Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges
Abstract
:1. Introduction
2. Microfluidic POC Devices
2.1. Introduction
2.2. Advantages of Microfluidic POC Devices in Medical Laboratory
2.3. Classification of Microfluidic POC Equipment Types
2.3.1. Microfluidic Equipment Made of PDMS
2.3.2. Paper-Based Microfluidic Devices
2.3.3. 3D-Printed Microfluidic Devices
2.3.4. Mobile Sensors Based on Integrated Microfluidic Devices and Smart Phones
2.3.5. Handheld Centrifugal Microfluidic Devices
2.3.6. Microfluidic POC Devices Using DEP Technology
2.4. Advantages over Non-Microfluidic POC Devices
3. Microfluidic POC in Early Diagnosis of Infectious Diseases
3.1. Introduction
3.2. Application of Infectious Disease Detection
3.3. Prospects for the Detection of Infectious Diseases
4. Microfluidic POC in Early Diagnosis of CVDs
4.1. Introduction
4.2. Detection of Multiple Biomarkers for CVD
4.3. Prospects for the Detection of CVDs
5. Microfluidic POC in Early Diagnosis of Tumors
5.1. Introduction
5.2. Applications for Cancer Detection
5.3. Prospects for the Detection of Cancer
6. Microfluidic POC in Early Diagnosis of Chronic Diseases
6.1. Introduction
6.2. Application of Chronic Disease Detection
6.3. Prospects for the Detection of Chronic Diseases
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, A.S.; Nerurkar, S.N.; Tan, W.C.C.; Goh, D.; Lai, C.P.T.; Poh, S.Y.J. The Virological, immunological, and imaging approaches for COVID-19 diagnosis and research. SLAS Technol. 2020, 25, 522–544. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Koirala, J.; Acharya, S.; Rijal, N. Impact of healthy life, education and living standard on spread of COVID-19 in developed and underdeveloped countries. Soc. Sci. Electron. Publ. 2021. [Google Scholar] [CrossRef]
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Weekly Epidemiological Update; World Health Organization: Geneva, Switzerland, 2021; Volume 77, pp. 1–3. [Google Scholar]
- Bonanni, P.; Cantón, R.; Gill, D.; Halfon, P.; Liebert, U.G.; Crespo, K.A.N.; Martín, J.J.P.; Trombetta, C.M. The role of serology testing to strengthen vaccination initiatives and policies for COVID-19 in Europe. COVID 2021, 1, 20–38. [Google Scholar] [CrossRef]
- Burki, T. COVID-19 vaccine mandates in Europe. Lancet Infect. Dis. 2022, 22, 27–28. [Google Scholar] [CrossRef]
- Raju, S.P.; Chu, X. Rapid low-cost microfluidic detection in point of care diagnostics. J. Med. Syst. 2018, 42, 184. [Google Scholar] [CrossRef]
- Aimi, F.; Procopio, M.G.; Flores, M.T.A.; Brouland, J.P.; Leval, L. De Microfluidic-based immunohistochemistry for breast cancer diagnosis: A comparative clinical study. Virchows Arch. 2019, 475, 313–323. [Google Scholar] [CrossRef]
- Pedde, R.D.; Li, H.; Borchers, C.H.; Akbari, M. Microfluidic-mass spectrometry interfaces for translational proteomics. Trends Biotechnol. 2017, 35, 954–970. [Google Scholar] [CrossRef]
- Su, W.; Li, H.; Chen, W.; Qin, J. Microfluidic strategies for label-free exosomes isolation and analysis. TrAC Trends Anal. Chem. 2019, 118, 686–698. [Google Scholar] [CrossRef]
- Wu, J.; He, Z.; Chen, Q.; Lin, J.M. Biochemical analysis on microfluidic chips. TrAC Trends Anal. Chem. 2016, 80, 213–231. [Google Scholar] [CrossRef]
- Park, J.; Han, D.H.; Park, J.K. Towards practical sample preparation in point-of-care testing: User-friendly microfluidic devices. Lab Chip 2020, 20, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef] [Green Version]
- Choong, C.-L.; Milne, W.I.; Teo, K.B.K. Review: Carbon nanotube for microfluidic lab-on-a-chip application. Int. J. Mater. Form. 2008, 1, 117–125. [Google Scholar] [CrossRef]
- Park, S.; Zhang, Y.; Lin, S.; Wang, T.H.; Yang, S. Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol. Adv. 2011, 29, 830–839. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Garrigos, A.; Maruthamuthu, M.K.; Ault, A.; Davidson, J.L.; Rudakov, G.; Pillai, D.; Koziol, J.; Schoonmaker, J.P.; Johnson, T.; Verma, M.S. On-farm colorimetric detection of Pasteurella multocida, Mannheimia haemolytica, and Histophilus somni in crude bovine nasal samples. Vet. Res. 2021, 52, 126. [Google Scholar] [CrossRef]
- Salehipour Masooleh, H.; Ghavami Lahiji, M.; Ciancio, A.; Tayebi, L. Microfluidic Technologies Using Oral Factors: Saliva-Based Studies. In Applications of Biomedical Engineering in Dentistry; Springer: Cham, Switzerland, 2020; pp. 339–358. ISBN 978-3-030-21582-8. [Google Scholar]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Liu, H.; Lei, Y. A critical review: Recent advances in “digital” biomolecule detection with single copy sensitivity. Biosens. Bioelectron. 2021, 177, 112901. [Google Scholar] [CrossRef]
- Voller, A.; Bartlett, A.; Bidwell, D.E.; Clark, M.F.; Adams, A.N. The detection of viruses by enzyme-linked immunosorbent assay (ELISA). J. Gen. Virol. 1976, 33, 165–167. [Google Scholar] [CrossRef]
- Nascimento, E.J.M.; George, J.K.; Velasco, M.; Bonaparte, M.I.; Zheng, L.; DiazGranados, C.A.; Marques, E.T.A.; Huleatt, J.W. Development of an anti-dengue NS1 IgG ELISA to evaluate exposure to dengue virus. J. Virol. Methods 2018, 257, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Nurtop, E.; Villarroel, P.; Pastorino, B.; Ninove, L.; Gallian, P. Correction to: Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol. J. 2019, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Nachbagauer, R.; Ermler, M.E.; Bunduc, P.; Amanat, F.; Izikson, R.; Cox, M.; Palese, P.; Eichelberger, M.; Krammer, F. Analysis of anti-influenza virus neuraminidase antibodies in children, adults, and the elderly by ELISA and enzyme inhibition: Evidence for original antigenic sin. MBio 2017, 8, e02281-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beavis, K.G.; Matushek, S.M.; Abeleda, A.P.F.; Bethel, C.; Hunt, C.; Gillen, S.; Moran, A.; Tesic, V. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. J. Clin. Virol. 2020, 129, 104468. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.; Ainsworth, M.; Anand, R.; Andersson, M.I.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Bell, J.I.; Berry, T.; et al. Antibody testing for COVID-19: A report from the National COVID Scientific Advisory Panel. Wellcome Open Res 2020, 5, 139. [Google Scholar] [CrossRef]
- Iha, K.; Inada, M.; Kawada, N.; Nakaishi, K.; Watabe, S.; Tan, Y.H.; Shen, C.; Ke, L.Y.; Yoshimura, T.; Ito, E. Ultrasensitive ELISA developed for diagnosis. Diagnostics 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J.; et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Rissin, D.M.; Fournier, D.R.; Piech, T.; Patel, P.P.; Wilson, D.H.; Duffy, D.C. Single molecule enzyme-linked immunosorbent assays: Theoretical considerations. J. Immunol. Methods 2012, 378, 102–115. [Google Scholar] [CrossRef] [Green Version]
- Kan, C.W.; Tobos, C.I.; Rissin, D.M.; Wiener, A.D.; Duffy, D.C. Digital enzyme-linked immunosorbent assays with sub-attomolar detection limits based on low numbers of capture beads combined with high efficiency bead analysis. Lab Chip 2020, 20, 2122–2135. [Google Scholar] [CrossRef]
- Park, S.; Yossifon, G. Combining dielectrophoresis and concentration polarization-based preconcentration to enhance bead-based immunoassay sensitivity. Nanoscale 2019, 11, 9436–9443. [Google Scholar] [CrossRef]
- Rissin, D.M.; Kan, C.W.; Song, L.; Rivnak, A.J.; Fishburn, M.W.; Shao, Q.; Piech, T.; Ferrell, E.P.; Meyer, R.E.; Campbell, T.G.; et al. Multiplexed single molecule immunoassays. Lab Chip 2013, 13, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.; Chandler, D.J.; Dunbar, S.A. The genesis and evolution of bead-based multiplexing. Methods 2019, 158, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Reslova, N.; Michna, V.; Kasny, M.; Mikel, P.; Kralik, P. xMAP technology: Applications in detection of pathogens. Front. Microbiol. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef]
- Hanash, S. Disease proteomics. Nature 2003, 422, 226–232. [Google Scholar] [CrossRef]
- Nilsson, T.; Mann, M.; Aebersold, R.; Yates, J.R.; Bairoch, A.; Bergeron, J.J.M. Mass spectrometry in high-throughput proteomics: Ready for the big time. Nat. Methods 2010, 7, 681–685. [Google Scholar] [CrossRef]
- Percy, A.J.; Byrns, S.; Pennington, S.R.; Holmes, D.T.; Anderson, N.L.; Agreste, T.M.; Duffy, M.A. Clinical translation of MS-based, quantitative plasma proteomics: Status, challenges, requirements, and potential. Expert Rev. Proteom. 2016, 13, 673–684. [Google Scholar] [CrossRef]
- Azzouz, A.; Hejji, L.; Kim, K.H.; Kukkar, D.; Souhail, B.; Bhardwaj, N.; Brown, R.J.C.; Zhang, W. Advances in surface plasmon resonance–based biosensor technologies for cancer biomarker detection. Biosens. Bioelectron. 2022, 197, 113767. [Google Scholar] [CrossRef]
- Moznuzzaman, M.; Khan, I.; Islam, M. Nano-layered surface plasmon resonance-based highly sensitive biosensor for virus detection: A theoretical approach to detect SARS-CoV-2. AIP Adv. 2021, 11, 65023. [Google Scholar] [CrossRef]
- Liu, C.; Xue, N.; Cai, H.; Sun, J.; Qi, Z.; Zhao, P.; Xiong, F.; Geng, Z.; Jiang, L.; Li, L. Nanoparticles enhanced self-driven microfludic biosensor. Micromachines 2020, 11, 350. [Google Scholar] [CrossRef]
- Lim, W.Y.; Thevarajah, T.M.; Goh, B.T.; Khor, S.M. Paper microfluidic device for early diagnosis and prognosis of acute myocardial infarction via quantitative multiplex cardiac biomarker detection. Biosens. Bioelectron. 2019, 128, 176–185. [Google Scholar] [CrossRef]
- Lin, Q.; Wen, D.; Wu, J.; Liu, L.; Wu, W.; Fang, X.; Kong, J. Microfluidic immunoassays for sensitive and simultaneous detection of IgG/IgM/Antigen of SARS-CoV-2 within 15 min. Anal. Chem. 2020, 92, 9454–9458. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.C.; Herman, J.H.; Angell, J.B. A Gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Mcdonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward practical application of paper-based microfluidics for medical diagnostics: State-of-the-art and challenges. Lab Chip 2017, 17, 1206–1249. [Google Scholar] [CrossRef]
- Bircsak, K.M.; Debiasio, R.; Miedel, M.; Alsebahi, A.; Gough, A. A 3D microfluidic liver model for high throughput compound toxicity screening in the organoplate. Toxicology 2021, 450, 152667. [Google Scholar] [CrossRef]
- Yang, K.; Peretz-Soroka, H.; Liu, Y.; Lin, F. Novel developments in mobile sensing based on the integration of microfluidic devices and smartphones. Lab Chip 2016, 16, 943–958. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Yang, J.; Xue, J.; Zhu, P.; Liu, L.; Li, S. Detection of SARS-CoV-2 RNA residue on object surfaces in nucleic acid testing laboratory using droplet digital PCR. Sci. Total Environ. 2020, 742, 140370. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Freitas, D.N.; Mongersun, A.; Chau, H.; Araci, I.E. Tunable soft lithography molds enable rapid-prototyping of multi-height channels for microfluidic large-scale integration. J. Micromech. Microeng. 2019, 29, 035009. [Google Scholar] [CrossRef]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft lithography for microfluidics: A Review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Sameoto, D.; Wasay, A. Materials selection and manufacturing of thermoplastic elastomer microfluidics. Proc. SPIE 2015, 9320, 932001. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.; Wang, L.; Xiao, K.; Wen, W. A simple method for fabricating multi-layer PDMS structures for 3D microfluidic chips. Lab Chip 2010, 10, 1199–1203. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, H.; Zhou, W.; Ma, L.; Perez, S.; Ibarra, A.; Xu, F.; Zhan, S.; Li, X.J. Recent advances in microfluidic platforms for single-cell analysis in cancer biology, diagnosis and therapy. TrAC Trends Anal. Chem. 2019, 117, 13–26. [Google Scholar] [CrossRef]
- Magro, L.; Escadafal, C.; Garneret, P.; Jacquelin, B.; Kwasiborski, A.; Manuguerra, J.C.; Monti, F.; Sakuntabhai, A.; Vanhomwegen, J.; Lafaye, P.; et al. Paper microfluidics for nucleic acid amplification testing (NAAT) of infectious diseases. Lab Chip 2017, 17, 2347–2371. [Google Scholar] [CrossRef] [Green Version]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Yang, Y.; Chu, J.; He, B. Emerging paper microfluidic devices. Analyst 2019, 144, 6497–6511. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Jiang, N.; Tamayol, A.; Ruiz-Esparza, G.U.; Zhang, Y.S.; Medina-Pando, S.; Gupta, A.; Wolffsohn, J.S.; Butt, H.; Khademhosseini, A.; et al. Paper-based microfluidic system for tear electrolyte analysis. Lab Chip 2017, 17, 1137–1148. [Google Scholar] [CrossRef] [Green Version]
- Becker, H. Hype, hope and hubris: The quest for the killer application in microfluidics. Lab Chip 2009, 9, 2119–2122. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Du, Z.; Xu, Y.; Sun, W. The crossing and integration between microfluidic technology and 3D printing for organ-on-chips. J. Mater. Chem. B 2018, 6, 6191–6206. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics Nirveek. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.N.; Shu, Y.; Xiong, B.; Chen, Y.; Chen, Y.; Tian, Q.; Michael, S.A.; Shen, B.; Wu, H. Simple, cost-effective 3D printed microfluidic components for disposable, point-of-care colorimetric analysis. ACS Sens. 2016, 1, 227–234. [Google Scholar] [CrossRef]
- Zoupanou, S.; Chiriacò, M.S.; Tarantini, I.; Ferrara, F. Innovative 3D microfluidic tools for on-chip fluids and particles manipulation: From design to experimental validation. Micromachines 2021, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.N.; Tan, M.J.A.; Wu, H. Point-of-care testing: Applications of 3D printing. Lab Chip 2017, 17, 2713–2739. [Google Scholar] [CrossRef]
- Song, J.; Mauk, M.G.; Hackett, B.A.; Cherry, S.; Bau, H.H.; Liu, C. Instrument-free point-of-care molecular detection of zika virus. Anal. Chem. 2016, 88, 7289–7294. [Google Scholar] [CrossRef] [Green Version]
- Kadimisetty, K.; Song, J.; Doto, A.M.; Hwang, Y.; Peng, J.; Mauk, M.G.; Bushman, F.D.; Gross, R.; Jarvis, J.N.; Liu, C. Fully 3D printed integrated reactor array for point-of-care molecular diagnostics. Biosens. Bioelectron. 2018, 109, 156–163. [Google Scholar] [CrossRef]
- Wojtczak, J.; Bonadonna, P. Pocket mobile smartphone system for the point-of-care submandibular ultrasonography. Am. J. Emerg. Med. 2013, 31, 573–577. [Google Scholar] [CrossRef]
- Álvaro, S.; Marín, G.; Vincent, S.; Marín, Á.G.; Hoeve, W.V.; García-Sánchez, P.; Convine, N.; Rosser-James, A.; Tyler, M.; Bandoo, K.; et al. Label-free biodetection using a smartphone. Lab Chip 2013, 15, 4491–4498. [Google Scholar]
- Xu, X.; Akay, A.; Wei, H.; Wang, S.; Pingguan-Murphy, B.; Erlandsson, B.E.; Li, X.; Lee, W.; Hu, J.; Wang, L.; et al. Advances in smartphone-based point-of-care diagnostics. Proc. IEEE 2015, 103, 236–247. [Google Scholar] [CrossRef]
- Kaarj, K.; Akarapipad, P.; Yoon, J.Y. Simpler, faster, and sensitive zika virus assay using smartphone detection of loop-mediated isothermal amplification on paper microfluidic chips. Sci. Rep. 2018, 8, 12438. [Google Scholar] [CrossRef] [PubMed]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper-plastic hybrid microfluidic device for smartphone-based colorimetric analysis of urine. Anal. Chem. 2017, 89, 13160–13166. [Google Scholar] [CrossRef]
- Mielczarek, W.S.; Obaje, E.A.; Bachmann, T.T.; Kersaudy-Kerhoas, M. Microfluidic blood plasma separation for medical diagnostics: Is it worth it? Lab Chip 2016, 16, 3441–3448. [Google Scholar] [CrossRef] [Green Version]
- Bond, M.; Richards-Kortum, R. Diagnostics for global health: Hand-spun centrifuge. Nat. Biomed. Eng. 2017, 1, 17. [Google Scholar] [CrossRef]
- Al-Faqheri, W.; Thio, T.H.G.; Qasaimeh, M.A.; Dietzel, A.; Madou, M.; Al-Halhouli, A. Particle/cell separation on microfluidic platforms based on centrifugation effect: A review. Microfluid. Nanofluid. 2017, 21, 102. [Google Scholar] [CrossRef]
- Dineva, M.A.; Mahilum-Tapay, L.; Lee, H. Sample preparation: A challenge in the development of point-of-care nucleic acid-based assays for resource-limited settings. Analyst 2007, 132, 1193–1199. [Google Scholar] [CrossRef]
- Bhamla, M.S.; Benson, B.; Chai, C.; Katsikis, G.; Johri, A.; Prakash, M. Hand-powered ultralow-cost paper centrifuge. Nat. Biomed. Eng. 2017, 1, 9. [Google Scholar] [CrossRef]
- Li, B.; Qi, J.; Fu, L.; Han, J.; Choo, J.; deMello, A.J.; Lin, B.; Chen, L. Integrated hand-powered centrifugation and paper-based diagnosis with blood-in/answer-out capabilities. Biosens. Bioelectron. 2020, 165, 112282. [Google Scholar] [CrossRef]
- Papadakis, G.; Pantazis, A.K.; Ntogka, M.; Parasyris, K.; Theodosi, G.I.; Kaprou, G.; Gizeli, E. 3D-printed point-of-care platform for genetic testing of infectious diseases directly in human samples using acoustic sensors and a smartphone. ACS Sens. 2019, 4, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Byagathvalli, G.; Pomerantz, A.F.; Sinha, S.; Standeven, J.; Bhamla, M.S. A 3D-printed hand-powered centrifuge for molecular biology. PLoS Biol. 2019, 17, e3000251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandika, M. A conversation with Manu Prakash. ACS Cent. Sci. 2017, 3, 148–149. [Google Scholar] [CrossRef]
- Pohl, H.A. The motion and precipitation of suspensoids in divergent electric fields. J. Appl. Phys. 1951, 22, 869–871. [Google Scholar] [CrossRef]
- Zaman, M.A.; Wu, M.; Padhy, P.; Jensen, M.A.; Hesselink, L.; Davis, R.W. Modeling brownian microparticle trajectories in lab-on-a-chip devices with time varying dielectrophoretic or optical forces. Micromachines 2021, 12, 1265. [Google Scholar] [CrossRef]
- Han, K.H.; Frazier, A.B. Lateral-driven continuous dielectrophoretic microseparators for blood cells suspended in a highly conductive medium. Lab Chip 2008, 8, 1079–1086. [Google Scholar] [CrossRef]
- Demircan, Y.; Özgür, E.; Külah, H. Dielectrophoresis: Applications and future outlook in point of care. Electrophoresis 2013, 34, 1008–1027. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lai, C.P.K.; Chen, C.; Lee, G. Bin Isolation and recovery of extracellular vesicles using optically-induced dielectrophoresis on an integrated microfluidic platform. Lab Chip 2021, 21, 1475–1483. [Google Scholar] [CrossRef]
- Sahin, O.; Elitas, M.; Yapici, M.K. Simulation of dielectrophoresis based separation of red blood cells (RBC) from bacteria cells. In Proceedings of the 2020 21st International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems (EuroSimE), Cracow, Poland, 5–8 July 2020. [Google Scholar] [CrossRef]
- Hu, J.; Cui, X.; Gong, Y.; Xu, X.; Gao, B.; Wen, T.; Lu, T.J.; Xu, F. Portable microfluidic and smartphone-based devices for monitoring of cardiovascular diseases at the point of care. Biotechnol. Adv. 2016, 34, 305–320. [Google Scholar] [CrossRef]
- Reyes, D.R.; Van Heeren, H.; Guha, S.; Herbertson, L.; Tzannis, A.P.; Ducree, J.; Bissig, H.; Becker, H. Accelerating innovation and commercialization through standardization of microfluidic-based medical devices. Lab Chip 2021, 21, 9–21. [Google Scholar] [CrossRef]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic point-of-care testing: Commercial landscape and future directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, T.; Guo, J.; Lu, H.; Yao, Y.; Chen, X.; Zhang, X.; Sui, G.; Guan, M. A LAMP-based microfluidic module for rapid detection of pathogen in cryptococcal meningitis. Talanta 2022, 236, 122827. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Bhuiyan, N.H.; Shim, J.S. Fully integrated rapid microfluidic device translated from conventional 96-well ELISA kit. Sci. Rep. 2021, 11, 1986. [Google Scholar] [CrossRef] [PubMed]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.I.; Haswell, S.; Gibson, I. Lab-on-a-chip or chip-in-a-lab: Challenges of commercialization lost in translation. Proced. Technol. 2015, 20, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed point-of-care testing—xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: A retrospective observational study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Lamb, L.E.; Bartolone, S.N.; Ward, E.; Chancellor, M.B. Rapid detection of novel coronavirus/Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by reverse transcription-loop-mediated isothermal amplification. PLoS ONE 2020, 15, e0234682. [Google Scholar] [CrossRef]
- Gates, B. Responding to COVID-19—A once-in-a-century pandemic? N. Engl. J. Med. 2020, 382, 1677–1679. [Google Scholar] [CrossRef]
- BEDYŃSKI, W. Liminality: Black death 700 years later. what lessons are for us from the medieval pandemic? Soc. Regist. 2020, 4, 129–144. [Google Scholar] [CrossRef]
- Ratre, Y.K.; Vishvakarma, N.K.; Bhaskar, L.V.K.S.; Verma, H.K. Dynamic propagation and impact of pandemic influenza A (2009 H1N1) in children: A detailed review. Curr. Microbiol. 2020, 77, 3809–3820. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; Chu, C.M.; Cheng, V.C.C.; Chan, K.S.; Hung, I.F.N.; Poon, L.L.M.; Law, K.I.; Tang, B.S.F.; Hon, T.Y.W.; Chan, C.S.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, K.R.; Mykhalovskiy, E.; Worthington, C.; Gómez-Ramírez, O.; Gilbert, M.; Grace, D. Pay to skip the line: The political economy of digital testing services for HIV and other sexually transmitted infections. Soc. Sci. Med. 2021, 268, 113571. [Google Scholar] [CrossRef] [PubMed]
- De Groote, M.A.; Sterling, D.G.; Hraha, T.; Russell, T.M.; Green, L.S.; Wall, K.; Kraemer, S.; Ostroff, R.; Janjic, N.; Ochsner, U.A. Discovery and validation of a six-marker serum protein signature for the diagnosis of active pulmonary tuberculosis. J. Clin. Microbiol. 2017, 55, 3057–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- Chen, Q.; He, Z.; Mao, F.; Pei, H.; Cao, H.; Liu, X. Diagnostic technologies for COVID-19: A review. RSC Adv. 2020, 10, 35257–35264. [Google Scholar] [CrossRef]
- Tayyab, M.; Sami, M.A.; Raji, H.; Mushnoori, S.; Javanmard, M. Potential microfluidic devices for COVID-19 antibody detection at point-of-care (POC): A review. IEEE Sens. J. 2021, 21, 4007–4017. [Google Scholar] [CrossRef]
- To, K.K.W.; Tsang, O.T.Y.; Yip, C.C.Y.; Chan, K.H.; Wu, T.C.; Chan, J.M.C.; Leung, W.S.; Chik, T.S.H.; Choi, C.Y.C.; Kandamby, D.H.; et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020, 71, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Davidson, J.L.; Wang, J.; Maruthamuthu, M.K.; Dextre, A.; Pascual-Garrigos, A.; Mohan, S.; Putikam, S.V.S.; Osman, F.O.I.; McChesney, D.; Seville, J.; et al. A paper-based colorimetric molecular test for SARS-CoV-2 in saliva. Biosens. Bioelectron. X 2021, 9, 100076. [Google Scholar] [CrossRef]
- Wang, J.; Dextre, A.; Pascual-Garrigos, A.; Davidson, J.L.; McChesney, D.; Seville, J.; Verma, M.S. Fabrication of a paper-based colorimetric molecular test for SARS-CoV-2. MethodsX 2021, 8, 101586. [Google Scholar] [CrossRef]
- Farshidfar, N.; Hamedani, S. The potential role of smartphone-based microfluidic systems for rapid detection of COVID-19 using saliva specimen. Mol. Diagn. Ther. 2020, 24, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Pérez, L.; Li, X.; Liu, Y.; Xu, P.; Boritz, E.A.; Mullins, J.I.; Abate, A.R. Droplet microfluidic sequencing of HIV genomes and integration sites. bioRxiv 2020, 20, 314120. [Google Scholar] [CrossRef]
- Soares, R.R.G.; Varela, J.C.; Neogi, U.; Ciftci, S.; Ashokkumar, M.; Pinto, I.F.; Nilsson, M.; Madaboosi, N.; Russom, A. Sub-attomole detection of HIV-1 using padlock probes and rolling circle amplification combined with microfluidic affinity chromatography. Biosens. Bioelectron. 2020, 166, 112442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chang, L.; Wang, L. Nucleic acid testing and molecular characterization of HIV infections. Eur. J. Clin. Microbiol. 2019, 38, 829–842. [Google Scholar] [CrossRef]
- Yang, W.; Yang, D.; Gong, S.; Dong, X.; Liu, L.; Yu, S.; Zhang, X.; Ge, S.; Wang, D.; Xia, N.; et al. An immunoassay cassette with a handheld reader for HIV urine testing in point-of-care diagnostics. Biomed. Microdevices 2020, 22, 39. [Google Scholar] [CrossRef]
- Cao, X.F.; Li, Y.; Xin, H.N.; Zhang, H.R.; Pai, M.; Gao, L. Application of artificial intelligence in digital chest radiography reading for pulmonary tuberculosis screening. Chronic Dis. Transl. Med. 2021, 7, 35–40. [Google Scholar] [CrossRef]
- Mbano, I.M.; Mandizvo, T.; Rogich, J.; Kunota, T.T.R.; Mackenzie, J.S.; Pillay, M.; Balagaddé, F.K. Light forge: A microfluidic DNA melting-based tuberculosis test. J. Appl. Lab. Med. 2020, 5, 440–453. [Google Scholar] [CrossRef]
- Bashshur, R.; Doarn, C.R.; Frenk, J.M.; Kvedar, J.C.; Woolliscroft, J.O. Telemedicine and the COVID-19 pandemic, lessons for the future. Telemed. e-Health 2020, 26, 571–573. [Google Scholar] [CrossRef] [Green Version]
- Alterovitz, G.; Alterovitz, W.L.; Cassell, G.H.; Zhang, L.; Dunker, A.K. AI for infectious disease modelling and therapeutics. Biocomputing 2021, 2020, 91–94. [Google Scholar]
- Overton, C.E.; Stage, H.B.; Ahmad, S.; Curran-Sebastian, J.; Dark, P.; Das, R.; Fearon, E.; Felton, T.; Fyles, M.; Gent, N.; et al. Using statistics and mathematical modelling to understand infectious disease outbreaks: COVID-19 as an example. Infect. Dis. Model. 2020, 5, 409–441. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Cruz, K.R.; Vásques, E.M.M.; de Oliveira, O.N. Microfluidic point-of-care devices: New trends and future prospects for ehealth diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshadri, D.R.; Davies, E.V.; Harlow, E.R.; Hsu, J.J.; Knighton, S.C.; Walker, T.A.; Voos, J.E.; Drummond, C.K. Wearable sensors for COVID-19: A call to action to harness our digital infrastructure for remote patient monitoring and virtual assessments. Front. Digit. Health 2020, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Coombes, C.E.; Gregory, M.E. The current and future use of telemedicine in infectious diseases practice. Curr. Infect. Dis. Rep. 2019, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, X. A portable paper-based microfluidic platform for multiplexed electrochemical detection of human immunodeficiency virus and hepatitis C virus antibodies in serum. Biomicrofluidics 2016, 10, 024119. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in medicine. Adv. Mater. 2018, 30, e1706910. [Google Scholar] [CrossRef]
- Yadav, A.K.; Verma, D.; Kumar, A.; Kumar, P.; Solanki, P.R. The perspectives of biomarker-based electrochemical immunosensors, artificial intelligence and the Internet of Medical Things toward COVID-19 diagnosis and management. Mater. Today Chem. 2021, 20, 100443. [Google Scholar] [CrossRef]
- Li, D. 5G and intelligence medicine—How the next generation of wireless technology will reconstruct healthcare? Precis. Clin. Med. 2019, 2, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Hong, Z.; Li, N.; Li, D.; Li, J.; Li, B.; Xiong, W.; Lu, L.; Li, W.; Zhou, D. Telemedicine during the COVID-19 pandemic: Experiences from Western China. J. Med. Internet Res. 2020, 22, e19577. [Google Scholar] [CrossRef]
- Lees, J.S.; Welsh, C.E.; Celis-Morales, C.A.; Mackay, D.; Lewsey, J.; Gray, S.R.; Lyall, D.M.; Cleland, J.G.; Gill, J.; Jhund, P.S. Author correction: Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat. Med. 2020, 26, 1308. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Chen, J.; Liu, J.; Wang, R.; Xu, X.; Yao, J.; Guo, J. Smartphone-based blood lipid data acquisition for cardiovascular disease management in internet of medical things. IEEE Access 2019, 7, 75276–75283. [Google Scholar] [CrossRef]
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S.; et al. World health organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, D.; Miao, F.; Liu, H.; Li, Y. Personalized hemodynamic modeling of the human cardiovascular system: A reduced-order computing model. IEEE Trans. Biomed. Eng. 2020, 67, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Crosby, J.R.; Decook, K.J.; Tran, P.L.; Smith, R.G.; Larson, D.F.; Khalpey, Z.I.; Burkhoff, D.; Slepian, M.J. Physiological characterization of the SynCardia total artificial heart in a mock circulation system. ASAIO J. 2015, 61, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y.; Ali, F. Atherosclerotic cardiovascular disease: A review of initiators and protective factors. Inflammopharmacology 2016, 24, 1–10. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular disease in the developing world: Prevalences, patterns, and the potential of early disease detection. J. Am. Coll. Cardiol. 2012, 60, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Tian, S.; Zhao, W.; Liu, K.; Ma, X.; Guo, J. Multiplexed detection of biomarkers in lateral-flow immunoassays. Analyst 2020, 145, 2828–2840. [Google Scholar] [CrossRef]
- Ouyang, M.; Tu, D.; Tong, L.; Sarwar, M.; Bhimaraj, A.; Li, C.; Coté, G.L.; Di Carlo, D. A review of biosensor technologies for blood biomarkers toward monitoring cardiovascular diseases at the point-of-care. Biosens. Bioelectron. 2021, 171, 112621. [Google Scholar] [CrossRef]
- Deng, H.; Zhou, X.; Liu, Q.; Li, B.; Liu, H.; Huang, R.; Xing, D. Paperfluidic chip device for small RNA extraction, amplification, and multiplexed analysis. ACS Appl. Mater. Interfaces 2017, 9, 41151–41158. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, H.; Xu, F.; Wang, X.; Sun, W. Microfluidics in cardiovascular disease research: State of the art and future outlook. Microsyst. Nanoeng. 2021, 7, 19. [Google Scholar] [CrossRef]
- Yan, J.J.; Yang, Q.L.; Li, W.L.; Yu, J.; Xie, J.; Xiang, J.J.; Wang, H. Two desired epitopes of cTnI benefit for preparation of standardized monoclonal antibodies. Chirality 2019, 31, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, Y.; Liu, Y.; Liu, H.; Fu, L.; Wen, J.; Li, J.; Wei, P.; Chen, L. A graphene oxide/gold nanoparticle-based amplification method for SERS immunoassay of cardiac troponin I. Analyst 2019, 144, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, L.; Hu, Y.; Li, Z.; Liu, J.; He, J.; Cui, H. Multiplexed chemiluminescence determination of three acute myocardial infarction biomarkers based on microfluidic paper-based immunodevice dual amplified by multifunctionalized gold nanoparticles. Talanta 2020, 207, 120346. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, S.; Jang, I.; Noviana, E.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Electrochemical paper-based analytical device for multiplexed, point-of-care detection of cardiovascular disease biomarkers. Sens. Actuators B Chem. 2021, 330, 129336. [Google Scholar] [CrossRef]
- Sinha, A.; Tai, T.Y.; Li, K.H.; Gopinathan, P.; Chung, Y.D.; Sarangadharan, I.; Ma, H.P.; Huang, P.C.; Shiesh, S.C.; Wang, Y.L.; et al. An integrated microfluidic system with field-effect-transistor sensor arrays for detecting multiple cardiovascular biomarkers from clinical samples. Biosens. Bioelectron. 2019, 129, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Rama, E.C.; Costa-García, A. Screen-printed electrochemical immunosensors for the detection of cancer and cardiovascular biomarkers. Electroanalysis 2016, 28, 1700–1715. [Google Scholar] [CrossRef]
- Khan, R.; Khurshid, Z.; Yahya, I.A.F. Advancing point-of-care (PoC) testing using human saliva as liquid biopsy. Diagnostics 2017, 7, 39. [Google Scholar] [CrossRef]
- Abdul Rehman, S.; Khurshid, Z.; Hussain Niazi, F.; Naseem, M.; Al Waddani, H.; Sahibzada, H.A.; Sannam Khan, R. Role of salivary biomarkers in detection of cardiovascular diseases (CVD). Proteomes 2017, 5, 21. [Google Scholar] [CrossRef]
- Ramasamy, M.; Varadan, V. (Keynote) Wireless wearable and implantable monitoring and therapeutic systems for cardiac and neurological disorders. ECS Trans. 2018, 86, 21–30. [Google Scholar] [CrossRef]
- Vishwanatham, A.; Narendra, C.; Abhishek, S.R.; Ramakrishna, C.R.; Sanagapati, S.S.S.; Mohanty, S. Smart and wearable ECG monitoring system as a Point of Care (POC) device. Int. Symp. Adv. Netw. Telecommun. Syst. ANTS 2018, 2018, 11–14. [Google Scholar] [CrossRef]
- Dinter, F.; Burdukiewicz, M.; Schierack, P.; Lehmann, W.; Nestler, J.; Dame, G.; Rödiger, S. Simultaneous detection and quantification of DNA and protein biomarkers in spectrum of cardiovascular diseases in a microfluidic microbead chip. Anal. Bioanal. Chem. 2019, 411, 7725–7735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.-H.; Chen, P.-S.; Huang, C.-C.; Hung, Y.-T.; Lee, M.-Y.; Lin, W.-H.; Lin, Y.-C.; Lee, A.Y.-L. Unlocking the mystery of the therapeutic effects of chinese medicine on cancer. Front. Pharmacol. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, Q. Understanding the global cancer statistics 2018: Implications for cancer control. Sci. China Life Sci 2019, 64, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lai, H.C.; Liou, T.M.; Hsu, K.F.; Chou, C.Y.; Lee, G.B. A DNA methylation assay for detection of ovarian cancer cells using a HpaII/MspI digestion-based PCR assay in an integrated microfluidic system. Microfluid. Nanofluid. 2013, 15, 575–585. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [Green Version]
- Munge, B.S.; Stracensky, T.; Gamez, K.; Dibiase, D.; Rusling, J.F. Multiplex immunosensor arrays for electrochemical detection of cancer biomarker proteins. Electroanalysis 2016, 28, 2644–2658. [Google Scholar] [CrossRef]
- Nunna, B.B.; Mandal, D.; Zhuang, S.; Lee, E.S. A standalone micro biochip to monitor the cancer progression by measuring cancer antigens as a point-of-care (POC) device for enhanced cancer management. In Proceedings of the 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT), Bethesda, MD, USA, 6–8 November 2017; pp. 212–215. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Yu, W.; Zhang, Y.; Wang, J. Biogenesis, functions, and clinical implications of circular RNAs in non-small cell lung cancer. Mol. Ther. Nucleic Acids 2022, 27, 50–72. [Google Scholar] [CrossRef]
- Yang, Q.; Cheng, L.; Hu, L.; Lou, D.; Zhang, T.; Li, J.; Zhu, Q.; Liu, F. An integrative microfluidic device for isolation and ultrasensitive detection of lung cancer-specific exosomes from patient urine. Biosens. Bioelectron. 2020, 163, 112290. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Filella, X.; Albaladejo, M.D.; Allué, J.A.; Castaño, M.A.; Morell-Garcia, D.; Ruiz, M.À.; Santamaría, M.; Torrejón, M.J.; Giménez, N. Prostate cancer screening: Guidelines review and laboratory issues. Clin. Chem. Lab. Med. 2019, 57, 1474–1487. [Google Scholar] [CrossRef]
- Brawer, M.K.; Beatie, J.; Wener, M.H.; Vessella, R.L.; Preston, S.D.; Lange, P.H. Screening for Prostatic Carcinoma with Prostate Specific Antigen: Results of the Second Year. J. Urol. 1993, 150, 106–109. [Google Scholar] [CrossRef]
- Mandal, N.; Pakira, V.; Samanta, N.; Das, N.; Chakraborty, S.; Pramanick, B.; RoyChaudhuri, C. PSA detection using label free graphene FET with coplanar electrodes based microfluidic point of care diagnostic device. Talanta 2021, 222, 121581. [Google Scholar] [CrossRef] [PubMed]
- Rezqalla, J.; Alshatti, M.; Ibraheem, A.; Omar, D.; Al-Failakawi, H.; Alhaqqan, S.; Alghurair, S.; Akhtar, S. Human Papillomavirus (HPV): Unawareness of causal role HPV infection in cervical cancer, HPV vaccine availability and HPV vaccine uptake among female schoolteachers in a Middle Eastern country. J. Infect. Public Health 2021, 14, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, M. Analytical molecular diagnosis of cervical cancer via paper microfluidic chip. Proceedings 2018, 2, 1556. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Kang, B.; Son, H.Y.; Mun, B.; Huh, Y.M.; Rho, H.W.; Kang, T.; Moon, J.; Lee, J.J.; Seo, S.B.; et al. Microfluidic device for one-step detection of breast cancer-derived exosomal mRNA in blood using signal-amplifiable 3D nanostructure. Biosens. Bioelectron. 2022, 197, 113753. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, W.; Yang, F. Cancer liquid biopsy using integrated microfluidic exosome analysis platforms. Biotechnol. J. 2020, 15, e1900225. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, C.; Bai, S.; Gao, Y.; Metcalfe, G.; Cheng, W.; Zhu, Y. Multiplexed detection of cancer biomarkers using a microfluidic platform integrating single bead trapping and acoustic mixing techniques. Nanoscale 2018, 10, 20196–20206. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, Y.; Cao, Y.; Hong, Z.; Chai, Y. Recent advances in microfluidic technology and applications for anti-cancer drug screening. TrAC Trends Anal. Chem. 2021, 134, 116118. [Google Scholar] [CrossRef]
- Sayani, S.; Muzammil, M.; Saleh, K.; Muqeet, A.; Zaidi, F.; Shaikh, T. Addressing cost and time barriers in chronic disease management through telemedicine: An exploratory research in select low- and middle-income countries. Ther. Adv. Chronic Dis. 2019, 10, 2040622319891587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameh, S.; Klipstein-Grobusch, K.; D’Ambruoso, L.; Kahn, K.; Tollman, S.M.; Gómez-Olivé, F.X. Quality of integrated chronic disease care in rural South Africa: User and provider perspectives. Health Policy Plan. 2017, 32, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, R.; Dennis, S.; Hasan, I.; Slewa, J.; Chen, W.; Tian, D.; Bobba, S.; Zwar, N. A systematic review of chronic disease management interventions in primary care. BMC Fam. Pract. 2018, 19, 11. [Google Scholar] [CrossRef]
- Cockerham, W.C.; Hamby, B.W.; Oates, G.R. The social determinants of chronic disease. Am. J. Prev. Med. 2017, 52, S5–S12. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Taparia, N.; Platten, K.C.; Anderson, K.B.; Sniadecki, N.J. A microfluidic approach for hemoglobin detection in whole blood. AIP Adv. 2017, 7, 105102. [Google Scholar] [CrossRef] [Green Version]
- Plevniak, K.; Campbell, M.; Myers, T.; Hodges, A.; He, M. 3D printed auto-mixing chip enables rapid smartphone diagnosis of anemia. Biomicrofluidics 2016, 10, 054113. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Wu, J.; Ma, Z.; Peretz-Soroka, H.; Zhang, M.; Komenda, P.; Tangri, N.; Liu, Y.; Rigatto, C.; Lin, F. Rapid and low-cost CRP measurement by integrating a paper-based microfluidic immunoassay with smartphone (CRP-Chip). Sensors 2017, 17, 684. [Google Scholar] [CrossRef]
- Ray, A.; Esparza, S.; Wu, D.; Hanudel, M.R.; Joung, H.A.; Gales, B.; Tseng, D.; Salusky, I.B.; Ozcan, A. Measurement of serum phosphate levels using a mobile sensor. Analyst 2020, 145, 1841–1848. [Google Scholar] [CrossRef]
- Redman, E.A.; Ramos-Payan, M.; Mellors, J.S.; Ramsey, J.M. Analysis of hemoglobin glycation using microfluidic CE-MS: A rapid, mass spectrometry compatible method for assessing diabetes management. Anal. Chem. 2016, 88, 5324–5330. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Lakshmipriya, T.; Chen, Y.; Phang, W.M.; Hashim, U. Aptamer-based “point-of-care testing”. Biotechnol. Adv. 2016, 34, 198–208. [Google Scholar] [CrossRef]
- Chang, K.W.; Li, J.; Yang, C.H.; Shiesh, S.C.; Lee, G. Bin an integrated microfluidic system for measurement of glycated hemoglobin Levels by using an aptamer-antibody assay on magnetic beads. Biosens. Bioelectron. 2015, 68, 397–403. [Google Scholar] [CrossRef]
- Choobbari, M.L.; Rad, M.B.; Jahanshahi, A.; Ghourchian, H. A sample volume independent paper microfluidic device for quantifying glucose in real human plasma. Microfluid. Nanofluid. 2020, 24, 221883649. [Google Scholar] [CrossRef]
- Villiger, M.; Stoop, R.; Vetsch, T.; Hohenauer, E.; Pini, M.; Clarys, P.; Pereira, F.; Clijsen, R. Evaluation and review of body fluids saliva, sweat and tear compared to biochemical hydration assessment markers within blood and urine. Eur. J. Clin. Nutr. 2018, 72, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Sandhu, S.V.; Bansal, H.; Sharma, D. Comparison of salivary and serum glucose levels in diabetic patients. J. Diabetes Sci. Technol. 2015, 9, 91–96. [Google Scholar] [CrossRef]
- Castro, L.F.; de Freitas, S.V.; Duarte, L.C.; de Souza, J.A.C.; Paixão, T.R.L.C.; Coltro, W.K.T. Salivary diagnostics on paper microfluidic devices and their use as wearable sensors for glucose monitoring. Anal. Bioanal. Chem. 2019, 411, 4919–4928. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Chen, C.; Sheng, T.; Liu, P.; Zhang, G. A smartphone-assisted microfluidic chemistry analyzer using image-based colorimetric assays for multi-index monitoring of diabetes and hyperlipidemia. Anal. Chim. Acta 2019, 1052, 105–112. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable biosensors: An alternative and practical approach in healthcare and disease monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Gong, M.M.; Sinton, D. Turning the page: Advancing paper-based microfluidics for broad diagnostic application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, X.; Chen, Y.; Li, J.; Shao, H.; Lu, Y.; Pan, L.; Xu, Y.; Cheng, J. Self-served and fully automated biochemical detection of finger-prick blood at home using a portable microfluidic analyzer. Sens. Actuators B Chem. 2020, 303, 127235. [Google Scholar] [CrossRef]
- Addario, G.; Djudjaj, S.; Farè, S.; Boor, P.; Moroni, L.; Mota, C. Microfluidic bioprinting towards a renal in vitro model. Bioprinting 2020, 20, e00108. [Google Scholar] [CrossRef]
- Essaouiba, A.; Okitsu, T.; Kinoshita, R.; Jellali, R.; Shinohara, M.; Danoy, M.; Legallais, C.; Sakai, Y.; Leclerc, E. Development of a pancreas-liver organ-on-chip coculture model for organ-to-organ interaction studies. Biochem. Eng. J. 2020, 164, 107783. [Google Scholar] [CrossRef]
- Mahoney, E.; Kun, J.; Smieja, M.; Fang, Q. Review—Point-of-care urinalysis with emerging sensing and imaging technologies. J. Electrochem. Soc. 2020, 167, 037518. [Google Scholar] [CrossRef]

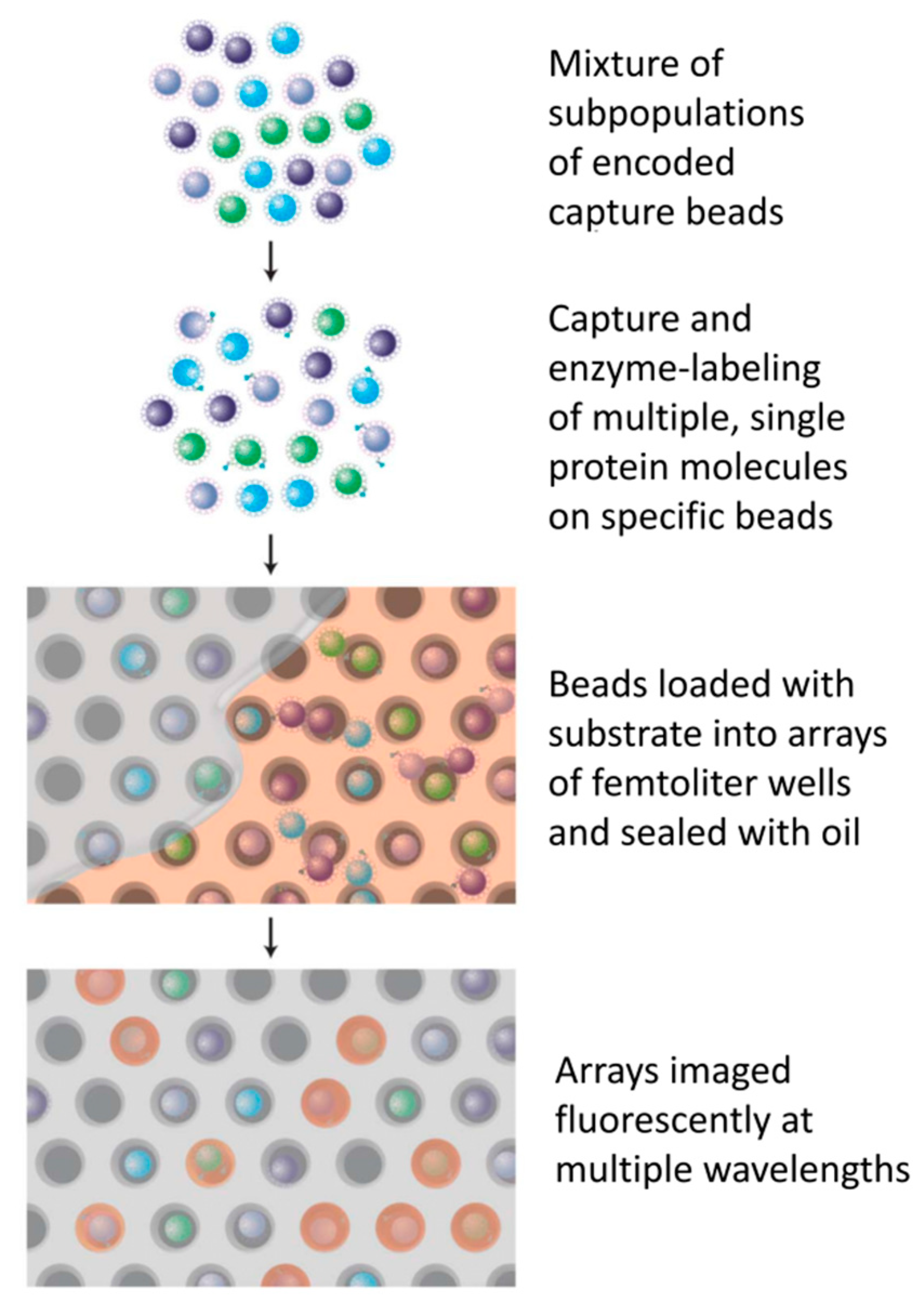
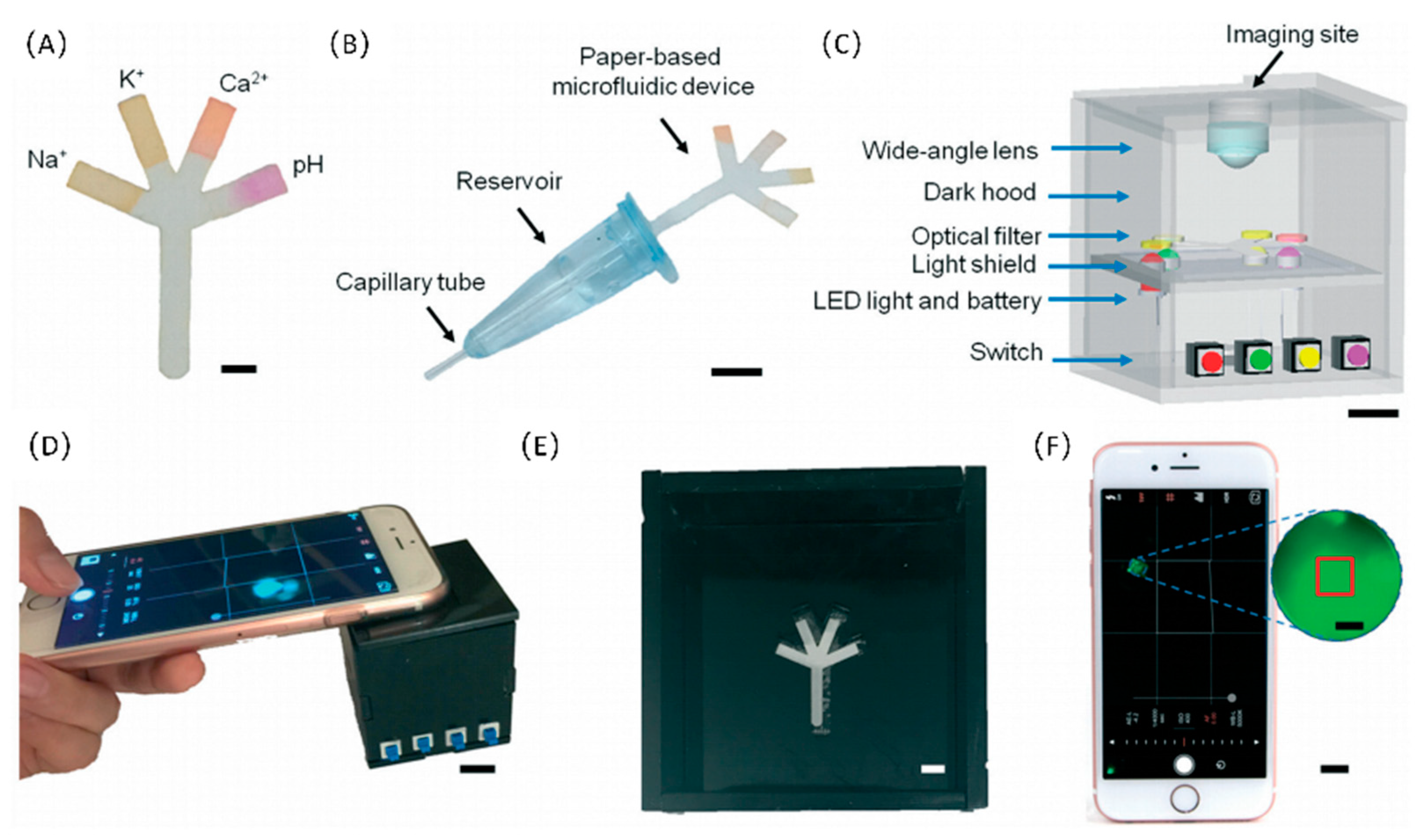


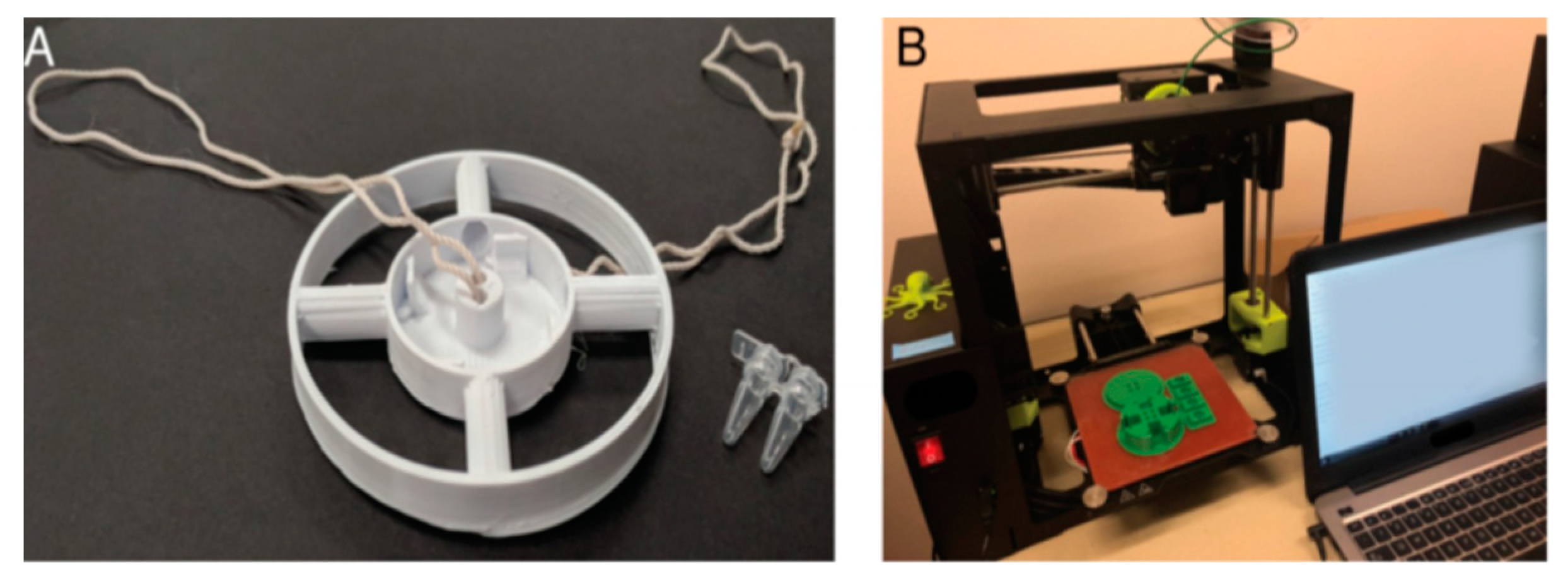
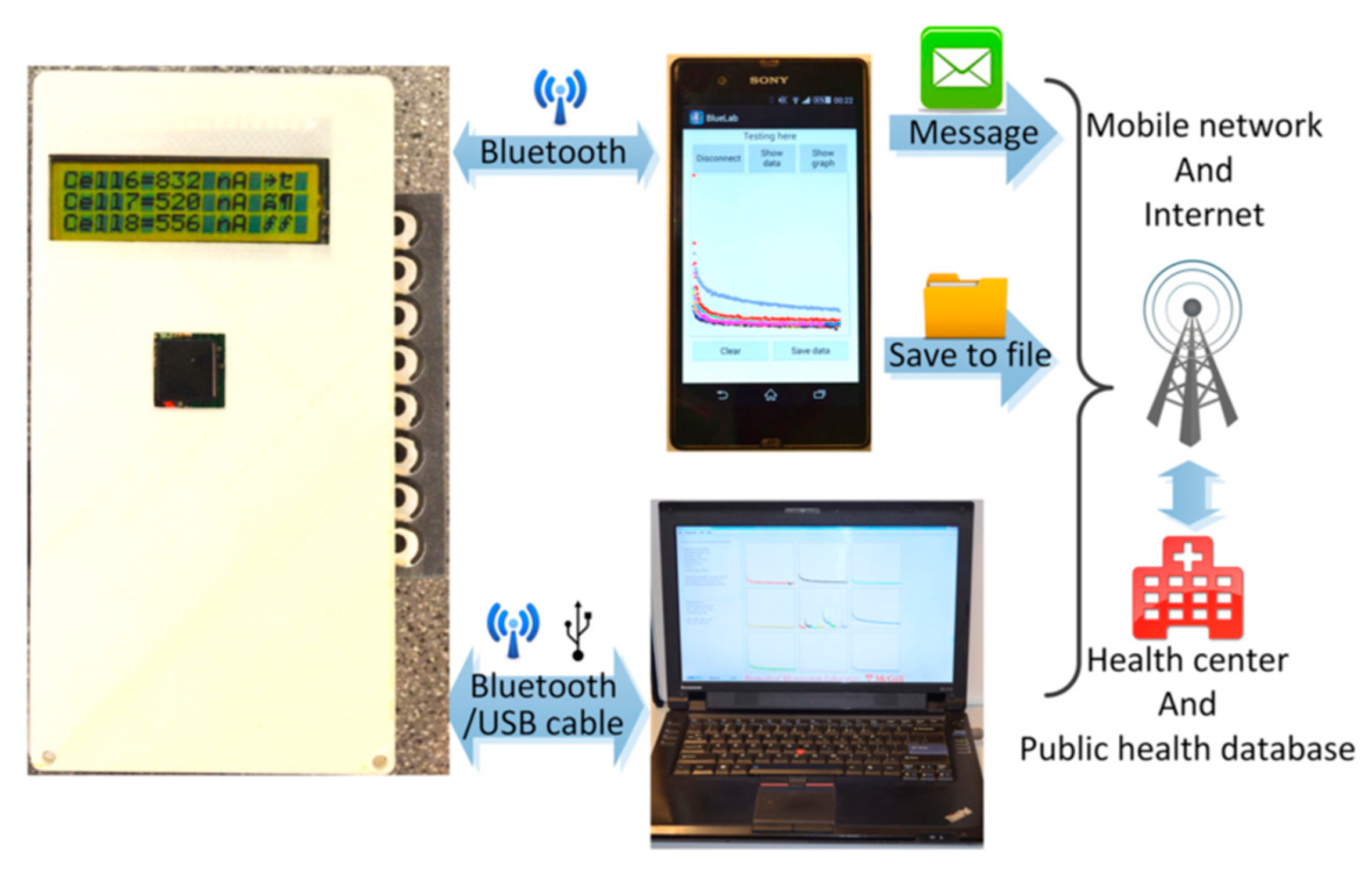

| Microfluidic POC Equipment Types | Constituent | Manufacturing Method | Comment | Ref. |
|---|---|---|---|---|
| Microfluidic equipment made of PDMS | PDMS polymer | Soft lithography | PDMS and soft lithography technology are of great significance to microfluidic devices. | [54] |
| Paper-based microfluidic device | Paper | Patterning technology | Paper chips are fast and inexpensive analysis platforms for the early diagnosis and treatment of diseases. | [61] |
| 3D-printed microfluidic device | Thermoplastic polymers (used for FDM) or photocurable resins (used for SLA) | 3D printing technology | 3D printing technology can make microfluidic devices faster and leverage experimental findings to realize commercial devices. | [69] |
| Mobile sensors based on integrated microfluidic devices and smartphones | Microfluidic chip, smart phone, external sensors | Integration of a mobile phone and microfluidic chip | Data and image processing capabilities of integrated system are key for POC detection. | [92] |
| Handheld centrifugal microfluidic device | PDMS, plastic, paper, and 3D-printed devices | Soft lithography or 3D printing technology | New opportunities for electricity-free POC diagnostics. | [81] |
| Microfluidic POC devices using DEP technology | PDMS polymer, DEP technology | Soft lithography | DEP technology has great potential in the applications of microfluidic POC devices. | [89] |
| Attribute Value | The Advantages of Microfluidic POC Devices | Ref. |
|---|---|---|
| Integration | Microfluidic POC devices have better integration. | [94] |
| Compatibility | The mature technology of the laboratory is easy to migrate to the microfluidic POC devices. | [95,96] |
| Commerciality | There are many categories, which can adapt to more disease detection occasions. The design and manufacturing are more modular and easier to be put into use in large-scale manufacturing. | [97,98,99] |
| Classification | Detection Target | Composition | Sample | Comment | Ref. |
|---|---|---|---|---|---|
| ZIKV | Zika virus | 3D-printed microfluidic device | Saliva | The device can achieve rapid detection of ZIKV. | [70] |
| Wax paper microfluidic chip and smartphone | Serum | The color change of ZIKV RNA in the microfluidic detection area can be seen within 15 min, and virus detection can be completed with a smartphone camera. | [75] | ||
| COVID-19 | IgG, IgM and antigens of SARS-CoV-2 | Microfluidic system integrated with a diagnostic microchip and portable fluorescence detector | Serum or pharyngeal swabs | The assay had high sensitivity and specificity. | [44] |
| SARS-CoV-2 | Paper-based microfluidic device using LAMP | Saliva | This device can detect the virus in a short time and has high analytical sensitivity and specificity. | [112] | |
| Paper-based microfluidic device using RT-LAMP | Saliva | The device can be used as a supplement to current point-of-care and community testing procedures. | [113] | ||
| Microfluidic system based on smartphone | Saliva | The system could have a substantial influence on the epidemiology of the disease. | [114] | ||
| AIDS | HIV | Microfluidic immunoassay box with a handheld optical reader | Urine | The system can provide more convenient, easier to operate, and more affordable HIV urine testing in POC diagnostics. | [118] |
| Tuberculosis | Tuberculosis virus | Microfluidic platform and linear workflow of HRMA | Mycobacteria tuberculosis Isolates | A promising prototype for a fast, low-cost diagnostic alternative for detection of drug resistant strains of tuberculosis in resource-constrained settings. | [120] |
| Classification | Detection Target | Composition | Sample | Comment | Ref. |
|---|---|---|---|---|---|
| Acute myocardial infarction (AMI) | Glycogen phosphorylase isoenzyme BB (GPBB), cTnT, and CK-MB | Paper-based microfluidic device | Serum | The platform has potential for the early diagnosis of AMI. | [43] |
| cTnI, H-FABP, and copeptin | 3D microfluidic paper analysis device (μPAD) | Serum | The developed platform has great potential for the early diagnosis of AMI. | [146] | |
| Detection of multiple biomarkers for cardiovascular disease | C-reactive protein (CRP), troponin I (cTnI), and procalcitonin (PCT) | Paper-based microfluidic | Serum | The proposed immunosensor can be a great alternative for the early detection of cardiovascular diseases at the point of care. | [147] |
| C-reactive protein (CRP), N-terminal pro b-type natriuretic peptide (NT-proB NP), cardiac troponin I (cTnl), and fibrinogen | Integrated microfluidic POC system with a field-effect transistor (FET) sensor | Serum | The sensor is promising for next-generation point-of-care devices assaying multiple CVDs biomarkers in clinical samples. | [148] |
| Classification | Detection Target | Composition | Sample | Comment | Ref. |
|---|---|---|---|---|---|
| Cancer biomarker detection | Carcinoembryonic antigens (CEA) and alpha fetoproteins (AFP) | Fully Integrated hand-powered centrifuge and analysis paper-based microfluidic device () | Serum | The system successfully performed ELISA analysis of carcinoembryonic antigen and alpha fetoprotein from human blood samples. | [82] |
| DNA methylation of tumor suppressor genes | PDMS microfluidic device | Cells, ascites, and serums | This developed microsystem may be promising for rapid and early diagnosis of cancers. | [157] | |
| CA-125 biomarker | A POC system combining a biochip and microfluidics | Blood through finger puncture | The system facilitates monitoring cancer progression and enables enhanced cancer management. | [160] | |
| Lung cancer (LC) | Lung cancer specific exosomes | An integrative microfluidic device | Urine | The device has high sensitivity and specificity in isolating and detecting cancer-specific exosomes from patients’ urine. | [162] |
| Prostate cancer (PCa) | PSA concentration | System integrating dielectrophoresis (DEP), graphene field-effect transistors (FETs) and a compact disc–based microfluidic | Serum | The system was validated satisfactorily with commercially available existing systems using human serum samples. | [165] |
| Cervical cancer | HPV 16 and HPV 18 | Paper-based microfluidic chip | Serum | This low-cost POC device requires less than 40 min to complete the test and has a low limit of detection. | [168] |
| Breast cancer | ERBB2 | A microfluidic chip-based exosomal mRNA sensor | Serum | The system was proven to be effective for cancer diagnosis and liquid biopsies. | [169] |
| Classification | Detection Target | Composition | Sample | Comment | Ref. |
|---|---|---|---|---|---|
| Anemia | Hemoglobin concentration | Microfluidic system that uses optical methods | Serum | The system provides an approach that uses microfluidic detection of hemoglobin levels that can be integrated with other microfluidic approaches for blood analysis. | [179] |
| Hemoglobin concentration | System integrating a 3D-printed microfluidic chip with a smartphone | Serum | This work presents a novel diagnostic strategy for advancing personalized medicine and mobile healthcare. | [180] | |
| Chronic heart disease and chronic kidney disease | C-reactive protein (CRP) | System integrating a paper-based microfluidic immunoassay and a smartphone | Serum | The system has potential for future clinical POC chronic disease diagnosis and risk stratification through parallel measurements of a panel of protein biomarkers. | [181] |
| Advanced chronic kidney disease (CKD) | Phosphate concentration | Paper-based microfluidic device and a 3D-printed smartphone attachment | Serum | The device can potentially be used on a daily basis by patients at home. | [182] |
| Diabetes | Glycated hemoglobin | PDMS microfluidic device | Serum | The system enables earlier diabetes screening and diagnosis at a lower cost and earlier phase, minimizing the risk of diabetic complications. | [185] |
| Paper-based microfluidic device using colorimetry | Saliva | Paper-based microfluidic devices have great potential for salivary diagnostics. | [189] | ||
| Glucose | System integrating a paper-based microfluidic device and a smartphone | Serum | The paper-based microfluidic device is not susceptible to changes of sample volume. | [186] | |
| Paper-based microfluidic device using colorimetry | Saliva | Paper-based microfluidic devices have great potential for salivary diagnostics | [189] | ||
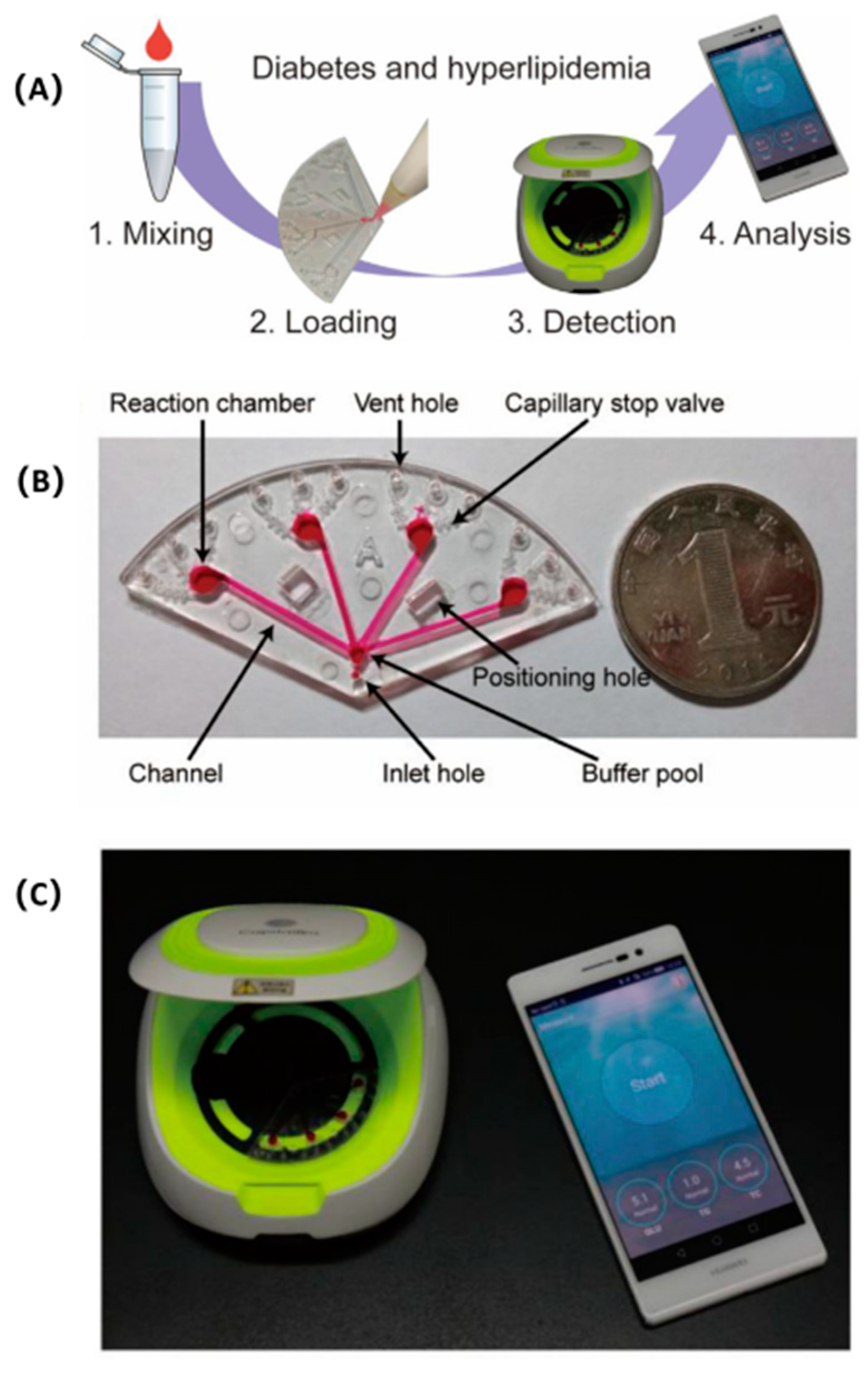
| Diabetes and hyperlipidemia | Glucose (GLU), triglycerides (TG) and total cholesterol (TC) | Smartphone-assisted microfluidic chemical analyzer | Serum | This study demonstrated the feasibility of performing multi-index monitoring of diabetes. | [190] |
| Early dry eye disease | The electrolytes in tears | Paper-based microfluidic device and a smartphone | Tears | The system demonstrates the feasibility for the detection of early-stage dry eye, differential diagnosis of dry eye sub-types, and the severity of the condition. | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. https://doi.org/10.3390/s22041620
Yang S-M, Lv S, Zhang W, Cui Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors. 2022; 22(4):1620. https://doi.org/10.3390/s22041620
Chicago/Turabian StyleYang, Shih-Mo, Shuangsong Lv, Wenjun Zhang, and Yubao Cui. 2022. "Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges" Sensors 22, no. 4: 1620. https://doi.org/10.3390/s22041620
APA StyleYang, S.-M., Lv, S., Zhang, W., & Cui, Y. (2022). Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors, 22(4), 1620. https://doi.org/10.3390/s22041620







