Usability of Functional Electrical Stimulation in Upper Limb Rehabilitation in Post-Stroke Patients: A Narrative Review
Abstract
:1. Introduction
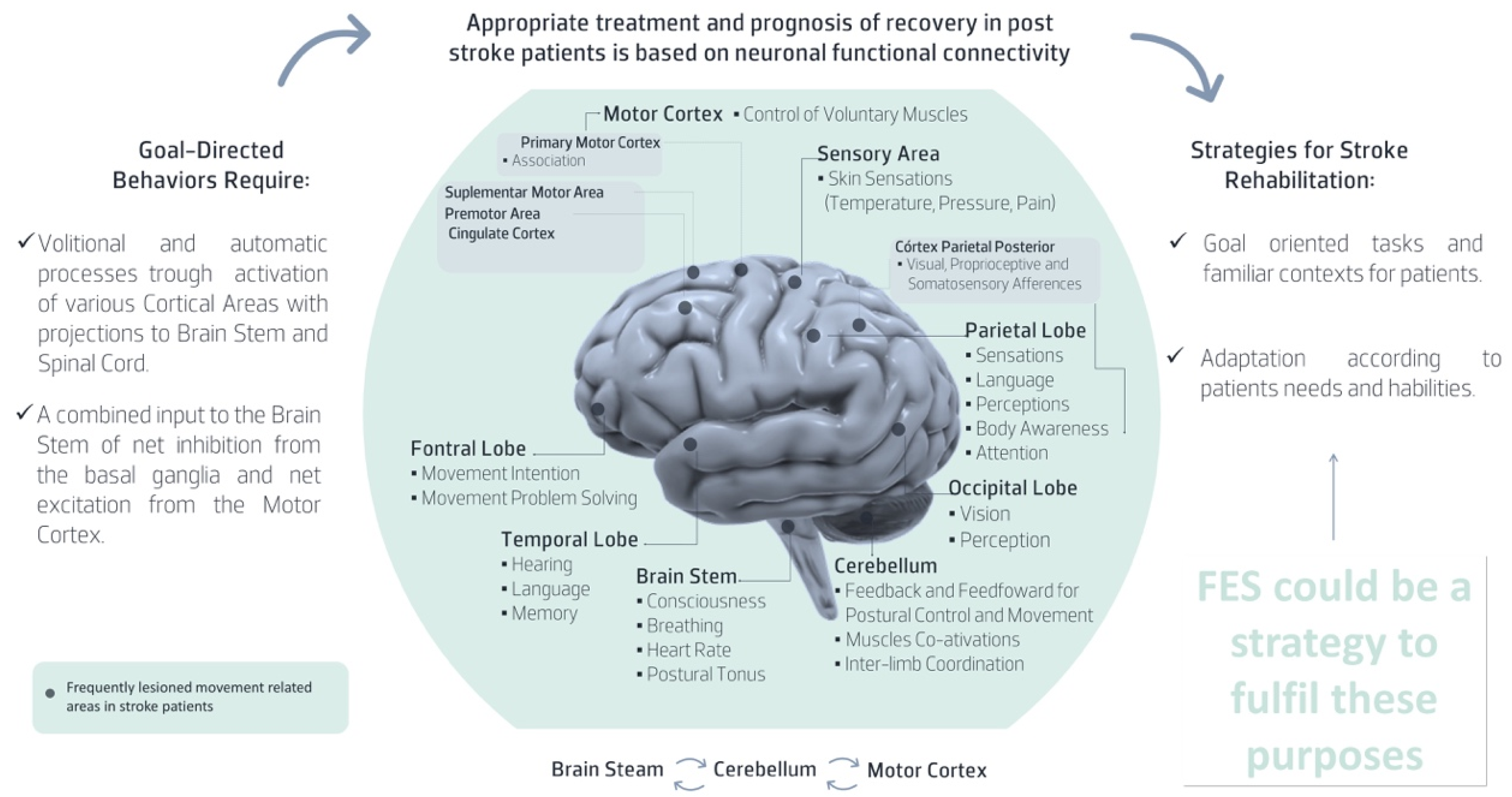
2. UL Post Stroke Rehabilitation Factors That Should Be Considered in FES Usability
- the possibility of desynchronized activation of different portions of the muscle [74];
- the possibility of individual adjustment of the stimulation location [77];
- the possibility of varying the stimulation parameters and patterns of stimulation, namely frequency, amplitude, pulse duration and stimulation channel, to recruit the more adequated synergy [77].
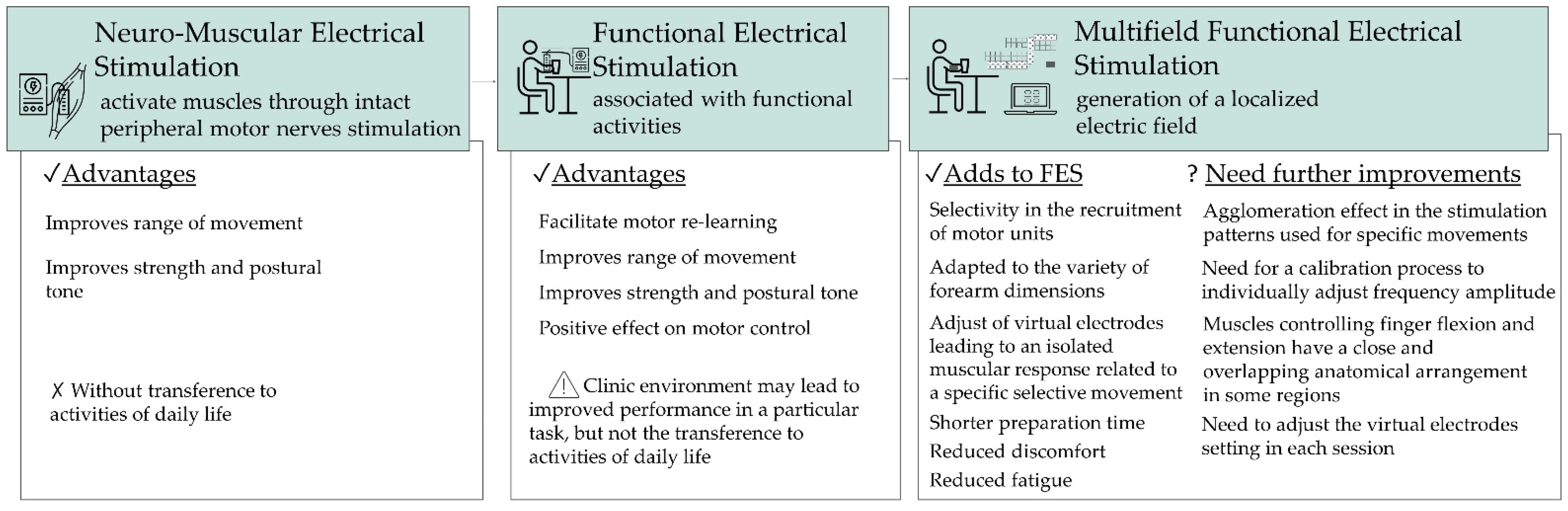
3. FES Usability in Post-Stroke Patients
3.1. FES User Satisfaction
3.2. FES Effectiveness
3.2.1. Clinical Measures
3.2.2. Laboratory Measures
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, Q.; Li, J.; Zhang, W.; He, Y.; Zhou, Y. Disability Status and Its Influencing Factors Among Stroke Patients in Northeast China: A 3-Year Follow-Up Study. Neuropsychiatr. Dis. Treat. 2021, 17, 2567–2573. [Google Scholar] [CrossRef]
- Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke; Royal College of Physicians: London, UK, 2012. [Google Scholar]
- Lawrence, E.S.; Coshall, C.; Dundas, R.; Stewart, J.; Rudd, A.G.; Howard, R.; Wolfe, C.D.A. Estimates of the Prevalence of Acute Stroke Impairments and Disability in a Multiethnic Population. Stroke 2001, 32, 1279–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwakkel, G.; Kollen, B.J.; van der Grond, J.; Prevo, A.J. Probability of Regaining Dexterity in the Flaccid Upper Limb: Impact of Severity of Paresis and Time since Onset in Acute Stroke. Stroke 2003, 34, 2181–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, C.E.; Edwards, D.F.; Birkenmeier, R.L.; Dromerick, A.W. Estimating Minimal Clinically Important Differences of Upper-Extremity Measures Early After Stroke. Arch. Phys. Med. Rehabil. 2008, 89, 1693–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Sousa, A.S.P.; Silva, C.C.; Santos, R.; Tavares, J.; Sousa, F. The role of the ipsilesional side in the rehabilitation of post-stroke subjects. Somatosens. Mot. Res. 2017, 34, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.; Silva, A.; de Sousa, A.S.P.; Pinheiro, A.R.; Bourlinova, C.; Silva, A.; Salazar, A.; Borges, C.; Crasto, C.; Correia, M.; et al. Co-activation of upper limb muscles during reaching in post-stroke subjects: An analysis of the contralesional and ipsilesional limbs. J. Electromyogr. Kinesiol. 2014, 24, 731–738. [Google Scholar] [CrossRef]
- Silva, C.; Pereira, S.; Ferreira, S.; Oliveira, N.; Santos, R. Anticipatory postural adjustments in the shoulder girdle in the reach movement performed in standing by post-stroke subjects. Somatosens. Mot. Res. 2018, 35, 124–130. [Google Scholar] [CrossRef]
- Johanne, D.; Malouin, F.; Richards, C.; Bourbonnais, D.; Rochette, A.; Bravo, G. Comparison of Changes in Upper and Lower Extremity Impairments and Disabilities after Stroke. Int. J. Rehabil. Res. 2003, 26, 109–116. [Google Scholar]
- Shelton, F.D.N.A.P.; Reding, M.J. Effect of Lesion Location on Upper Limb Motor Recovery After Stroke. Stroke 2001, 32, 107–112. [Google Scholar] [CrossRef]
- Franceschini, M.; La Porta, F.; Agosti, M.; Massucci, M. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur. J. Phys. Rehabil. Med. 2010, 46, 389–399. [Google Scholar] [PubMed]
- Lai, S.-M.; Studenski, S.; Duncan, P.; Perera, S. Persisting Consequences of Stroke Measured by the Stroke Impact Scale. Stroke 2002, 33, 1840–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingram, J.N.; Körding, K.P.; Howard, I.S.; Wolpert, D.M. The statistics of natural hand movements. Exp. Brain Res. 2008, 188, 223–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008. Cerebrovasc. Dis. 2008, 25, 457–507. [Google Scholar] [CrossRef] [PubMed]
- Gittler, M.; Davis, A.M. Guidelines for Adult Stroke Rehabilitation and Recovery. JAMA J. Am. Med. Assoc. 2018, 319, 820–821. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Popović, D.B.; Popović, M.B. Advances in the use of electrical stimulation for the recovery of motor function. Prog. Brain Res. 2011, 194, 215–225. [Google Scholar] [CrossRef]
- Eraifej, J.; Clark, W.; France, B.; Desando, S.; Moore, D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: A systematic review and meta-analysis. Syst. Rev. 2017, 6, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Howlett, O.A.; Lannin, N.; Ada, L.; McKinstry, C. Functional Electrical Stimulation Improves Activity After Stroke: A Systematic Review with Meta-Analysis. Arch. Phys. Med. Rehabil. 2015, 96, 934–943. [Google Scholar] [CrossRef]
- Cirstea, M.C.; Ptito, A.; Levin, M. Feedback and Cognition in Arm Motor Skill Reacquisition After Stroke. Stroke 2006, 37, 1237–1242. [Google Scholar] [CrossRef]
- Kristensen, M.G.H.; Busk, H.; Wienecke, T. Neuromuscular Electrical Stimulation improves Activities of Daily Living Post-Stroke: A Systematic Review and Meta-analysis. Arch. Rehabil. Res. Clin. Transl. 2021, 100167, in press. [Google Scholar] [CrossRef]
- Stroke Foundation. Clinical Guidelines for Stroke Management; Stroke Foundation: Melbourne, Australia, 2021. [Google Scholar]
- ISO 9241-11; Ergonomic Requirements for Office Work with Visual Display Terminals (Vdts)—Part 11: Guidance on Usability. ISO: Geneve, Switzerland, 1998. Available online: http://www.it.uu.se/edu/course/homepage/acsd/vt09/ISO9241part11.pdf (accessed on 20 December 2021).
- Seitz, R.J.; Matyas, T.A.; Carey, L.M. Neural Plasticity as a Basis for Motor Learning and Neurorehabilitation. Brain Impair. 2008, 9, 103–113. [Google Scholar] [CrossRef]
- Nudo, R.J. Postinfarct Cortical Plasticity and Behavioral Recovery. Stroke 2007, 38, 840–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nudo, R.J. Recovery after brain injury: Mechanisms and principles. Front. Hum. Neurosci. 2013, 7, 887. [Google Scholar] [CrossRef] [Green Version]
- Chae, J.; Bethoux, F.; Bohinc, T.; Dobos, L.; Davis, T.; Friedl, A. Neuromuscular Stimulation for Upper Extremity Motor and Functional Recovery in Acute Hemiplegia. Stroke 1998, 29, 975–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, J.; Pandyan, A.D.; Granat, M.; Cameron, M.; Stott, D.J. Electrical Stimulation of Wrist Extensors in Poststroke Hemiplegia. Stroke 1999, 30, 1384–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimberley, T.J.; Lewis, S.M.; Auerbach, E.; Dorsey, L.L.; Lojovich, J.M.; Carey, J.R. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp. Brain Res. 2004, 154, 450–460. [Google Scholar] [CrossRef]
- Maffiuletti, N.A. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur. J. Appl. Physiol. 2010, 110, 223–234. [Google Scholar] [CrossRef]
- Marquez-Chin, C.; Popovic, M.R. Functional Electrical Stimulation Therapy for Restoration of Motor Function after Spinal Cord Injury and Stroke: A Review. Biomed. Eng. Online 2020, 19, 1–25. [Google Scholar] [CrossRef]
- Barss, T.; Ainsley, E.N.; Claveria-Gonzalez, F.C.; Luu, M.J.; Miller, D.J.; Wiest, M.J.; Collins, D.F. Utilizing Physiological Principles of Motor Unit Recruitment to Reduce Fatigability of Electrically-Evoked Contractions: A Narrative Review. Arch. Phys. Med. Rehabil. 2018, 99, 779–791. [Google Scholar] [CrossRef]
- Kapadia, N.; Moineau, B.; Popovic, M.R. Functional Electrical Stimulation Therapy for Retraining Reaching and Grasping After Spinal Cord Injury and Stroke. Front. Neurosci. 2020, 14, 718. [Google Scholar] [CrossRef] [PubMed]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar] [PubMed]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef]
- Dobkin, B.H. Strategies for stroke rehabilitation. Lancet Neurol. 2004, 3, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Kleim, J.A.; Jones, T.A. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation after Brain Damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Cirstea, M.C.; Levin, M. Improvement of Arm Movement Patterns and Endpoint Control Depends on Type of Feedback During Practice in Stroke Survivors. Neurorehabil. Neural Repair 2007, 21, 398–411. [Google Scholar] [CrossRef]
- De Kroon, J.R.; Van Der Lee, J.H.; Ijzerman, M.J.; Lankhorst, G.J. Therapeutic electrical stimulation to improve motor control and functional abilities of the upper extremity after stroke: A systematic review. Clin. Rehabil. 2002, 16, 350–360. [Google Scholar] [CrossRef]
- Urton, M.L.; Kohia, M.; Davis, J.; Neill, M.R. Systematic Literature Review of Treatment Interventions for Upper Extremity Hemiparesis Following Stroke. Occup. Ther. Int. 2007, 14, 11–27. [Google Scholar] [CrossRef]
- de Kroon, J.; Ijzerman, M.; Chae, J.; Lankhorst, G.; Zilvold, G. Relation between Stimulation Characteristics and Clinical Outcome in Studies Using Electrical Stimulation to Improve Motor Control of the Upper Extremity in Stroke. J. Rehabil. Med. 2005, 37, 65–74. [Google Scholar] [CrossRef] [Green Version]
- van Peppen, R.P.; Kwakkel, G.; Wood-Dauphinee, S.; Hendriks, H.J.; Van der Wees Ph, J.; Dekker, J. The Impact of Physical Therapy on Functional Outcomes after Stroke: What’s the Evidence? Clin. Rehabil. 2004, 18, 833862. [Google Scholar] [CrossRef]
- Han, B.S.; Jang, S.H.; Chang, Y.; Byun, W.M.; Lim, S.K.; Kang, D.S. Functional Magnetic Resonance Image Finding of Cortical Activation by Neuromuscular Electrical Stimulation on Wrist Extensor Muscles. Am. J. Phys. Med. Rehabil. 2003, 82, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.V.; Alon, G.; Roys, S.R.; Gullapalli, R.P. Functional MRI determination of a dose-response relationship to lower extremity neuromuscular electrical stimulation in healthy subjects. Exp. Brain Res. 2003, 150, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Blickenstorfer, A.; Kleiser, R.; Keller, T.; Keisker, B.; Meyer, M.; Riener, R.; Kollias, S. Cortical and Subcortical Correlates of Functional Electrical Stimulation of Wrist Extensor and Flexor Muscles Revealed by Fmri. Hum. Brain Mapp. 2009, 30, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Iftime-Nielsen, S.D.; Christensen, M.S.; Vingborg, R.J.; Sinkjaer, T.; Roepstorff, A.; Grey, M.J. Interaction of electrical stimulation and voluntary hand movement in SII and the cerebellum during simulated therapeutic functional electrical stimulation in healthy adults. Hum. Brain Mapp. 2012, 33, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D. Functional Electrical Stimulation and rehabilitation—An hypothesis. Med. Eng. Phys. 2003, 25, 75–78. [Google Scholar] [CrossRef]
- Ridding, M.; Brouwer, B.; Miles, T.; Pitcher, J.; Thompson, P. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp. Brain Res. 2000, 131, 135–143. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.; Brooker, R.; Giacomin, P.; Ridding, M.; Miles, T. Time course of induction of increased human motor cortex excitability by nerve stimulation. NeuroReport 2002, 13, 1271–1273. [Google Scholar] [CrossRef]
- Kwakkel, G. Impact of intensity of practice after stroke: Issues for consideration. Disabil. Rehabil. 2006, 28, 823–830. [Google Scholar] [CrossRef]
- Arya, K.N.; Pandian, S.; Verma, R.; Garg, R. Movement therapy induced neural reorganization and motor recovery in stroke: A review. J. Bodyw. Mov. Ther. 2011, 15, 528–537. [Google Scholar] [CrossRef]
- Sheffler, L.R.; Chae, J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve 2007, 35, 562–590. [Google Scholar] [CrossRef]
- Seitz, R.J.; Donnan, G.A. Recovery Potential After Acute Stroke. Front. Neurol. 2015, 6, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothwell, J. Meet the Brain Neurophysiology. Int. Rev. Neurobiol. 2009, 86, 51–65. [Google Scholar] [PubMed]
- Rothwell, J. Overview of neurophysiology of movement control. Clin. Neurol. Neurosurg. 2012, 114, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Vaughan-Graham, J.; Silva, C.; Sousa, A.; Cunha, C.; Ferreira, R.; Barbosa, P.M. Stroke rehabilitation and research: Consideration of the role of the cortico-reticulospinal system. Somatosens. Mot. Res. 2018, 35, 148–152. [Google Scholar] [CrossRef]
- Matsuyama, K.; Mori, F.; Nakajima, K.; Drew, T.; Aoki, M.; Mori, S. Locomotor role of the corticoreticular–reticulospinal–spinal interneuronal system. Prog. Brain Res. 2004, 143, 239–249. [Google Scholar] [CrossRef]
- Schepens, B.; Drew, T. Independent and Convergent Signals from the Pontomedullary Reticular Formation Contribute to the Control of Posture and Movement During Reaching in the Cat. J. Neurophysiol. 2004, 92, 2217–2238. [Google Scholar] [CrossRef]
- Schepens, B.; Drew, T. Descending Signals from the Pontomedullary Reticular Formation Are Bilateral, Asymmetric, and Gated During Reaching Movements in the Cat. J. Neurophysiol. 2006, 96, 2229–2252. [Google Scholar] [CrossRef]
- Schepens, B.; Stapley, P.; Drew, T. Neurons in the Pontomedullary Reticular Formation Signal Posture and Movement Both as an Integrated Behavior and Independently. J. Neurophysiol. 2008, 100, 2235–2253. [Google Scholar] [CrossRef]
- Hughes, A.M.; Freeman, C.T.; Burridge, J.; Chappell, P.H.; Lewin, P.L.; Rogers, E. Feasibility of Iterative Learning Control Mediated by Functional Electrical Stimulation for Reaching After Stroke. Neurorehabil. Neural Repair 2009, 23, 559–568. [Google Scholar] [CrossRef]
- Geraldine, M.; Taylor, P.; Lane, R. Accelerometer-Triggered Electrical Stimulation for Reach and Grasp in Chronic Stroke Patients: A Pilot Study. Neurorehabil. Neural Repair 2011, 25, 774–780. [Google Scholar]
- Cuesta-Gómez, A.; Molina-Rueda, F.; Tejada, M.C.; Ojanguren, E.I.; Torricelli, D.; Miangolarra-Page, J.C. The Use of Functional Electrical Stimulation on the Upper Limb and Interscapular Muscles of Patients with Stroke for the Improvement of Reaching Movements: A Feasibility Study. Front. Neurol. 2017, 8, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, C.L.; Popovic, M.R. Functional Electrical Stimulation. IEEE Control Syst. 2008, 28, 40–50. [Google Scholar]
- Chipchase, L.; Schabrun, S.; Hodges, P. Peripheral electrical stimulation to induce cortical plasticity: A systematic review of stimulus parameters. Clin. Neurophysiol. 2011, 122, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, D.F. Central Contributions to Contractions Evoked by Tetanic Neuromuscular Electrical Stimulation. Exerc. Sport Sci. Rev. 2007, 35, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Robertson, V.; Ward, A.; Low, J.; Reed, A. Electrotherapy Explained: Principles and Practice; Butterworth Heinemann: Edinburgh, Scotland, 2006. [Google Scholar]
- Bickel, C.S.; Gregory, C.M.; Dean, J. Motor unit recruitment during neuromuscular electrical stimulation: A critical appraisal. Eur. J. Appl. Physiol. 2011, 111, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- John, C.; Sheffler, L. Neuromuscular Eletrical Stimulation for Motor Restoration in Hemiplegia. In Stroke Recovery and Rehabilitation; Harvey, R.L., Macko, R.F., Stein, J., Winstein, C.J., Zorowitz, R.D., Eds.; DemosMedical: New York, NY, USA, 2009. [Google Scholar]
- Peckham, P.H.; Knutson, J.S. Functional Electrical Stimulation for Neuromuscular Applications. Annu. Rev. Biomed. Eng. 2005, 7, 327–360. [Google Scholar] [CrossRef]
- Sousa, A.S.; da Silva, C.I.C.; Mesquita, I.A.; Silva, A.; Macedo, R.; Imatz-Ojanguren, E.; Hernandez, E.; Keller, T.; Moreira, J.; da Fonseca, P.F.P.; et al. Optimal Multi-Field Functional Electrical Stimulation Parameters for the “Drinking Task—Reaching phase” and Related Upper Limb Kinematics Repeatability in Post stroke Subjects. J. Hand Ther. 2021. [Google Scholar] [CrossRef]
- Sousa, A.S.; Mesquita, I.A.; da Silva, C.I.C.; Silva, A.; Macedo, R.; Imatz-Ojanguren, E.; Hernandez, E.; Keller, T.; Moreira, J.; da Fonseca, P.F.P.; et al. Optimal Multifield Functional Electrical Stimulation Parameters for the “Turn on the Light” Task and Related Upper Limb Kinematics Repeatability in Poststroke Subjects. Arch. Phys. Med. Rehabil. 2021, 102, 1180–1190. [Google Scholar] [CrossRef]
- Popovic-Maneski, L.P.; Kostic, M.; Bijelic, G.; Keller, T.; Mitrovic, S.; Konstantinovic, L.; Popovic, D. Multi-Pad Electrode for Effective Grasping: Design. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 648–654. [Google Scholar] [CrossRef]
- Malešević, J.; Štrbac, M.; Isaković, M.; Kojić, V.; Konstantinović, L.; Vidaković, A.; Dedijer, S.; Kostić, M.; Keller, T. Evolution of surface motor activation zones in hemiplegic patients during 20 sessions of FES therapy with multi-pad electrodes. Eur. J. Transl. Myol. 2016, 26, 6059. [Google Scholar] [CrossRef] [Green Version]
- Malešević, N.M.; Maneski, L.Z.P.; Ilić, V.; Jorgovanović, N.; Bijelić, G.; Keller, T.; Popović, D.B. A multi-pad electrode based functional electrical stimulation system for restoration of grasp. J. Neuroeng. Rehabil. 2012, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malešević, J.; Štrbac, M.; Isaković, M.; Kojić, V.; Konstantinović, L.; Vidaković, A.; Dujović, S.D.; Kostić, M.; Keller, T. Temporal and Spatial Variability of Surface Motor Activation Zones in Hemiplegic Patients During Functional Electrical Stimulation Therapy Sessions. Artif. Organs 2017, 41, E166–E177. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J. Usability Testing. In Handbook of Human Factors and Ergonomics; Salvendy, G., Ed.; John Wiley: New York, NY, USA, 2006. [Google Scholar]
- Rice, V.J.B. Human Factors in Medical Rehabilitation Equipment: Product Development and Usability Testing; Elsevier BV: Amsterdam, The Netherlands, 2008; pp. 151–172. [Google Scholar]
- Frøkjær, E.; Hertzum, M.; Hornbæk, K. Measuring Usability: Are Effectiveness, Efficiency, and Satisfaction Really Correlated? Association for Computing Machinery: New York, NY, USA, 2000. [Google Scholar]
- Imatz-Ojanguren, E.; Sánchez-Márquez, G.; Asiain-Aristu, J.R.; Cueto-Mendo, J.; Jaunarena-Goicoechea, E.; Zabaleta, H.; Keller, T. A Foot Drop Compensation Device Based on Surface Multi-Field Functional Electrical Stimulation—Usability Study in a Clinical Environment. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319862141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prenton, S.; Kenney, L.P.; Stapleton, C.; Cooper, G.; Reeves, M.L.; Heller, B.W.; Sobuh, M.; Barker, A.T.; Healey, J.; Good, T.R.; et al. Feasibility Study of a Take-Home Array-Based Functional Electrical Stimulation System with Automated Setup for Current Functional Electrical Stimulation Users With Foot-Drop. Arch. Phys. Med. Rehabil. 2014, 95, 1870–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, I.A.; Vieira Pinheiro, A.R.V.; Correia, M.V.; Costa da Silva, C.I.C. Methodological Considerations for Kinematic Analysis of Upper Limbs in Healthy and Post-Stroke Adults. Part II: A Systematic Review of Motion Capture Systems and Kinematic Metrics. Top. Stroke Rehabil. 2019, 26, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, S.M.; Dannenbaum, R.; Levin, M.F. Task-Specific Training with Trunk Restraint on Arm Recovery in Stroke: Randomized Control Trial. Stroke 2006, 37, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Levin, M.F.; Desrosiers, J.; Beauchemin, D.; Bergeron, N.; Rochette, A. Development and Validation of a Scale for Rating Motor Compensations Used for Reaching in Patients with Hemiparesis: The Reaching Performance Scale. Phys. Ther. 2004, 84, 8–22. [Google Scholar] [CrossRef]
- Raine, S. The Bobath Concept: Developments and Current Theoretical Underpinning; Wiley: Hoboken, NJ, USA, 2009; pp. 1–22. [Google Scholar]
- Wang, C.-H.; Hsieh, C.-L.; Dai, M.-H.; Chen, C.-H.; Lai, Y.-F. Inter-rater reliability and validity of the stroke rehabilitation assessment of movement (STREAM) instrument. J. Rehabil. Med. 2002, 34, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Carr, J.H.; Shepherd, R.B.; Nordholm, L.; Lynne, D. Investigation of a New Motor Assessment Scale for Stroke Patients. Phys. Ther. 1985, 65, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Gowland, C.; Stratford, P.; Ward, M.; Moreland, J.; Torresin, W.; van Hullenaar, S.; Sanford, J.; Barreca, S.; Vanspall, B.; Plews, N. Measuring Physical Impairment and Disability with the Chedoke-Mcmaster Stroke Assessment. Stroke 1993, 24, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Cauraugh, J.; Light, K.; Kim, S.; Thigpen, M.; Behrman, A. Chronic Motor Dysfunction after Stroke: Recovering Wrist and Finger Extension by Electromyography-Triggered Neuromuscular Stimulation. Stroke 2000, 31, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Cauraugh, J.H.; Kim, S.B. Chronic stroke motor recovery: Duration of active neuromuscular stimulation. J. Neurol. Sci. 2003, 215, 13–19. [Google Scholar] [CrossRef]
- Cauraugh, J.; Kim, S.B. Stroke Motor Recovery: Active Neuromuscular Stimulation and Repetitive Practice Schedules. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1562–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauraugh, J.H.; Kim, S. Two Coupled Motor Recovery Protocols Are Better Than One: Electromyogram-Triggered Neuromuscular Stimulation and Bilateral Movements. Stroke 2002, 33, 1589–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, Y.; Ogawa, S.; Tsujiuchi, K.; Muraoka, Y. A home-based rehabilitation program for the hemiplegic upper extremity by power-assisted functional electrical stimulation. Disabil. Rehabil. 2008, 30, 296–304. [Google Scholar] [CrossRef]
- Shin, H.K.; Cho, S.H.; Jeon, H.-S.; Lee, Y.-H.; Song, J.C.; Jang, S.H.; Lee, C.-H.; Kwon, Y.H. Cortical effect and functional recovery by the electromyography-triggered neuromuscular stimulation in chronic stroke patients. Neurosci. Lett. 2008, 442, 174–179. [Google Scholar] [CrossRef]
- Chan, M.K.-L.; Tong, R.K.-Y.; Chung, K.Y.-K. Bilateral Upper Limb Training with Functional Electric Stimulation in Patients with Chronic Stroke. Neurorehabil. Neural Repair 2008, 23, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Guzmán, A.D.L.; Dimbwadyo-Terrer, I.; Trincado-Alonso, F.; Monasterio-Huelin, F.; Torricelli, D.; Gil-Agudo, A. Quantitative assessment based on kinematic measures of functional impairments during upper extremity movements: A review. Clin. Biomech. 2014, 29, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, A.; Tartar, A.; Huseyinsinoglu, B.E.; Ertas, A.H. A clinically feasible kinematic assessment method of upper extremity motor function impairment after stroke. Measurement 2016, 80, 207–216. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Galán-Mercant, A.; Williams, J. The use of inertial sensors system for human motion analysis. Phys. Ther. Rev. 2010, 15, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Webster, D.; Celik, O. Systematic review of Kinect applications in elderly care and stroke rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, A.; Chess, D.; Johnson, J.; King, G. Accuracy of an electromagnetic tracking device: A study of the optimal operating range and metal interference. J. Biomech. 1996, 29, 791–793. [Google Scholar] [CrossRef]
- Van Tuijl, J.; Janssen-Potten, Y.; Seelen, H. Evaluation of upper extremity motor function tests in tetraplegics. Spinal Cord 2002, 40, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.A.; Willén, C.; Sunnerhagen, K.S. Kinematic Variables Quantifying Upper-Extremity Performance After Stroke During Reaching and Drinking from a Glass. Neurorehabil. Neural Repair 2010, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.A.; Willén, C.; Sunnerhagen, K.S. Movement Kinematics During Drinking Task Are Associated with the Activity Capacity Level after Stroke. Neurorehabil. Neural Repair 2012, 26, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, W.-K.; Lee, J.; Lee, H.-Y.; Park, D.S.; Ko, B.-W.; Kim, J. Kinematic analysis of upper extremity movement during drinking in hemiplegic subjects. Clin. Biomech. 2014, 29, 248–256. [Google Scholar] [CrossRef]
- Mesquita, I.A.; Pinheiro, A.R.V.; Correia, M.F.P.V.; Silva, C.I.C. Methodological Considerations for Kinematic Analysis of Upper Limbs in Healthy and Post-Stroke Adults. Part I: A Systematic Review of Sampling and Motor Tasks. Top. Stroke Rehabil. 2019, 26, 142–152. [Google Scholar] [CrossRef]
- Wagner, J.M.; Rhodes, J.A.; Patten, C. Reproducibility and Minimal Detectable Change of Three-Dimensional Kinematic Analysis of Reaching Tasks in People with Hemiparesis After Stroke. Phys. Ther. 2008, 88, 652–663. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA; London, UK, 2017. [Google Scholar]
- Thies, S.B.; Tresadern, P.A.; Kenney, L.P.; Smith, J.; Howard, D.; Goulermas, J.Y.; Smith, C.; Rigby, J. Movement variability in stroke patients and controls performing two upper limb functional tasks: A new assessment methodology. J. Neuroeng. Rehabil. 2009, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.S.; Bishop, M.D.; McGuirk, T.E.; Sethi, A.; Richards, L.G. Reliability of Upper Extremity Kinematics While Performing Different Tasks in Individuals with Stroke. J. Mot. Behav. 2011, 43, 121–130. [Google Scholar] [CrossRef]
- Finley, M.; Combs, S.; Carnahan, K.; Peacock, S.; Van Buskirk, A. Comparison of “Less Affected Limb” Reaching Kinematics in Individuals with Chronic Stroke and Healthy Age-Matched Controls. Phys. Occup. Ther. Geriatr. 2012, 30, 245–259. [Google Scholar] [CrossRef]
- Murphy, M.A.; Willén, C.; Sunnerhagen, K.S. Responsiveness of Upper Extremity Kinematic Measures and Clinical Improvement During the First Three Months After Stroke. Neurorehabil. Neural Repair 2013, 27, 844–853. [Google Scholar] [CrossRef] [PubMed]
- van Dokkum, L.; Hauret, I.; Mottet, D.; Froger, J.; Métrot, J.; Laffont, I. The Contribution of Kinematics in the Assessment of Upper Limb Motor Recovery Early After Stroke. Neurorehabil. Neural Repair 2014, 28, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.K.; Yamanaka, J.; Chilingaryan, G.; Levin, M.F. Validity of Movement Pattern Kinematics as Measures of Arm Motor Impairment Poststroke. Stroke 2010, 41, 2303–2308. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; Silva, C.C.; Ferreira, S.; Oliveira, N.; Santos, R.; Vilas-Boas, J.P.; Correia, M.V. Anticipatory postural adjustments during sitting reach movement in post-stroke subjects. J. Electromyogr. Kinesiol. 2014, 24, 165–171. [Google Scholar] [CrossRef] [Green Version]



| Clinical Measure | Tool |
|---|---|
| ADL |
|
| Funcional Motor Recovery |
|
| Muscle related |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, A.S.P.; Moreira, J.; Silva, C.; Mesquita, I.; Macedo, R.; Silva, A.; Santos, R. Usability of Functional Electrical Stimulation in Upper Limb Rehabilitation in Post-Stroke Patients: A Narrative Review. Sensors 2022, 22, 1409. https://doi.org/10.3390/s22041409
Sousa ASP, Moreira J, Silva C, Mesquita I, Macedo R, Silva A, Santos R. Usability of Functional Electrical Stimulation in Upper Limb Rehabilitation in Post-Stroke Patients: A Narrative Review. Sensors. 2022; 22(4):1409. https://doi.org/10.3390/s22041409
Chicago/Turabian StyleSousa, Andreia S. P., Juliana Moreira, Cláudia Silva, Inês Mesquita, Rui Macedo, Augusta Silva, and Rubim Santos. 2022. "Usability of Functional Electrical Stimulation in Upper Limb Rehabilitation in Post-Stroke Patients: A Narrative Review" Sensors 22, no. 4: 1409. https://doi.org/10.3390/s22041409
APA StyleSousa, A. S. P., Moreira, J., Silva, C., Mesquita, I., Macedo, R., Silva, A., & Santos, R. (2022). Usability of Functional Electrical Stimulation in Upper Limb Rehabilitation in Post-Stroke Patients: A Narrative Review. Sensors, 22(4), 1409. https://doi.org/10.3390/s22041409








