A Smart System for the Contactless Measurement of Energy Expenditure
Abstract
:1. Introduction
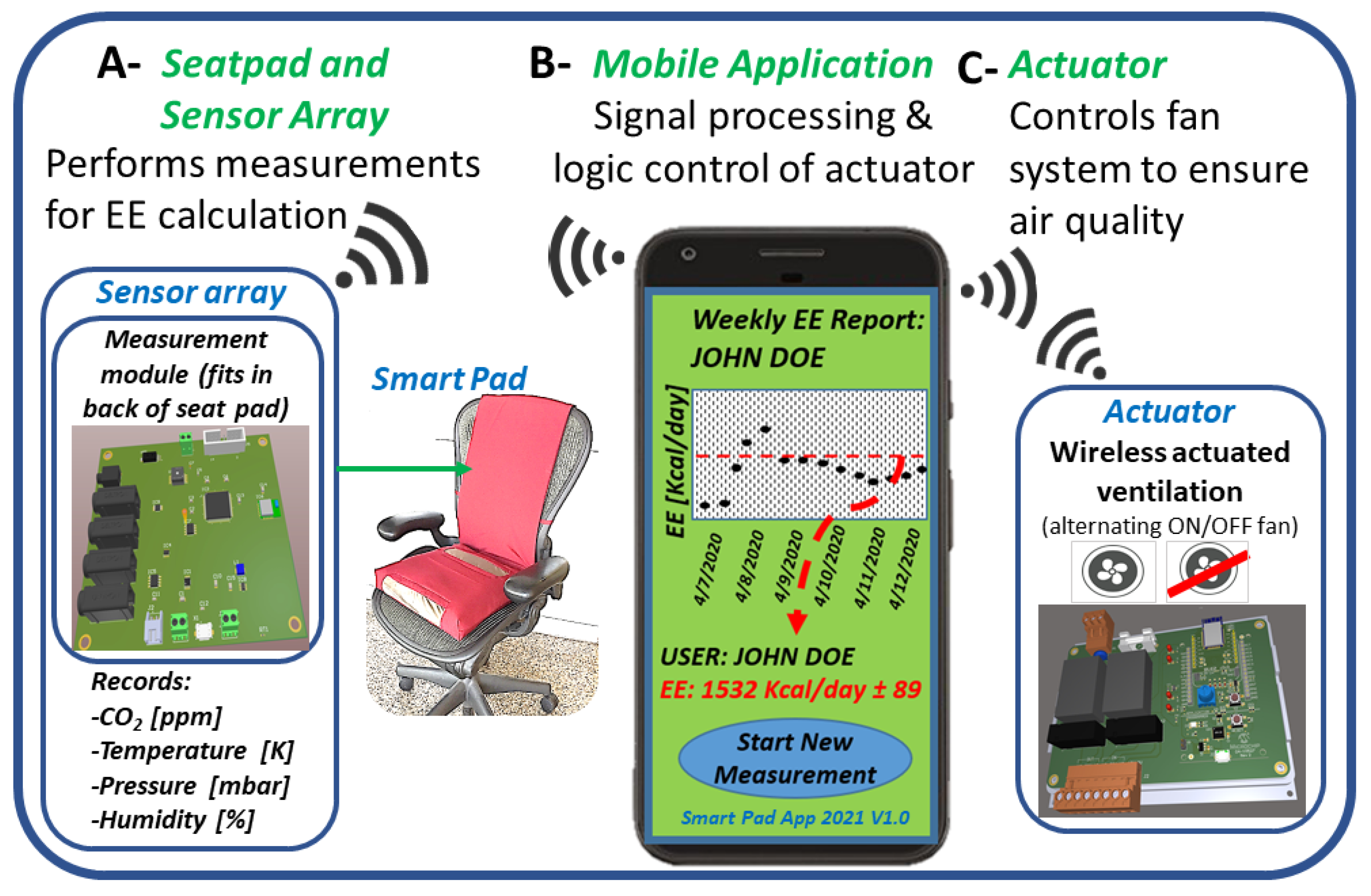
2. Materials and Methods
2.1. Smart Pad System Operating Equations and Measurement Technique
2.2. Smart Pad: Physical Characteristics, Design, and Testing Protocol
2.3. CO2 Measurements for REE Assessment
2.4. CO2 Measurements for Exercise Energy Expenditure Assessment
2.5. CO2 Measurements in Subjects of Varying Body Mass Index and REE
2.6. Evaluative Study of Room Air Exchange Rate (λAcc vs. λo)
2.7. Data Analysis
3. Results and Discussion
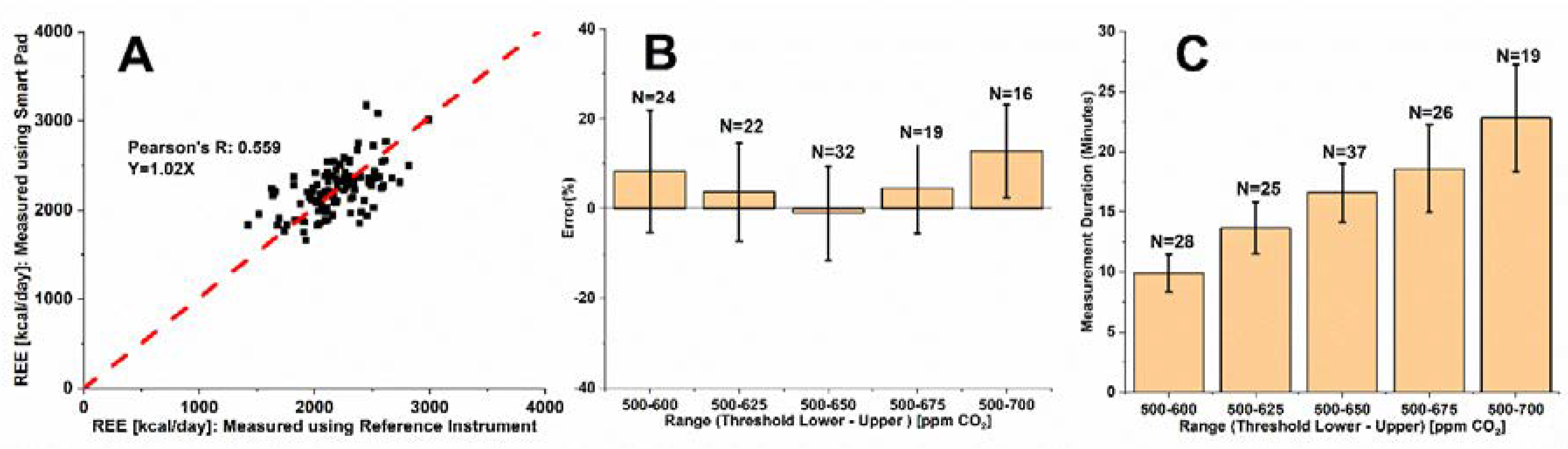
3.1. Optimization of CO2 Measurement Range for REE Assessment—Single Subject Study
3.2. Optimization of CO2 Measurement Range for Exercise Energy Expenditure Assessment—Single Subject Study
3.3. Smart Pad REE Assessment–Multiple Subject Study
3.4. Smart Pad REE Assessment—New Smart Pad System Model
3.4.1. Evaluation of Air Exchange Rate Assessment from Decay Model (λo) vs. Accumulation Model (λAcc)
3.4.2. New Smart Pad System’s Model Validation
| Indirect Calorimeter Medical Device (FDA Authorized for Prescription Use) | Single Breath Gas, VCO2 or VO2 (mL/min), Measurement Accuracy | Regulatory Considerations | Anatomical Contact Sites |
|---|---|---|---|
| Vyaire MasterScreen CPXTM | ±50 mL/min (VCO2) [63] | 510(k) Cleared [63] | Face |
| Vyaire Oxycon ProTM | ±50 mL/min (VCO2) [64] | 510(k) Cleared [64] | Entire head |
| Vyaire Vyntus CPXTM | ±50 mL/min (VCO2) [65] | 510(k) Cleared [65] | Entire head + torso |
| Vyaire Oxycon MobileTM | ±50 mL/min (VCO2) [66] | 510(k) Cleared [66] | Face |
| Microlife MedGemTM | Y = 0.83X (R = 0.81) (VO2) [67] | 510(k) Cleared [67] | Mouth |
| MGC Ultima CPXTM Indirect Calorimeter | ±3% (exhalation rate) | [53,68] Cleared | Face |
| Smart Pad: 14–19 Minute Measurement | ±45 mL/min (VCO2) | Meets 510(k) Standard | N/A (contactless) |
| Smart Pad: 14–19 Minute Measurement | y = 1.05x (R = 0.82) (VCO2) | Meets 510(k) Standard | N/A (contactless) |
4. Conclusions
5. Patent and Competing Interest Statement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity among Adults and Youth: United States, 2015–2016; National Center for Health Statistics: Hyattsville, MD, USA, 2017. [Google Scholar]
- De Gonzalez, A.B.; Hartge, P.; Cerhan, J.; Flint, A.J.; Hannan, L.; MacInnis, R.; Moore, S.; Tobias, G.; Anton-Culver, H.; Freeman, L.B.; et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.; Allison, D. Years of Life Lost Due to Obesity. JAMA J. Am. Med. Assoc. 2003, 289, 187–193. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, H.R. Update on treatment strategies for obesity. J. Clin. Endocrinol. Metab. 2013, 98, 1299–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passmore, R.; Durnin, J.V.G.A. Human Energy Expenditure. Physiol. Rev. 1955, 35, 801–840. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A. Measurement of energy expenditure. Public Health Nutr. 2005, 8, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988, 37, 287–301. [Google Scholar] [CrossRef]
- Haugen, H.A.; Chan, L.-N.; Li, F. Indirect Calorimetry: A Practical Guide for Clinicians. Nutr. Clin. Pract. 2007, 22, 377–388. [Google Scholar] [CrossRef]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Frankenfield, D.C. Bias and accuracy of resting metabolic rate equations in non-obese and obese adults. Clin. Nutr. 2013, 32, 976–982. [Google Scholar] [CrossRef]
- Mifflin, M.D.; Jeor, S.T.S.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Yue, D.; Deng, Y.; Scott, B.J. Comparison of Resting Metabolic Rates: Calculated using predictive equation and measured using Portable Indirect Calorimeter. Glob. J. Obes. Diabetes Metab. Syndr. 2019, 6, 010–016. [Google Scholar] [CrossRef] [Green Version]
- Cancello, R.; Soranna, D.; Brunani, A.; Scacchi, M.; Tagliaferri, A.; Mai, S.; Marzullo, P.; Zambon, A.; Invitti, C. Analysis of Predictive Equations for Estimating Resting Energy Expenditure in a Large Cohort of Morbidly Obese Patients. Front. Endocrinol. 2018, 9, 367. [Google Scholar] [CrossRef]
- Hasson, R.E.; Howe, C.A.; Jones, B.L.; Freedson, P.S. Accuracy of four resting metabolic rate prediction equations: Effects of sex, body mass index, age, and race/ethnicity. J. Sci. Med. Sport 2011, 14, 344–351. [Google Scholar] [CrossRef]
- Purcell, S.A.; Elliott, S.A.; Baracos, V.E.; Chu, Q.S.C.; Sawyer, M.B.; Mourtzakis, M.; Easaw, J.C.; Spratlin, J.L.; Siervo, M.; Prado, C.M. Accuracy of Resting Energy Expenditure Predictive Equations in Patients with Cancer. Nutr. Clin. Pract. 2019, 34, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Bertoli, S.; Battezzati, A.; Wells, J.; Lara, J.; Ferraris, C.; Tagliabue, A. Accuracy of predictive equations for the measurement of resting energy expenditure in older subjects. Clin. Nutr. 2014, 33, 613–619. [Google Scholar] [CrossRef]
- Chowdhury, E.A.; Western, M.J.; Nightingale, T.E.; Peacock, O.J.; Thompson, D. Assessment of laboratory and daily energy expenditure estimates from consumer multi-sensor physical activity monitors. PLoS ONE 2017, 12, e0171720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonson, D.C.; DeFronzo, R.A. Indirect calorimetry: Methodological and interpretative problems. Am. J. Physiol. Metab. 1990, 258, E399–E412. [Google Scholar] [CrossRef] [PubMed]
- Black, A.; Cole, T. Within- and between-subject variation in energy expenditure measured by the doubly-labelled water technique: Implications for validating reported dietary energy intake. Eur. J. Clin. Nutr. 2000, 54, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, J.A.; Watras, A.C.; O’Brien, M.J.; Luke, A.; Dobratz, J.R.; Earthman, C.P.; Schoeller, D.A. Assessing Validity and Reliability of Resting Metabolic Rate in Six Gas Analysis Systems. J. Am. Diet. Assoc. 2009, 109, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Grunwald, G.K.; Melanson, E.L.; Forster, J.E.; Seagle, H.M.; Sharp, T.A.; Hill, J.O. Comparison of Methods for Achieving 24-Hour Energy Balance in a Whole-Room Indirect Calorimeter. Obes. Res. 2003, 11, 752–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rising, R.; Whyte, K.J.; Albu, J.B.; Pi-Sunyer, X. Evaluation of a new whole room indirect calorimeter specific for measurement of resting metabolic rate. Nutr. Metab. 2015, 12, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.Y.; Smith, S.; Ravussin, E.; Krakoff, J.; Plasqui, G.; Tanaka, S.; Murgatroyd, P.; Brychta, R.; Bock, C.; Carnero, E.; et al. Room Indirect Calorimetry Operating and Reporting Standards (RICORS 1.0): A Guide to Conducting and Reporting Human Whole-Room Calorimeter Studies. Obesity 2020, 28, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, I.; Sprowls, M.; Deng, Y.; Kulick, D.; Destaillats, H.; Forzani, E.S. Assessing metabolic rate and indoor air quality with passive environmental sensors. J. Breath Res. 2018, 12, 036012. [Google Scholar] [CrossRef] [PubMed]
- Sprowls, M.; Victor, S.; Serhan, M.; Destaillats, H.; Wheatley-Guy, C.; Johnson, B.D.; Kulick, D.; Forzani, E.S. A system for contact free energy expenditure assessment under free-living conditions: Monitoring metabolism for weight loss using carbon dioxide emission. J. Breath Res. 2020, 15, 026004. [Google Scholar] [CrossRef] [PubMed]
- Haghi, M.; Neubert, S.; Geissler, A.; Fleischer, H.; Stoll, N.; Stoll, R.; Thurow, K. A Flexible and Pervasive IoT-Based Healthcare Platform for Physiological and Environmental Parameters Monitoring. IEEE Internet Things J. 2020, 7, 5628–5647. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Chou, E.-F.; Le, J.; Wong, S.; Chu, M.; Khine, M. Soft Wearable Pressure Sensors for Beat-to-Beat Blood Pressure Monitoring. Adv. Healthc. Mater. 2019, 8, 1900109. [Google Scholar] [CrossRef]
- Liu, N.-Y.; Cay-Durgun, P.; Lai, T.; Sprowls, M.; Thomas, L.; Lind, M.L.; Forzani, E. A Handheld, Colorimetric Optoelectronic Dynamics Analyzer for Measuring Total Ammonia of Biological Samples. IEEE J. Transl. Eng. Health Med. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Chan, J.; Rea, T.; Gollakota, S.; Sunshine, J.E. Contactless cardiac arrest detection using smart devices. Npj Digit. Med. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Deng, Y.; Sprowls, M.; Mora, S.J.; Kulick, D.; Tao, N.; Destaillats, H.; Forzani, E. An Unobstructive Sensing Method for Indoor Air Quality Optimization and Metabolic Assessment within Vehicles. Sensors 2020, 20, 7202. [Google Scholar] [CrossRef]
- Kamshilin, A.A.; Sidorov, I.S.; Babayan, L.; Volynsky, M.A.; Giniatullin, R.; Mamontov, O.V. Accurate measurement of the pulse wave delay with imaging photoplethysmography. Biomed. Opt. Express 2016, 7, 5138–5147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.R.; An, S.; Hwang, J. An integrated system of air sampling and simultaneous enrichment for rapid biosensing of airborne coronavirus and influenza virus. Biosens. Bioelectron. 2020, 170, 112656. [Google Scholar] [CrossRef]
- Mercuri, M.; Lorato, I.R.; Liu, Y.-H.; Wieringa, F.; Van Hoof, C.; Torfs, T. Vital-sign monitoring and spatial tracking of multiple people using a contactless radar-based sensor. Nat. Electron. 2019, 2, 252–262. [Google Scholar] [CrossRef]
- Shao, D.; Liu, C.; Tsow, F.; Yang, Y.; Du, Z.; Iriya, R.; Yu, H.; Tao, N. Noncontact Monitoring of Blood Oxygen Saturation Using Camera and Dual-Wavelength Imaging System. IEEE Trans. Biomed. Eng. 2016, 63, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Yang, Y.; Liu, C.; Tsow, F.; Yu, H.; Tao, N. Noncontact Monitoring Breathing Pattern, Exhalation Flow Rate and Pulse Transit Time. IEEE Trans. Biomed. Eng. 2014, 61, 2760–2767. [Google Scholar] [CrossRef]
- Dascalaki, E.G.; Lagoudi, A.; Balaras, C.A.; Gaglia, A.G. Air quality in hospital operating rooms. Build. Environ. 2008, 43, 1945–1952. [Google Scholar] [CrossRef]
- Yang, W.; Sohn, J.; Kim, J.; Son, B.; Park, J. Indoor air quality investigation according to age of the school buildings in Korea. J. Environ. Manag. 2009, 90, 348–354. [Google Scholar] [CrossRef]
- Bakó-Biró, Z.; Clements-Croome, D.; Kochhar, N.; Awbi, H.; Williams, M. Ventilation rates in schools and pupils’ performance. Build. Environ. 2012, 48, 215–223. [Google Scholar] [CrossRef]
- Bluyssen, P.M.; Fernandes, E.O.; Groes, L.; Clausen, G.; Fanger, P.O.; Bernhard, C.A.; Roulet, C.A.; Valbjørn, O. European Indoor Air Quality Audit Project in 56 Office Buildings. Indoor Air 1996, 6, 221–238. [Google Scholar] [CrossRef]
- Redlich, C.A.; Sparer, J.; Cullen, M.R. Sick-building syndrome. Lancet 1997, 349, 1013–1016. [Google Scholar] [CrossRef]
- Batterman, S. Review and Extension of CO2-Based Methods to Determine Ventilation Rates with Application to School Classrooms. Int. J. Environ. Res. Public Health 2017, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, O.; Mandin, C.; Ribéron, J.; Wyart, G. Air Stuffiness and Air Exchange Rate in French Schools and Day-Care Centres. Int. J. Vent. 2013, 12, 175–180. [Google Scholar] [CrossRef]
- Turanjanin, V.; Vucicevic, B.; Jovanovic, M.; Mirkov, N.; Lazović, I. Indoor CO2 measurements in Serbian schools and ventilation rate calculation. Energy 2014, 77, 290–296. [Google Scholar] [CrossRef]
- Claude-Alain, R.; Foradini, F. Simple and Cheap Air Change Rate Measurement Using CO2Concentration Decays. Int. J. Vent. 2002, 1, 39–44. [Google Scholar] [CrossRef]
- Cui, S.; Cohen, M.; Stabat, P.; Marchio, D. CO2 tracer gas concentration decay method for measuring air change rate. Build. Environ. 2015, 84, 162–169. [Google Scholar] [CrossRef]
- Gall, E.T.; Mishra, A.K.; Li, J.; Schiavon, S.; Laguerre, A. Impact of Cognitive Tasks on CO2 and Isoprene Emissions from Humans. Environ. Sci. Technol. 2021, 55, 139–148. [Google Scholar] [CrossRef]
- Tans, P.; Keeling, R. Trends in Atmospheric Carbon Dioxide: NOAA ESRL Global Monitoring Division. 2020. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/gl_trend.html (accessed on 23 December 2021).
- Cheong, K.; Chong, K. Development and application of an indoor air quality audit to an air-conditioned building in Singapore. Build. Environ. 2001, 36, 181–188. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Satish, U.; Mendell, M.J.; Shekhar, K.; Hotchi, T.; Sullivan, D.; Streufert, S.; Fisk, W.J. Is CO2 an Indoor Pollutant? Direct Effects of Low-to-Moderate CO2 Concentrations on Human Decision-Making Performance. Environ. Health Perspect. 2012, 120, 1671–1677. [Google Scholar] [CrossRef] [Green Version]
- FDA. Medgraphics Ultima System 510(k) Premarket Notification: U.S. Food and Drug Administration. 2006. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf6/K061731.pdf (accessed on 23 December 2021).
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The Thermic Effect of Food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Gorostiaga, E.; Maurer, C.; Eclache, J. Decrease in Respiratory Quotient During Exercise Following L-Carnitine Supplementation. Int. J. Sports Med. 1989, 10, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Issekutz, B.; Rodahl, K. Respiratory quotient during exercise. J. Appl. Physiol. 1961, 16, 606–610. [Google Scholar] [CrossRef]
- Haverinen-Shaughnessy, U.; Moschandreas, D.J.; Shaughnessy, R.J. Association between substandard classroom ventilation rates and students’ academic achievement. Indoor Air 2010, 21, 121–131. [Google Scholar] [CrossRef] [PubMed]
- ASTM D 6245. Standard Guide for Using Indoor Carbon Dioxide Concentrations to Evaluate Indoor Air Quality and Ventilation: American Society for Testing and Materials (ASTM). 2007. Available online: https://www.astm.org/ (accessed on 7 February 2022).
- Auerswald, S.; Hörberg, C.; Pflug, T.; Pfafferott, J.; Bongs, C.; Henning, H.-M. Experimental Investigation of the Air Exchange Effectiveness of Push-Pull Ventilation Devices. Energies 2020, 13, 5817. [Google Scholar] [CrossRef]
- Lyden, K.; Swibas, T.; Catenacci, V.; Guo, R.; Szuminsky, N.; Melanson, E.L. Estimating Energy Expenditure Using Heat Flux Measured at a Single Body Site. Med. Sci. Sports Exerc. 2014, 46, 2159–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselac, A.; Srebric, J. Comparison of Air Exchange Efficiency and Contaminant Removal Effectiveness as IAQ Indices ASHRAE Transactions. 2003. Available online: https://www.caee.utexas.edu/prof/novoselac/Publications/Novoselac_ASHRAE_Transactions_2003.pdf (accessed on 7 February 2022).
- Altman, D.G.; Bland, J.M. Standard deviations and standard errors. BMJ 2005, 331, 903. [Google Scholar] [CrossRef] [Green Version]
- FDA. MasterScreen CPXTM 510(k) Premarket Notification (K072323). U.S. Food and Drug Administration. 2007. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf7/K072323.pdf (accessed on 23 December 2021).
- FDA. OxyconTM Pro 510(k) Premarket Notification (K992214): U.S. Food and Drug Administration. 2000. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf/K992214.pdf (accessed on 23 December 2021).
- FDA. VyntusTM/SentrySuite Product Line 510(k) Premarket Notification (K133925): U.S. Food and Drug Administration. 2014. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf13/K133925.pdf (accessed on 23 December 2021).
- FDA. OxyconTM Mobile 510(k) Premarket Notification (K023120): U.S. Food and Drug Administration. 2003. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf/K023120.pdf (accessed on 23 December 2021).
- FDA. ReeVue Indirect Calorimeter, Model#8100 510(k) Premarket Notification. In Korr Medical Technologies Incorporated (Ed.): U.S. Food and Drug Administration. 2003. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf2/K021490.pdf (accessed on 23 December 2021).
- Cardiorespiratory Diagnostic System, Medical Graphics, Ultima Series. Available online: https://mgcdiagnostics.com/images/uploads/documents/Ultima_CPX_sellsheet_060155-001.pdf (accessed on 23 December 2021).
- Thomson, G.W. The Antoine Equation for Vapor-pressure Data. Chem. Rev. 1946, 38, 1–39. [Google Scholar] [CrossRef]
- Matarese, L.E. Indirect calorimetry: Technical aspects. J. Am. Diet Assoc. 1997, 97 (Suppl. 2), S154–S160. [Google Scholar] [CrossRef]
- Marra, M.; Scalfi, L.; Contaldo, F.; Pasanisi, F. Fasting Respiratory Quotient as a Predictor of Long-Term Weight Changes in Non-Obese Women. Ann. Nutr. Metab. 2004, 48, 189–192. [Google Scholar] [CrossRef]
- A McClave, S.; Lowen, C.C.; Kleber, M.J.; McConnell, J.W.; Jung, L.Y.; Goldsmith, L.J. Clinical use of the respiratory quotient obtained from indirect calorimetry. J. Parenter. Enter. Nutr. 2003, 27, 21–26. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprowls, M.; Victor, S.; Mora, S.J.; Osorio, O.; Pyznar, G.; Destaillats, H.; Wheatley-Guy, C.; Johnson, B.; Kulick, D.; Forzani, E. A Smart System for the Contactless Measurement of Energy Expenditure. Sensors 2022, 22, 1355. https://doi.org/10.3390/s22041355
Sprowls M, Victor S, Mora SJ, Osorio O, Pyznar G, Destaillats H, Wheatley-Guy C, Johnson B, Kulick D, Forzani E. A Smart System for the Contactless Measurement of Energy Expenditure. Sensors. 2022; 22(4):1355. https://doi.org/10.3390/s22041355
Chicago/Turabian StyleSprowls, Mark, Shaun Victor, Sabrina Jimena Mora, Oscar Osorio, Gabriel Pyznar, Hugo Destaillats, Courtney Wheatley-Guy, Bruce Johnson, Doina Kulick, and Erica Forzani. 2022. "A Smart System for the Contactless Measurement of Energy Expenditure" Sensors 22, no. 4: 1355. https://doi.org/10.3390/s22041355
APA StyleSprowls, M., Victor, S., Mora, S. J., Osorio, O., Pyznar, G., Destaillats, H., Wheatley-Guy, C., Johnson, B., Kulick, D., & Forzani, E. (2022). A Smart System for the Contactless Measurement of Energy Expenditure. Sensors, 22(4), 1355. https://doi.org/10.3390/s22041355






