Part-Per-Billion Level Chemical Sensing with a Gold-Based SERS-Active Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the SERS Substrate
2.3. SERS Measurements
2.3.1. Sample Preparation
2.3.2. Instrumentation
2.4. Data Analysis
3. Results and Discussion
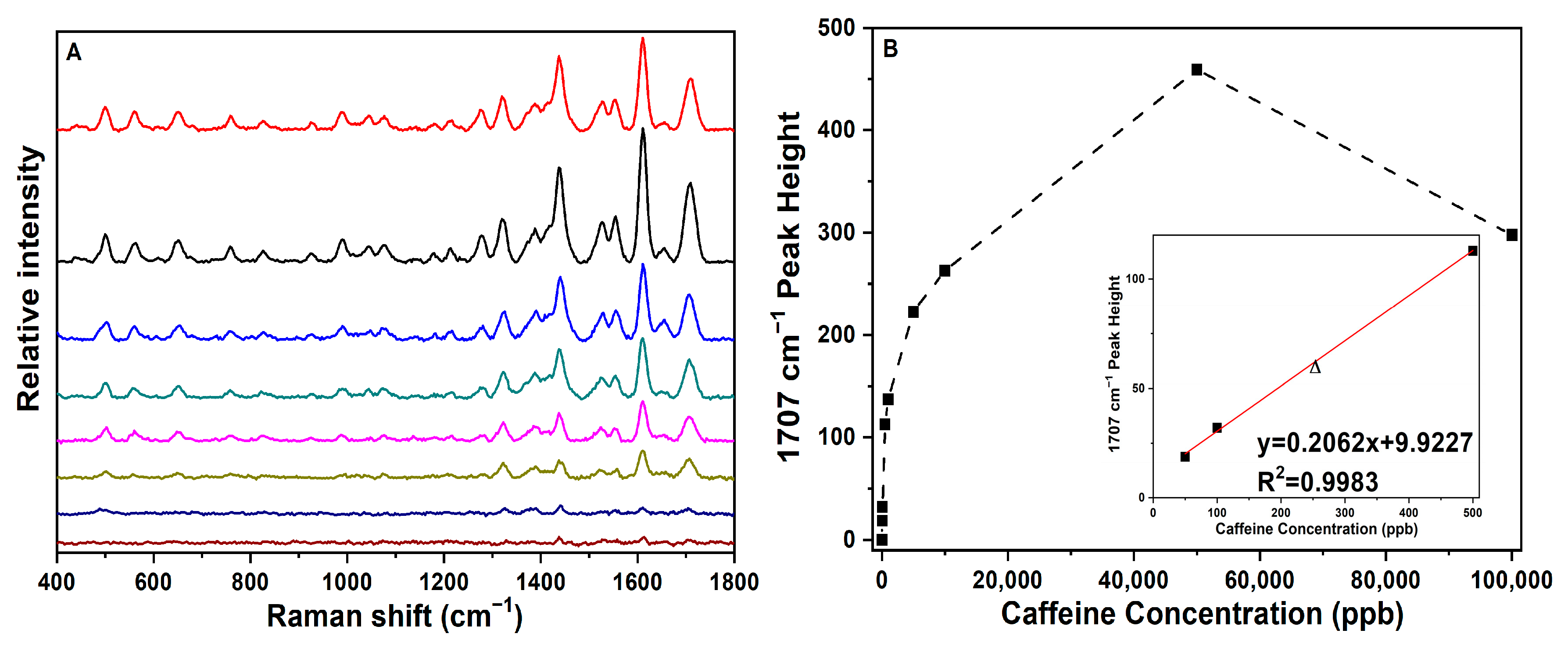
3.1. SERS Spectral Reference Measurement of Caffeine and Band Assignments
3.2. SERS LMC Determination, LOD Estimation, and Quantification of Caffeine
3.3. Effects of pH on Caffeine Measurements with the SERS-Active Substrate
3.4. Reproducibility of the SERS Substrate with Caffeine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-Enhanced Raman Scattering (SERS)—A Tool for Single Molecule Detection in Solution. In Single Molecule Detection in Solution: Methods and Applications; Enderlein, J., Keller, R.A., Zander, C., Eds.; VCH-Wiley: Weinheim, Germany, 2001. [Google Scholar]
- Creighton, J.A. Contributions to the early development of surface-enhanced Raman spectroscopy. Notes Rec. R. Soc. 2010, 64, 175–183. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-Enhanced Raman Spectroscopy: A Brief Perspective. In Surface-Enhanced Raman Scattering; Kneipp, K., Moskovits, M., Kneipp, H., Eds.; Springer: Cham, Switzerland, 2006; Volume 18, pp. 1–17. [Google Scholar]
- Pilot, R. SERS detection of food contaminants by means of portable Raman instruments. J. Raman Spectrosc. 2018, 49, 954–981. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Monitoring and characterization of polyaromatic compounds in the environment. Talanta 1998, 47, 943–969. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.; Bazan, G.; Bell, S.; Boisen, A.; Brolo, A.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muehlethaler, C.; Leona, M.; Lombardi, J.R. Towards a validation of surface-enhanced Raman scattering (SERS) for use in forensic science: Repeatability and reproducibility experiments. Forensic Sci. Int. 2016, 268, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.; Itzkan, I.; Dasari, R.; Feld, M. Single Molecule Detection Using Surface enhanced-Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Jones, R.; Hooper, D.; Zhang, L.; Wolverson, D.; Valev, V. Raman techniques: Fundamentals and frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef] [Green Version]
- Efremov, E.; Ariese, F.; Gooijer, C. Achievements in resonance Raman spectroscopy: Review of a technique with a distinct analytical chemistry potential. Anal. Chim. Acta 2008, 606, 119–134. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Asiala, S.; Schultz, Z. Surface enhanced Raman correlation spectroscopy of particles in solution. Anal. Chem. 2014, 86, 2625–2632. [Google Scholar] [CrossRef]
- Otto, A.; Mrozek, I.; Grabhorn, H.; Akemann, A. Surface-enhanced Raman scattering. J. Phys. Condens. Matter 1992, 4, 1143. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.; McQuillan, A. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Mosier-Boss, P. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, H.; Ke, H.; Dvoynenko, M.; Wang, J. Multipolar Resonances of Ag Nanoparticle Arrays in Anodic Aluminum Oxide Nanochannels for Enhanced Hot Spot Intensity and Signal-to-Background Ratio in Surface-Enhanced Raman Scattering. ACS Appl. Nano Mater. 2020, 3, 4477–4485. [Google Scholar] [CrossRef]
- Yang, Y.; Creedon, N.; Riordan, A.; Lovera, P. Surface Enhanced Raman spectroscopy: Applications in agriculture and food safety. Photonics 2021, 8, 568. [Google Scholar] [CrossRef]
- Galletto, P.; Brevet, P.; Girault, H.; Antoine, R.; Broyer, M. Enhancement of the Second Harmonic Response by Adsorbates on Gold Colloids: The Effect of Aggregation. J. Phys. Chem. B 1999, 103, 8706–8710. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, C.; Zhang, G. The aggregation effect of metal ions on silver sol and the fractal structure formed. Acta Phys. Sin. 1987, 36, 1289–1297. [Google Scholar]
- Singh, M.; Sinha, I.; Singh, A.; Mandal, R. Formation of fractal aggregates during green synthesis of silver nanoparticles. J. Nanopart. Res. 2011, 13, 69–76. [Google Scholar] [CrossRef]
- He, L.; Kim, N.; Li, H.; Hu, Z.; Lin, M. Use of a fractal-like gold nanostructure in surface-enhanced Raman spectroscopy for detection of selected food contaminants. J. Agric. Food Chem. 2008, 56, 9843–9847. [Google Scholar] [CrossRef]
- Huang, H.; Shende, C.; Sengupta, A.; Inscore, F.; Brouillette, C.; Smith, W.; Farquharson, S. Surface-enhanced Raman spectra of melamine and other chemicals using a 1550 nm (retina safe) laser. J. Raman Spectrosc. 2011, 43, 701–705. [Google Scholar] [CrossRef]
- Chen, X.; Gu, H.; Shen, G.; Dong, X.; Kang, J. Spectroscopic study of surface enhanced Raman scattering of caffeine on borohydride-reduced silver colloids. J. Mol. Struct. 2010, 975, 63–68. [Google Scholar] [CrossRef]
- Zheng, H.; Ni, D.; Yu, Z.; Liang, P.; Chen, H. Fabrication of flower-like silver nanostructures for rapid detection of caffeine using surface enhanced Raman spectroscopy. Sens. Actuators B Chem. 2016, 231, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.; Otero, J.; Tocón, I. Comparative Performance of Citrate, Borohydride, Hydroxylamine and Cyclodextrin Silver Sols for Detecting Ibuprofen and Caffeine Pollutants by Means of Surface-Enhanced Raman Spectroscopy. Nanomaterials 2020, 10, 2339. [Google Scholar] [CrossRef]
- Farquharson, S.; Brouillette, C.; Smith, W.; Shende, C. A Surface-Enhanced Raman Spectral Library of Important Drugs Associated with Point-of-Care and Field Applications. Front. Chem. 2019, 7, 706–721. [Google Scholar] [CrossRef]
- Turzhitsky, V.; Zhang, L.; Horowitz, G.L.; Vitkin, E.; Khan, U.; Zakharov, Y.; Qiu, L.; Itzkan, I.; Perelman, L.T. Picoanalysis of drugs in biofluids with quantitative label-free surface-enhanced Raman spectroscopy. Small 2018, 14, 1802392. [Google Scholar] [CrossRef]
- Velička, M.; Zacharovas, E.; Adomavičiūtė, S.; Šablinskas, V. Detection of caffeine intake by means of EC-SERS spectroscopy of human saliva. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 118956. [Google Scholar] [CrossRef]
- Alharbi, O.; Xu, Y.; Goodacre, R. Simultaneous Multiplexed Quantification of Caffeine and its Major Metabolites Theobromine and Paraxanthine Using Surface-Enhanced Raman Scattering. Anal. Bioanal. Chem. 2015, 407, 8253–8261. [Google Scholar] [CrossRef] [Green Version]
- Farquharson, S.; Dana, K.; Shende, C.; Gladding, Z.; Newcomb, J.; Dascher, J.; Petrakis, I.; Arias, A. Rapid Identification of Buprenorphine in Patient Saliva. J. Anal. Bioanal. Tech. 2017, 8, 368–372. [Google Scholar] [CrossRef]
- Pavel, I.; Szeghalmi, A.; Moigno, D.; Cînta, S.; Kiefer, W. Theoretical and pH dependent surface enhanced Raman spectroscopy study on caffeine. Biopolym. Biospectrosc. Sect. 2002, 72, 25–37. [Google Scholar] [CrossRef]
- Fox, J.; Waverka, K.; Verbeck, G. Gold-plating of mylar lift films to capitalize on surface enhanced Raman spectroscopy for chemical extraction of drug residues. Forensic Sci. Int. 2012, 216, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Tang, R.; Xu, J.; Feng, L. Chemical nanosensors based on molecularly-imprinted polymers doped with silver nanoparticles for the rapid detection of caffeine in wastewater. Anal. Chim. Acta 2018, 1034, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Chio, W.; Liu, J.; Jones, T.; Perumal, J.; Dinish, U.; Parkin, I.; Malini, O.; Lee, T. SERS multiplexing of methylxanthine drug isomers via host-guest size matching and machine learning. J. Mater. Chem. C 2021, 9, 12624–12632. [Google Scholar] [CrossRef]
- Inscore, F.; Shende, C.; Sengupta, A.; Huang, H.; Farquharson, S. Detection of Drugs of Abuse In Saliva by Surface-Enhanced Raman Spectroscopy (SERS). Appl. Spectrosc. 2011, 65, 1004–1008. [Google Scholar] [CrossRef]
- Matas, M.; Edwards, H.; Lawson, E.; Shields, L.; York, P. FT-Raman spectroscopic investigation of a pseudopolymorphic transition in caffeine hydrate. J. Molec. Struct. 1998, 440, 97–104. [Google Scholar] [CrossRef]
- Baranska, M.; Proniewicz, L. Raman mapping of caffeine alkaloid. Vib. Spectrosc. 2008, 48, 153–157. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, V. Ab initio and DFT studies of the structure and vibrational spectra of anhydrous caffeine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 45–50. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sankari, G.; Ponnusamy, S. Vibrational spectral investigation on xanthine and its derivatives—Theophylline, caffeine and theobromine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 117–127. [Google Scholar] [CrossRef]
- Belay, A.; Ture, K.; Redi, M.; Asfaw, A. Measurement of caffeine in coffee beans with UV/vis spectrometer. Food Chem. 2008, 108, 310–315. [Google Scholar] [CrossRef]
- Mullen, K.; Carron, K. Adsorption of chlorinated ethylenes at 1-octadecanethiol-modified silver surfaces. Anal. Chem. 1994, 66, 478–483. [Google Scholar] [CrossRef]


| Raman (cm−1) | SERS (cm−1) | Δ (cm−1) | Vibrational Assignments |
|---|---|---|---|
| 445 s | 442 w | −3 | N-C-C deformation |
| 483 s | 499 m | +16 | C-N-C deformation |
| 556 vs | 557 m | +1 | O=C-N deformation (or pyridine ring breathing mode) |
| 610 vw | 610 vw | 0 | o.p. CH deformation |
| 644 s | 650 m | +6 | O=C-N deformation |
| 699 vw | 679 vw sh | −20 | pyrimidine, imidazole ring deformation |
| 741 s | 758 m | +17 | O=C-C deformation |
| 764 vw sh | - | ||
| 802 m | 822 w | +20 | N-C-H deformation |
| 928 m | 925 w | −3 | imidazole ring deformation |
| 975 w | 987 m | +12 | pyrimidine ring deformation |
| 1023 w | 1044 m | +21 | i.p. C-C deformation |
| 1072 m | 1076 m | +4 | H-C=N bending |
| 1135 w | 1138 vw | +3 | CH3 bending |
| 1190 vw | 1179 w | −11 | CH bending |
| 1241 s | 1217 w | −24 | C-N stretching |
| 1285 s | 1278 m | −7 | C-N stretching |
| 1329 vs | 1319 s | −10 | imidazole trigonal ring stretching |
| 1361 s | 1369 m sh | +8 | C=N, C-N stretching |
| 1384 w sh | 1387 s sh | +3 | CH2 bending |
| 1408 m | 1411 m sh | +3 | C-N sym. stretching |
| 1458 m | 1437 vs | −21 | CH2 bending + imidazole ring stretching |
| 1500 m sh | 1526 s | +26 | CHn bending + C-N stretching |
| 1554 w | 1552 s | −2 | imidazole, pyrimidine ring stretching |
| 1600 s | 1610 vs | +10 | C=C sym. stretching |
| 1657 m | 1655 w | −2 | o.p. C=O stretching |
| 1698 s | 1707 vs | +9 | i.p C=O stretching |
| 1 ppm | Batch1 | Batch2 | Average | ||
| cap1 | cap2 | cap3 | cap4 | ||
| spot1 | 118 | 160 | 125 | 152 | |
| spot2 | 116 | 174 | 179 | 170 | |
| spot3 | 142 | 160 | 159 | 164 | |
| spot4 | 143 | 111 | 170 | 167 | |
| spot5 | 148 | 116 | 184 | 174 | |
| spot6 | 167 | 152 | 176 | 118 | |
| spot7 | 132 | 156 | 163 | 117 | |
| spot8 | 105 | 158 | 163 | 109 | |
| spot9 | 125 | 163 | 135 | 134 | |
| spot10 | 122 | 162 | 114 | 110 | |
| Avg | 131.8 | 151.2 | 156.8 | 141.5 | 145.325 |
| Stddev | 18.4 | 20.7 | 24.0 | 26.7 | 22.4 |
| %Dev | 13.93% | 13.69% | 15.29% | 18.83% | 15.43% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wu, L.; Pei, J.; Li, X.; Li, H.; Inscore, F. Part-Per-Billion Level Chemical Sensing with a Gold-Based SERS-Active Substrate. Sensors 2022, 22, 1778. https://doi.org/10.3390/s22051778
Zhang T, Wu L, Pei J, Li X, Li H, Inscore F. Part-Per-Billion Level Chemical Sensing with a Gold-Based SERS-Active Substrate. Sensors. 2022; 22(5):1778. https://doi.org/10.3390/s22051778
Chicago/Turabian StyleZhang, Tingting, Liyun Wu, Junchang Pei, Xuefeng Li, Haowen Li, and Frank Inscore. 2022. "Part-Per-Billion Level Chemical Sensing with a Gold-Based SERS-Active Substrate" Sensors 22, no. 5: 1778. https://doi.org/10.3390/s22051778
APA StyleZhang, T., Wu, L., Pei, J., Li, X., Li, H., & Inscore, F. (2022). Part-Per-Billion Level Chemical Sensing with a Gold-Based SERS-Active Substrate. Sensors, 22(5), 1778. https://doi.org/10.3390/s22051778






