Generation and Characterization of Stable Redox-Reporter Mammalian Cell Lines of Biotechnological Relevance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Growth and Metabolite Analysis
2.4. hGM-CSF Activity
2.5. Generation of Expression Vectors
2.6. Generation of Stable Redox Reporter Cell Lines
2.7. Flow Cytometry Analysis
2.8. Confocal Microscopy
2.9. Physiological Assays
2.9.1. Dose–Response Curve for H2O2
2.9.2. Treatment with rhGM-CSF
2.9.3. Glucose and FBS Deprivation
2.10. Redox Sensor Calibration
2.11. Statistical Analysis
3. Results
3.1. Generation of Transgenic Cell Lines Expressing the Redox Biosensor
3.2. The Redox Reporter Cell Lines Respond in a Dynamic and Sensitive Fashion to Redox Stimuli
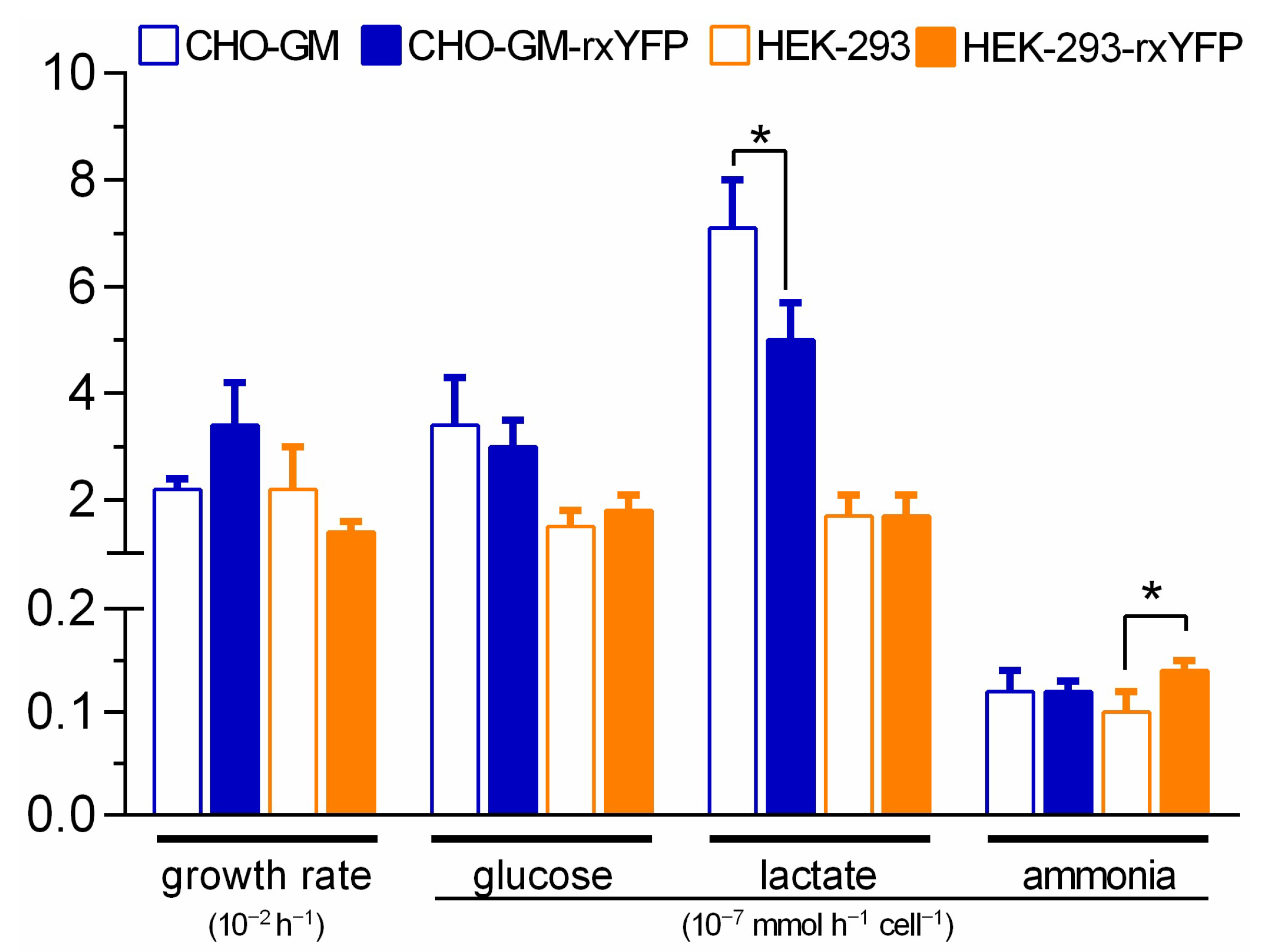
3.3. The Expression of the Redox Biosensor Does Not Affect the Phenotype of the Host Cells
3.4. Monitoring Intracellular Redox Changes in CHO-GM-rxYFP Batch Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian Cell Culture for Production of Recombinant Proteins: A Review of the Critical Steps in their Biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Takagi, Y.; Kikuchi, T.; Wada, R.; Omasa, T. The Enhancement of Antibody Concentration and Achievement of High Cell Density CHO Cell Cultivation by Adding Nucleoside. Cytotechnology 2017, 69, 511–521. [Google Scholar] [CrossRef]
- Tihanyi, B.; Nyitray, L. Recent Advances in CHO Cell Line Development for Recombinant Protein Production. Drug Discov. Today Technol. 2020, 38, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, V.; Andersen, M.R.; Malphettes, L. Oxidative Stress-Alleviating Strategies to Improve Recombinant Protein Production in CHO Cells. Biotechnol. Bioeng. 2019, 117, 1172–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinterkörner, G.; Brugger, G.; Müller, D.; Hesse, F.; Kunert, R.; Katinger, H.; Borth, N. Improvement of the Energy Metabolism of Recombinant CHO Cells by Cell Sorting for Reduced Mitochondrial Membrane Potential. J. Biotechnol. 2007, 129, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Follstad, B.D.; Wang, D.I.C.; Stephanopoulos, G. Mitochondrial Membrane Potential Selects Hybridomas Yielding High Viability in Fed-Batch Cultures. Biotechnol. Prog. 2002, 18, 1–5. [Google Scholar] [CrossRef]
- Johansson, C.; Holmgren, A.; Berndt, C.; Lönn, M.; Hudemann, C.; Lillig, C.H. Thiol Redox Control via Thioredoxin and Glutaredoxin Systems. Biochem. Soc. Trans. 2005, 33, 1375–1377. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.; Holmgren, A. Glutaredoxins: Glutathione-Dependent Redox Enzymes with Functions Far Beyond a Simple Thioredoxin Backup System. Antioxid. Redox Signal. 2004, 6, 63–74. [Google Scholar] [CrossRef]
- Ogata, F.T.; Branco, V.; Vale, F.F.; Coppo, L. Glutaredoxin: Discovery, Redox Defense and Much More. Redox Biol. 2021, 43, 101975. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione Catalysis and the Reaction Mechanisms of Glutathione-Dependent Enzymes. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DePonte, M. The Incomplete Glutathione Puzzle: Just Guessing at Numbers and Figures? Antioxid. Redox Signal. 2017, 27, 1130–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, F.Q.; Buettner, G. Redox Environment of the Cell as Viewed through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Go, Y.-M.; Jones, D.P. Redox Compartmentalization in Eukaryotic Cells. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [Green Version]
- Montero, D.; Tachibana, C.; Winther, J.R.; Appenzeller-Herzog, C. Intracellular Glutathione Pools Are Heterogeneously Concentrated. Redox Biol. 2013, 1, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Comini, M.A. Measurement and Meaning of Cellular Thiol: Disufhide Redox Status. Free Radic. Res. 2016, 50, 246–271. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, H.; Henriksen, A.; Hansen, F.G.; Winther, J.R. Shedding Light on Disulfide Bond Formation: Engineering a Redox Switch in Green Fluorescent Protein. EMBO J. 2001, 20, 5853–5862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dooley, C.T.; Dore, T.M.; Hanson, G.T.; Jackson, W.C.; Remington, S.J.; Tsien, R.Y. Imaging Dynamic Redox Changes in Mammalian Cells with Green Fluorescent Protein Indicators. J. Biol. Chem. 2004, 279, 22284–22293. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Ai, H.-W. Development of Redox-Sensitive Red Fluorescent Proteins for Imaging Redox Dynamics in Cellular Compartments. Anal. Bioanal. Chem. 2016, 408, 2901–2911. [Google Scholar] [CrossRef]
- Piattoni, C.V.; Sardi, F.; Klein, F.; Pantano, S.; Bollati-Fogolin, M.; Comini, M. New Red-Shifted Fluorescent Biosensor for Monitoring Intracellular Redox Changes. Free Radic. Biol. Med. 2019, 134, 545–554. [Google Scholar] [CrossRef]
- Meyer, A.J.; Dick, T.P. Fluorescent Protein-Based Redox Probes. Antioxid. Redox Signal. 2010, 13, 621–650. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Dick, T.P.; Meyer, A.J.; Morgan, B. Dissecting Redox Biology Using Fluorescent Protein Sensors. Antioxid. Redox Signal. 2016, 24, 680–712. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Petsko, G.A.; Liu, C.F. Crystal Structure of Green Fluorescent Protein Clover and Design of Clover-Based Redox Sensors. Structure 2018, 26, 225–237.e3. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Makar, M.; Wang, M.X.; Ai, H. Monitoring Thioredoxin Redox with a Genetically Encoded Red Fluorescent Biosensor. Nat. Chem. Biol. 2017, 13, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseler, A.; Dhalleine, T.; Rouhier, N.; Couturier, J. Arabidopsis Thaliana 3-Mercaptopyruvate Sulfurtransferases Interact with and are Protected by Reducing Systems. J. Biol. Chem. 2021, 296, 100429. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and Colorimetric Probes for Detection of Thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef]
- Conte, M.L.; Carroll, K.S. The Chemistry of Thiol Oxidation and Detection; Springer: Dordrecht, The Netherlands, 2013; pp. 1–42. [Google Scholar] [CrossRef]
- Peng, H.; Chen, W.; Cheng, Y.; Hakuna, L.; Strongin, R.; Wang, B. Thiol Reactive Probes and Chemosensors. Sensors 2012, 12, 15907–15946. [Google Scholar] [CrossRef] [Green Version]
- Winther, J.R.; Thorpe, C. Quantification of Thiols and Disulfides. Biochim. Biophys. Acta-Gen. Subj. 2013, 1840, 838–846. [Google Scholar] [CrossRef] [Green Version]
- Lalonde, M.-E.; Durocher, Y. Therapeutic Glycoprotein Production in Mammalian Cells. J. Biotechnol. 2017, 251, 128–140. [Google Scholar] [CrossRef]
- Bandaranayake, A.D.; Almo, S.C. Recent Advances in Mammalian Protein Production. FEBS Lett. 2014, 588, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Durocher, Y. High-Level and High-tThroughput Recombinant Protein Production by Transient Transfection of Suspension-Growing Human 293-EBNA1 Cells. Nucleic Acids Res. 2002, 30, E9. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Chin, C.S.H.; Lim, Z.F.S.; Ng, S.K. HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front. Bioeng. Biotechnol. 2021, 9, 796991. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D. The Granulocyte-Macrophage Colony-Stimulating Factors. Science 1985, 229, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.O. Emerging Applications of Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor. Blood 1998, 92, 4491–4508. [Google Scholar] [CrossRef] [PubMed]
- Forno, G.; Bollati-Fogolín, M.; Oggero, M.; Kratje, R.; Etcheverrigaray, M.; Conradt, H.S.; Nimtz, M. N- and O-Linked Carbohydrates and Glycosylation Site Occupancy in Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor Secreted by a Chinese Hamster Ovary Cell Line. JBIC J. Biol. Inorg. Chem. 2004, 271, 907–919. [Google Scholar] [CrossRef]
- Berthelot, M.P.E. Berthelot’s Reaction Mechanism. Rep. Chim. Appl. 1859, 2884. [Google Scholar]
- Weisbart, R.; Kwan, L.; Golde, D.; Gasson, J. Human GM-CSF Primes Neutrophils for Enhanced Oxidative Metabolism in Response to the Major Physiological Chemoattractants. Blood 1987, 69, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Selvarasu, S.; Ho, Y.S.; Chong, W.P.K.; Wong, N.S.C.; Yusufi, F.N.K.; Lee, Y.Y.; Yap, M.G.S.; Lee, D.-Y. Combined in Silico Modeling and Metabolomics Analysis to Characterize Fed-Batch CHO Cell Culture. Biotechnol. Bioeng. 2012, 109, 1415–1429. [Google Scholar] [CrossRef]
- López-Mirabal, H.R.; Winther, J.R. Redox Characteristics of the Eukaryotic Cytosol. Biochim. Biophys Acta 2008, 1783, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Banach-Latapy, A.; He, T.; Dardalhon, M.; Vernis, L.; Chanet, R.; Huang, M.-E. Redox-Sensitive YFP Sensors for Monitoring Dynamic Compartment-Specific Glutathione Redox State. Free Radic. Biol. Med. 2013, 65, 436–445. [Google Scholar] [CrossRef]
- Huang, B.K.; Sikes, H.D. Quantifying Intracellular Hydrogen Peroxide Perturbations in Terms of Concentration. Redox Biol. 2014, 2, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.R.; Yang, S. Hydrogen Peroxide: A Signaling Messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.P.K.; Thng, S.H.; Hiu, A.P.; Lee, D.-Y.; Chan, E.C.Y.; Ho, Y.S. LC-MS-Based Metabolic Characterization of High Monoclonal Antibody-Producing Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2012, 109, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Orellana, C.A.; Marcellin, E.; Schulz, B.L.; Nouwens, A.S.; Gray, P.P.; Nielsen, L.K. High-Antibody-Producing Chinese Hamster Ovary Cells Up-Regulate Intracellular Protein Transport and Glutathione Synthesis. J. Proteome Res. 2015, 14, 609–618. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Raducanu, D.; Gloriant, T.; Gordin, D.M.; Cinca, I. Effects of Cold-Rolling Deformation on Texture Evolution and Mechanical Properties of Ti–29Nb–9Ta–10Zr Alloy. Mater. Sci. Eng. 2013, 586, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Makino, N.; Sasaki, K.; Hashida, K.; Sakakura, Y. A Metabolic Model Describing the H2O2 Elimination by Mammalian Cells Including H2O2 Permeation through Cytoplasmic and Peroxisomal Membranes: Comparison with Experimental Data. Biochim. Biophys. Acta Gen. Subj. 2004, 1673, 149–159. [Google Scholar] [CrossRef]
- Björnberg, O.; Østergaard, H.; Winther, J.R. Mechanistic Insight Provided by Glutaredoxin within a Fusion to Redox-Sensitive Yellow Fluorescent Protein. Biochemistry 2006, 45, 2362–2371. [Google Scholar] [CrossRef]
- Gutscher, M.; Pauleau, A.-L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-Time Imaging of the Intracellular Glutathione Redox Potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef]
- Maulucci, G.; Labate, V.; Mele, M.; Panieri, E.; Arcovito, G.; Galeotti, T.; Østergaard, H.; Winther, J.R.; De Spirito, M.; Pani, G. High-Resolution Imaging of Redox Signaling in Live Cells Through an Oxidation-Sensitive Yellow Fluorescent Protein. Sci. Signal. 2008, 1, pl3. [Google Scholar] [CrossRef]
- Kyriakopoulos, S.; Polizzi, K.M.; Kontoravdi, C. Dynamic Profiling of Amino Acid Transport and Metabolism in Chinese Hamster Ovary Cell Culture. BMC Proc. 2013, 7, P97. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, N.; Dickman, M.B.; Becker, D.F. Proline Modulates the Intracellular Redox Environment and Protects Mammalian Cells against Oxidative Stress. Free Radic. Biol. Med. 2008, 44, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowaloff, E.M.; Phang, J.M.; Granger, A.S.; Downing, S.J. Regulation of Proline Oxidase Activity by Lactate. Proc. Natl. Acad. Sci. USA 1977, 74, 5368–5371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donald, S.P.; Sun, X.Y.; Hu, C.A.; Yu, J.; Mei, J.M.; Valle, D.; Phang, J.M. Proline Oxidase, Encoded by p53-Induced Gene-6, Catalyzes the Generation of Proline-Dependent Reactive Oxygen Species. Cancer Res. 2001, 61, 1810–1815. [Google Scholar]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Templeton, N.; Dean, J.; Reddy, P.; Young, J.D. Peak Antibody Production Is Associated with Increased Oxidative Metabolism in an Industrially Relevant Fed-Batch CHO Cell Culture. Biotechnol. Bioeng. 2013, 110, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.A.; Emborg, C. Extra- and Intracellular Amino Acid Concentrations in Continuous Chinese Hamster Ovary Cell Culture. Appl. Microbiol. Biotechnol. 1994, 41, 560–564. [Google Scholar] [CrossRef]
- Hill, C.P.; Osslund, T.D.; Eisenberg, D. The Structure of Granulocyte-Colony-Stimulating Factor and its Relationship to other Growth Factors. Proc. Natl. Acad. Sci. USA 1993, 90, 5167–5171. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthi, S.; Jessop, C.E.; Bulleid, N.J. The Role of Glutathione in Disulphide Bond Formation and Endoplasmic-Reticulum-Generated Oxidative Stress. EMBO Rep. 2006, 7, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Altamirano, C.; Paredes, C.; Illanes, A.; Cairó, J.; Gòdia, F. Strategies for Fed-Batch Cultivation of t-PA Producing CHO Cells: Substitution of Glucose and Glutamine and Rational Design of Culture Medium. J. Biotechnol. 2004, 110, 171–179. [Google Scholar] [CrossRef]
- Altamirano, C.; Illanes, A.; Becerra, S.; Cairó, J.J.; Gòdia, F. Considerations on the Lactate Consumption by CHO Cells in the Presence of Galactose. J. Biotechnol. 2006, 125, 547–556. [Google Scholar] [CrossRef]
- Becker, M.; Junghans, L.; Teleki, A.; Bechmann, J.; Takors, R. The Less the Better: How Suppressed Base Addition Boosts Production of Monoclonal Antibodies with Chinese Hamster Ovary Cells. Front. Bioeng. Biotechnol. 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Fogolı́n, M.B.; Wagner, R.; Etcheverrigaray, M.; Kratje, R. Impact of Temperature Reduction and Expression of Yeast Pyruvate Carboxylase on hGM-CSF-Producing CHO Cells. J. Biotechnol. 2004, 109, 179–191. [Google Scholar] [CrossRef]
- Dorai, H.; Kyung, Y.S.; Ellis, D.; Kinney, C.; Lin, C.; Jan, D.; Moore, G.; Betenbaugh, M.J. Expression of Anti-Apoptosis Genes Alters Lactate Metabolism of Chinese Hamster Ovary Cells in Culture. Biotechnol. Bioeng. 2009, 103, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Bulté, D.B.; Palomares, L.A.; Parra, C.G.; Martínez, J.A.; Contreras, M.A.; Noriega, L.G.; Ramírez, O.T. Overexpression of the Mitochondrial Pyruvate Carrier Reduces Lactate Production and Increases Recombinant Protein Productivity in CHO Cells. Biotechnol. Bioeng. 2020, 117, 2633–2647. [Google Scholar] [CrossRef]
- Orellana, C.A.; Marcellin, E.; Gray, P.P.; Nielsen, L.K. Overexpression of the Regulatory Subunit of Glutamate-Cysteine Ligase Enhances Monoclonal Antibody Production in CHO Cells. Biotechnol. Bioeng. 2017, 114, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, V.; Schoof, E.M.; Malphettes, L.; Andersen, M.R.; Workman, C.T. Characterization of Glutathione Proteome in CHO Cells and its Relationship with Productivity and Cholesterol Synthesis. Biotechnol. Bioeng. 2020, 117, 3448–3458. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.P.; Yusufi, F.N.; Lee, D.-Y.; Reddy, S.G.; Wong, N.S.; Heng, C.K.; Yap, M.G.; Ho, Y.S. Metabolomics-Based Identification of Apoptosis-Inducing Metabolites in Recombinant Fed-Batch CHO Culture Media. J. Biotechnol. 2011, 151, 218–224. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelmuter, K.; Tiscornia, I.; Comini, M.A.; Bollati-Fogolín, M. Generation and Characterization of Stable Redox-Reporter Mammalian Cell Lines of Biotechnological Relevance. Sensors 2022, 22, 1324. https://doi.org/10.3390/s22041324
Perelmuter K, Tiscornia I, Comini MA, Bollati-Fogolín M. Generation and Characterization of Stable Redox-Reporter Mammalian Cell Lines of Biotechnological Relevance. Sensors. 2022; 22(4):1324. https://doi.org/10.3390/s22041324
Chicago/Turabian StylePerelmuter, Karen, Inés Tiscornia, Marcelo A. Comini, and Mariela Bollati-Fogolín. 2022. "Generation and Characterization of Stable Redox-Reporter Mammalian Cell Lines of Biotechnological Relevance" Sensors 22, no. 4: 1324. https://doi.org/10.3390/s22041324
APA StylePerelmuter, K., Tiscornia, I., Comini, M. A., & Bollati-Fogolín, M. (2022). Generation and Characterization of Stable Redox-Reporter Mammalian Cell Lines of Biotechnological Relevance. Sensors, 22(4), 1324. https://doi.org/10.3390/s22041324






