sensors in Parkinson’s Disease
Abstract
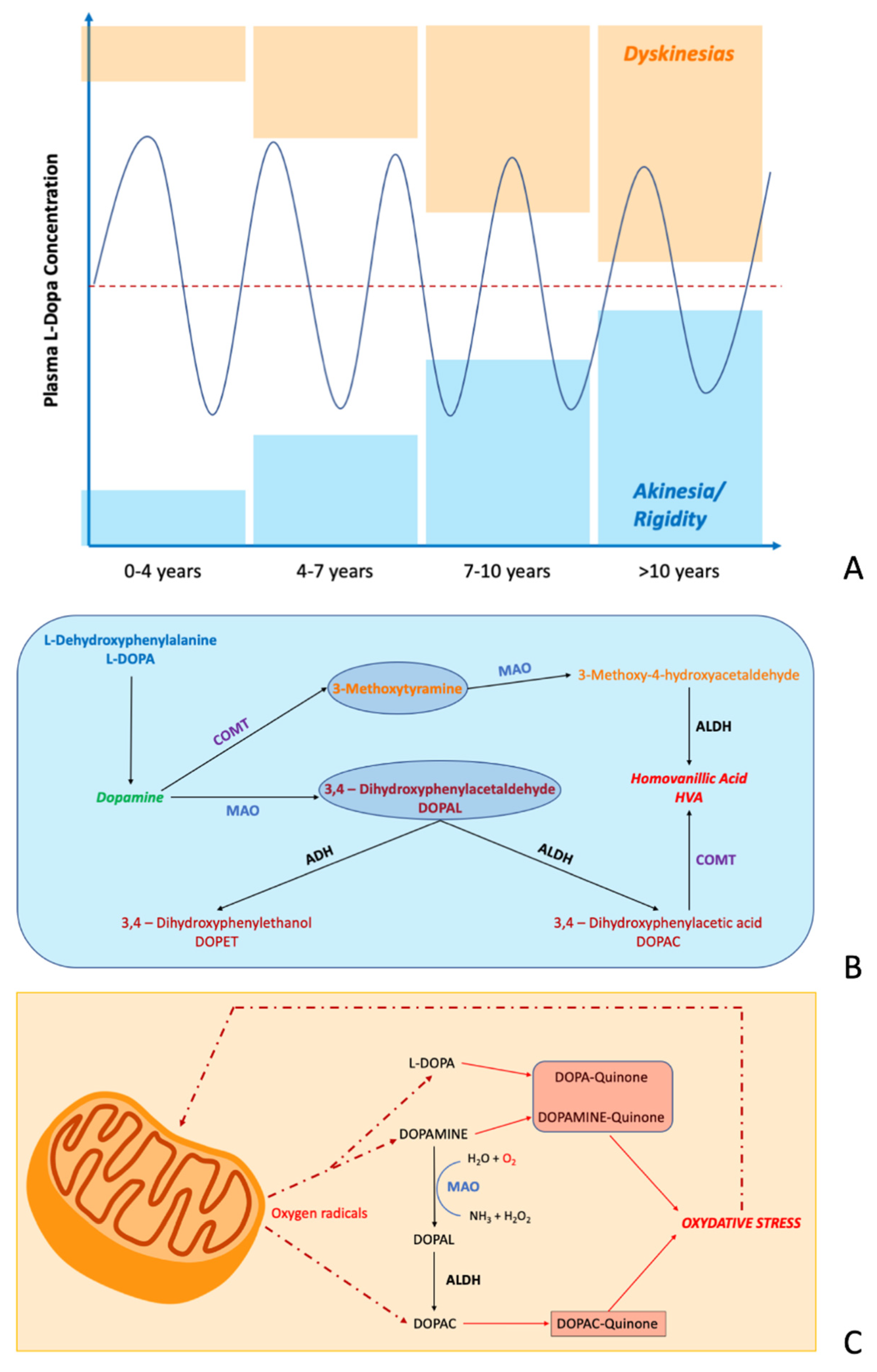
:1. Introduction
2. Hunting Biomarkers for Diagnosis and Follow-Up in PD
3. Electrochemical Biosensors
4. Electrochemical Biosensors in PD
5. Clinical Prospects in PD
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.; Lang, A.E.; Halliday, G.; Goetz, C.G.; Chan, P.; Slow, E.; Seppi, K.; et al. Validation of the MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2018, 33, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathology of Parkinson Disease. Parkinsonism Relat. Disord. 2018, 46 (Suppl. 1), S30–S33. [Google Scholar] [CrossRef]
- Fox, S.H.; Brotchie, J.M.; Lang, A.E. Non-Dopaminergic Treatments in Development for Parkinson’s Disease. Lancet Neurol. 2008, 7, 927–938. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological Treatment of Parkinson Disease: A Review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Abbott, A. Levodopa: The Story so Far. Nature 2010, 466, S6–S7. [Google Scholar] [CrossRef]
- Widnell, K. Pathophysiology of Motor Fluctuations in Parkinson’s Disease. Mov. Disord. 2005, 20 (Suppl. 11), S17–S22. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Hauser, R.A.; Pahwa, R.; Isaacson, S.H.; Fernandez, H.H.; Lew, M.; Saint-Hilaire, M.; Pourcher, E.; Lopez-Manzanares, L.; Waters, C.; et al. Safety and Efficacy of CVT-301 (Levodopa Inhalation Powder) on Motor Function during off Periods in Patients with Parkinson’s Disease: A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Neurol. 2019, 18, 145–154. [Google Scholar] [CrossRef]
- Olanow, C.W.; Factor, S.A.; Espay, A.J.; Hauser, R.A.; Shill, H.A.; Isaacson, S.; Pahwa, R.; Leinonen, M.; Bhargava, P.; Sciarappa, K.; et al. Apomorphine Sublingual Film for off Episodes in Parkinson’s Disease: A Randomised, Double-Blind, Placebo-Controlled Phase 3 Study. Lancet Neurol. 2020, 19, 135–144. [Google Scholar] [CrossRef]
- Huot, P.; Kang, W.; Kim, E.; Bédard, D.; Belliveau, S.; Frouni, I.; Kwan, C. Levodopa-Induced Dyskinesia: A Brief Review of the Ongoing Clinical Trials. Neurodegener. Dis. Manag. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sage, J.I.; Mark, M.H.; McHale, D.M.; Sonsalla, P.K.; Vitagliano, D. Benefits of Monitoring Plasma Levodopa in Parkinson’s Disease Patients with Drug-Induced Chorea. Ann. Neurol. 1991, 29, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Contin, M.; Martinelli, P. Pharmacokinetics of Levodopa. J. Neurol. 2010, 257, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core-Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano. 2018, 12, 2466–2473. [Google Scholar] [CrossRef]
- Lan, X.; She, J.; Lin, D.-A.; Xu, Y.; Li, X.; Yang, W.-F.; Lui, V.W.Y.; Jin, L.; Xie, X.; Su, Y.-X. Microneedle-Mediated Delivery of Lipid-Coated Cisplatin Nanoparticles for Efficient and Safe Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 33060–33069. [Google Scholar] [CrossRef]
- Xie, X.; Pascual, C.; Lieu, C.; Oh, S.; Wang, J.; Zou, B.; Xie, J.; Li, Z.; Xie, J.; Yeomans, D.C.; et al. Analgesic Microneedle Patch for Neuropathic Pain Therapy. ACS Nano. 2017, 11, 395–406. [Google Scholar] [CrossRef]
- Samavat, S.; Lloyd, J.; O’Dea, L.; Zhang, W.; Preedy, E.; Luzio, S.; Teng, K.S. Uniform Sensing Layer of Immiscible Enzyme-Mediator Compounds Developed via a Spray Aerosol Mixing Technique towards Low Cost Minimally Invasive Microneedle Continuous Glucose Monitoring Devices. Biosens. Bioelectron. 2018, 118, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Microneedle-Based Biosensor for Minimally-Invasive Lactate Detection. Biosens. Bioelectron. 2019, 123, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, A.M.V.; Windmiller, J.R.; Mishra, R.K.; Wang, J. Continuous Minimally-Invasive Alcohol Monitoring Using Microneedle Sensor Arrays. Biosens. Bioelectron. 2017, 91, 574–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucher, E.S.; Wightman, R.M. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montague, P.R.; Kishida, K.T. Computational Underpinnings of Neuromodulation in Humans. Cold Spring Harb. Symp. Quant. Biol. 2018, 83, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Teymourian, H.; De la Paz, E.; Sempionatto, J.R.; Mahato, K.; Sonsa-ard, T.; Huang, N.; Longardner, K.; Litvan, I.; Wang, J. Non-Invasive Sweat-Based Tracking of L-Dopa Pharmacokinetic Profiles Following an Oral Tablet Administration. Angew. Chem. Int. Ed. 2021, 60, 19074–19078. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, e1701150. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.R.; Munni, M.N.; Campbell, L.; Mostofa, G.; Dobson, L.; Shittu, M.; Pattanayek, S.K.; Uddin, J.; Das, D.B. Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges. Pharmaceutics 2021, 13, 1132. [Google Scholar] [CrossRef]
- Liu, G.-S.; Kong, Y.; Wang, Y.; Luo, Y.; Fan, X.; Xie, X.; Yang, B.-R.; Wu, M.X. Microneedles for Transdermal Diagnostics: Recent Advances and New Horizons. Biomaterials 2020, 232, 119740. [Google Scholar] [CrossRef]
- Kim, J.H.; Suh, Y.J.; Park, D.; Yim, H.; Kim, H.; Kim, H.J.; Yoon, D.S.; Hwang, K.S. Technological Advances in Electrochemical Biosensors for the Detection of Disease Biomarkers. Biomed. Eng. Lett. 2021, 11, 309–334. [Google Scholar] [CrossRef]
- Erdem, Ö.; Eş, I.; Akceoglu, G.A.; Saylan, Y.; Inci, F. Recent Advances in Microneedle-Based Sensors for Sampling, Diagnosis and Monitoring of Chronic Diseases. Biosensors 2021, 11, 296. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C. Microneedle, Bio-Microneedle and Bio-Inspired Microneedle: A Review. J. Control Release 2017, 251, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventrelli, L.; Marsilio Strambini, L.; Barillaro, G. Microneedles for Transdermal Biosensing: Current Picture and Future Direction. Adv. Healthc. Mater. 2015, 4, 2606–2640. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.Q.; Miller, P.R.; Taylor, R.M.; Boyd, G.; Mach, P.M.; Rosenzweig, C.N.; Baca, J.T.; Polsky, R.; Glaros, T. Proteomic Characterization of Dermal Interstitial Fluid Extracted Using a Novel Microneedle-Assisted Technique. J. Proteome. Res. 2018, 17, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bobrinetskiy, I.; Radovic, M.; Rizzotto, F.; Vizzini, P.; Jaric, S.; Pavlovic, Z.; Radonic, V.; Nikolic, M.V.; Vidic, J. Advances in Nanomaterials-Based Electrochemical Biosensors for Foodborne Pathogen Detection. Nanomaterials 2021, 11, 2700. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Cheng, H.; Lehr, J.; Patil, A.V.; Davis, J.J. Graphene Oxide Interfaces in Serum Based Autoantibody Quantification. Anal. Chem. 2015, 87, 346–350. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Nyholm, D.; Lennernäs, H.; Gomes-Trolin, C.; Aquilonius, S.-M. Levodopa Pharmacokinetics and Motor Performance during Activities of Daily Living in Patients with Parkinson’s Disease on Individual Drug Combinations. Clin. Neuropharmacol. 2002, 25, 89–96. [Google Scholar] [CrossRef]
- Blandini, F.; Martignoni, E.; Pacchetti, C.; Desideri, S.; Rivellini, D.; Nappi, G. Simultaneous Determination of L-Dopa and 3-O-Methyldopa in Human Platelets and Plasma Using High-Performance Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B Biomed. Sci. Appl. 1997, 700, 278–282. [Google Scholar] [CrossRef]
- Wynne, A.; Finnerty, N. Ascorbic Acid Rejection Characteristics of Modified Platinum Electrodes: A Shelf Life Investigation. Chemosensors 2015, 3, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Kudur Jayaprakash, G.; Swamy, B.E.K.; Sánchez, J.P.M.; Li, X.; Sharma, S.C.; Lee, S.-L. Electrochemical and Quantum Chemical Studies of Cetylpyridinium Bromide Modified Carbon Electrode Interface for Sensor Applications. J. Mol. Liq. 2020, 315, 113719. [Google Scholar] [CrossRef]
- Müller, T.; Thiede, H.M. Bound, Free, and Total L-Dopa Measurement in Plasma of Parkinson’s Disease Patients. J. Neural. Transm. 2019, 126, 1417–1420. [Google Scholar] [CrossRef]
- Kujawska, M.; Bhardwaj, S.K.; Mishra, Y.K.; Kaushik, A. Using Graphene-Based Biosensors to Detect Dopamine for Efficient Parkinson’s Disease Diagnostics. Biosensors 2021, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Ma, Y.; Parajuli, R.R.; Balogun, Y.; Lai, W.Y.-C.; He, H. A Nonoxidative Sensor Based on a Self-Doped Polyaniline/Carbon Nanotube Composite for Sensitive and Selective Detection of the Neurotransmitter Dopamine. Anal. Chem. 2007, 79, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Jiang, X. A Facile One-Pot Synthesis of Copper Sulfide-Decorated Reduced Graphene Oxide Composites for Enhanced Detecting of H2O2 in Biological Environments. Anal. Chem. 2013, 85, 8095–8101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Cheng, C.; Liu, X.; Jiang, H.; Wang, X. Highly Sensitive Electrochemical Biosensor for Evaluation of Oxidative Stress Based on the Nanointerface of Graphene Nanocomposites Blended with Gold, Fe3O4, and Platinum Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 18441–18449. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Ultrasensitive and Selective Organic FET-Type Nonenzymatic Dopamine Sensor Based on Platinum Nanoparticles-Decorated Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2017, 9, 39526–39533. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.H.; Nolan, J.K.; Park, H.; Lam, S.; Fattah, M.; Page, J.C.; Joe, H.-E.; Jun, M.B.G.; Lee, H.; Kim, S.J.; et al. Facile Fabrication of Flexible Glutamate Biosensor Using Direct Writing of Platinum Nanoparticle-Based Nanocomposite Ink. Biosens. Bioelectron. 2019, 131, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Asif, M.; Azeem, M.; Ashraf, G.; Wang, Z.; Xiao, F.; Liu, H. Self-Stacking of Exfoliated Charged Nanosheets of LDHs and Graphene as Biosensor with Real-Time Tracking of Dopamine from Live Cells. Anal. Chim. Acta 2019, 1047, 197–207. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, Y.; Hao, Y.; Zhao, L.; Sun, S.; Zhang, Y.; Ye, B.; Xu, M. “Turn-on” Ratiometric Electrochemical Detection of H2O2 in One Drop of Whole Blood Sample via a Novel Microelectrode Sensor. Biosens. Bioelectron. 2020, 165, 112402. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, Y.; Shao, Y.; Li, B.; Wu, Y.; Zhang, W.; Zhou, Y.; Yu, Z.; Lu, L.; Wang, X.; et al. Solid-Phase Microextraction Integrated Nanobiosensors for the Serial Detection of Cytoplasmic Dopamine in a Single Living Cell. Biosens. Bioelectron. 2021, 175, 112915. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, X.; Zou, Z.; Yu, L.; Hu, F.; Li, Y.; Guo, C.; Li, C.M. Screen-Printed Analytical Strip Constructed with Bacteria-Templated Porous N-Doped Carbon Nanorods/Au Nanoparticles for Sensitive Electrochemical Detection of Dopamine Molecules. Biosens. Bioelectron. 2021, 186, 113303. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, G.; Suppa, A.; Mancinelli, R.; Belvisi, D.; Fabbrini, A.; Costanzo, M.; Formica, A.; Onori, P.; Fabbrini, G.; Berardelli, A. Salivary Alpha-Synuclein in the Diagnosis of Parkinson’s Disease and Progressive Supranuclear Palsy. Parkinsonism Relat. Disord. 2019, 63, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, G.; Latorre, A.; Suppa, A.; Nardi, M.; Pietracupa, S.; Mancinelli, R.; Fabbrini, G.; Colosimo, C.; Gaudio, E.; Berardelli, A. Abnormal Salivary Total and Oligomeric Alpha-Synuclein in Parkinson’s Disease. PLoS ONE 2016, 11, e0151156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonini, A.; Odin, P.; Pahwa, R.; Aldred, J.; Alobaidi, A.; Jalundhwala, Y.J.; Kukreja, P.; Bergmann, L.; Inguva, S.; Bao, Y.; et al. The Long-Term Impact of Levodopa/Carbidopa Intestinal Gel on ’Off’-Time in Patients with Advanced Parkinson’s Disease: A Systematic Review. Adv. Ther. 2021, 38, 2854–2890. [Google Scholar] [CrossRef]
- Murata, M.; Mihara, M.; Hasegawa, K.; Jeon, B.; Tsai, C.-H.; Nishikawa, N.; Oeda, T.; Yokoyama, M.; Robieson, W.Z.; Chatamra, K.; et al. Safety and Efficacy of Levodopa-Carbidopa Intestinal Gel: Results from an Open-Label Extension Study in Japanese, Korean and Taiwanese Patients with Advanced Parkinson’s Disease. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418759315. [Google Scholar] [CrossRef] [Green Version]
- Foltynie, T.; Magee, C.; James, C.; Webster, G.J.M.; Lees, A.J.; Limousin, P. Impact of Duodopa on Quality of Life in Advanced Parkinson’s Disease: A UK Case Series. Parkinson’s Dis. 2013, 2013, 362908. [Google Scholar] [CrossRef]
- Timpka, J.; Mundt-Petersen, U.; Odin, P. Continuous Dopaminergic Stimulation Therapy for Parkinson’s Disease—Recent Advances. Curr. Opin. Neurol. 2016, 29, 474–479. [Google Scholar] [CrossRef]
- Lee, K.H.; Lujan, J.L.; Trevathan, J.K.; Ross, E.K.; Bartoletta, J.J.; Park, H.O.; Paek, S.B.; Nicolai, E.N.; Lee, J.H.; Min, H.-K.; et al. WINCS Harmoni: Closed-Loop Dynamic Neurochemical Control of Therapeutic Interventions. Sci. Rep. 2017, 7, 46675. [Google Scholar] [CrossRef] [Green Version]
- Tokuçoğlu, F. Monitoring Physical Activity with Wearable Technologies. Noro. Psikiyatr. Ars. 2018, 55, S63–S65. [Google Scholar] [CrossRef]
- Suppa, A.; Kita, A.; Leodori, G.; Zampogna, A.; Nicolini, E.; Lorenzi, P.; Rao, R.; Irrera, F. L-DOPA and Freezing of Gait in Parkinson’s Disease: Objective Assessment through a Wearable Wireless System. Front. Neurol. 2017, 8, 406. [Google Scholar] [CrossRef]
- Zampogna, A.; Mileti, I.; Martelli, F.; Paoloni, M.; Del Prete, Z.; Palermo, E.; Suppa, A. Early Balance Impairment in Parkinson’s Disease: Evidence from Robot-Assisted Axial Rotations. Clin. Neurophysiol. 2021, 132, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Zampogna, A.; Mileti, I.; Palermo, E.; Celletti, C.; Paoloni, M.; Manoni, A.; Mazzetta, I.; Dalla Costa, G.; Pérez-López, C.; Camerota, F.; et al. Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders. Sensors 2020, 20, 3247. [Google Scholar] [CrossRef] [PubMed]
- Zampogna, A.; Manoni, A.; Asci, F.; Liguori, C.; Irrera, F.; Suppa, A. Shedding Light on Nocturnal Movements in Parkinson’s Disease: Evidence from Wearable Technologies. Sensors 2020, 20, 5171. [Google Scholar] [CrossRef]
- Yu, L.; Feng, L.; Xiong, L.; Li, S.; Xu, Q.; Pan, X.; Xiao, Y. Multifunctional Nanoscale Lanthanide Metal-Organic Framework Based Ratiometric Fluorescence Paper Microchip for Visual Dopamine Assay. Nanoscale 2021, 13, 11188–11196. [Google Scholar] [CrossRef] [PubMed]
- Hatcher-Martin, J.M.; Adams, J.L.; Anderson, E.R.; Bove, R.; Burrus, T.M.; Chehrenama, M.; Dolan O’Brien, M.; Eliashiv, D.S.; Erten-Lyons, D.; Giesser, B.S.; et al. Telemedicine in Neurology: Telemedicine Work Group of the American Academy of Neurology Update. Neurology 2020, 94, 30–38. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Ismail, Z.H.; Shapiai, M.I.; Yasin, M.N.M. Advancement in Biosensor: “Telediagnosis” and “Remote Digital Imaging”. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]

| Types of Biosensors | Measurement Methods | Sampling Methods | Fabrication Material | Fabrication Methods |
|---|---|---|---|---|
| Platforms | Impedance Potentiometry Amperometry (enzymatic) Voltammetry (non-enzymatic) | Vacuum Capillary Swelling | Polymers Silicon | Laser ablation Laser cutting |
| Solid MNs | Impedance Potentiometry Amperometry (enzymatic) Voltammetry (non-enzymatic) | Compression Absorption Vacuum | Silicon Ceramics Glass Metals | Laser ablation Laser cutting Casting Electroplating Lithography Wet and dry etching methods Metal injection molding Micromolding Two photon polymerization |
| Coated MNs | Impedance Potentiometry Amperometry (enzymatic) Voltammetry (non-enzymatic) | Capillary | Silicon Metals | Micromolding Dip coating Spray coating Layer-by-layer manufacturing |
| Dissolving MNs | Impedance Potentiometry Amperometry (enzymatic) Voltammetry (non-enzymatic) | Swelling | Cellulose Carbohydrates Sodium carboxymethyl | Mold based techniques Drawing lithography UV assisted fabrication Heat Droplet air blowing Fused deposition modeling Atomized spray process |
| Hollow MNs | Impedance Potentiometry Amperometry (enzymatic) Voltammetry (non-enzymatic) | Capillary Vacuum | Silicon Ceramics Polymers Glass Metals | MEMS Deep reactive ion etching Photolithographic Micromachining Pipette technique Deep X-ray lithography |
| Authors | Year | Type of Biosensor | Chemical Process | Experiment | Fluid | Biomarker | LODs |
|---|---|---|---|---|---|---|---|
| Ali et al. [43] | 2007 | poly (anilineboronic acid)/carbon nanotube composit | Dopamine oxidation | In vitro | Blood | Dopamine | - |
| Bai and Jiang [44] | 2013 | Copper sulfide-decorated reduced graphene oxide composites | CuS/RGO composite-based reaction | In vitro | - | H2O2 | - |
| Xu et al. [35] | 2015 | Cysteamine-graphene modified gold electrode nanocomposites | Carboxylic acid-induced covalent attachment | In vitro | Serum | α-synuclein | 1.2 pM |
| Wang et al. [45] | 2015 | Gold Fe3O4 Platinum Graphene-based nanocomposites | Catalytic reaction of Pt RGO/AuFe3O4-GCE | In vitro | Normal and tumor cells | H2O2 | 0.1 μM |
| Oh et al. [46] | 2017 | Organic field-effect-transistor-type nonenzymatic biosensor | Dopamine oxidation | In vivo | ISF | L-Dopa | 10 pM |
| Goud et al. [15] | 2019 | Orthogonal electrochemical/ biocatalytic hollow MN | Dopamine oxidation | In vivo/In vitro | ISF | L-Dopa | - |
| Nguyen et al. [47] | 2019 | Platinum-based nanocomposite | Glutamate oxidation | In vitro | Spinal cord sample | Glutamate | 0.2–0.5 μM |
| Aziz et al. 2019 [48] | 2019 | LDHs and graphene-based nanocomposite | Dopamine oxidation | In vitro | Living cells | Dopamine | 2.0 nM |
| Dong et al. [49] | 2020 | 5-(1,2-dithiolan-3-yl)-N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) phenyl) pent-anamide | One-step amide reaction | In vitro | Blood | H2O2 | 0.02 μM |
| Chang et al. [50] | 2021 | Nanobiosensor integrated with solid-phase microextractiontechnique | Dopamine oxidation | In vitro | Cytoplasm of single living cell | Dopamine | 10 pM |
| Moon et al. [25] | 2021 | Wearable electrochemical platform | L-Dopa oxidation | In vivo/In vitro | Sweat/Blood | L-Dopa | - |
| Shi et al. [51] | 2021 | N-doped carbon nanorods and Au nanoparticles based biosensor | Dopamine oxidation | In vivo | Serum | Dopamine | - |
| Kudur-Jayaprakash et al. [40] | 2021 | Cetyl pyridinium bromide (CPB) modified carbon paste electrode (CPBMCPE) biosensor | Dopamine/Uric Acid-Voltammetric oxidation | In vivo | Urine | Dopamine/Uric Acid | 38–42 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asci, F.; Vivacqua, G.; Zampogna, A.; D’Onofrio, V.; Mazzeo, A.; Suppa, A. Wearable Electrochemical Sensors in Parkinson’s Disease. Sensors 2022, 22, 951. https://doi.org/10.3390/s22030951
Asci F, Vivacqua G, Zampogna A, D’Onofrio V, Mazzeo A, Suppa A. Wearable Electrochemical Sensors in Parkinson’s Disease. Sensors. 2022; 22(3):951. https://doi.org/10.3390/s22030951
Chicago/Turabian StyleAsci, Francesco, Giorgio Vivacqua, Alessandro Zampogna, Valentina D’Onofrio, Adolfo Mazzeo, and Antonio Suppa. 2022. "Wearable Electrochemical Sensors in Parkinson’s Disease" Sensors 22, no. 3: 951. https://doi.org/10.3390/s22030951
APA StyleAsci, F., Vivacqua, G., Zampogna, A., D’Onofrio, V., Mazzeo, A., & Suppa, A. (2022). Wearable Electrochemical Sensors in Parkinson’s Disease. Sensors, 22(3), 951. https://doi.org/10.3390/s22030951










