HRV Monitoring Using Commercial Wearable Devices as a Health Indicator for Older Persons during the Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wearable Device
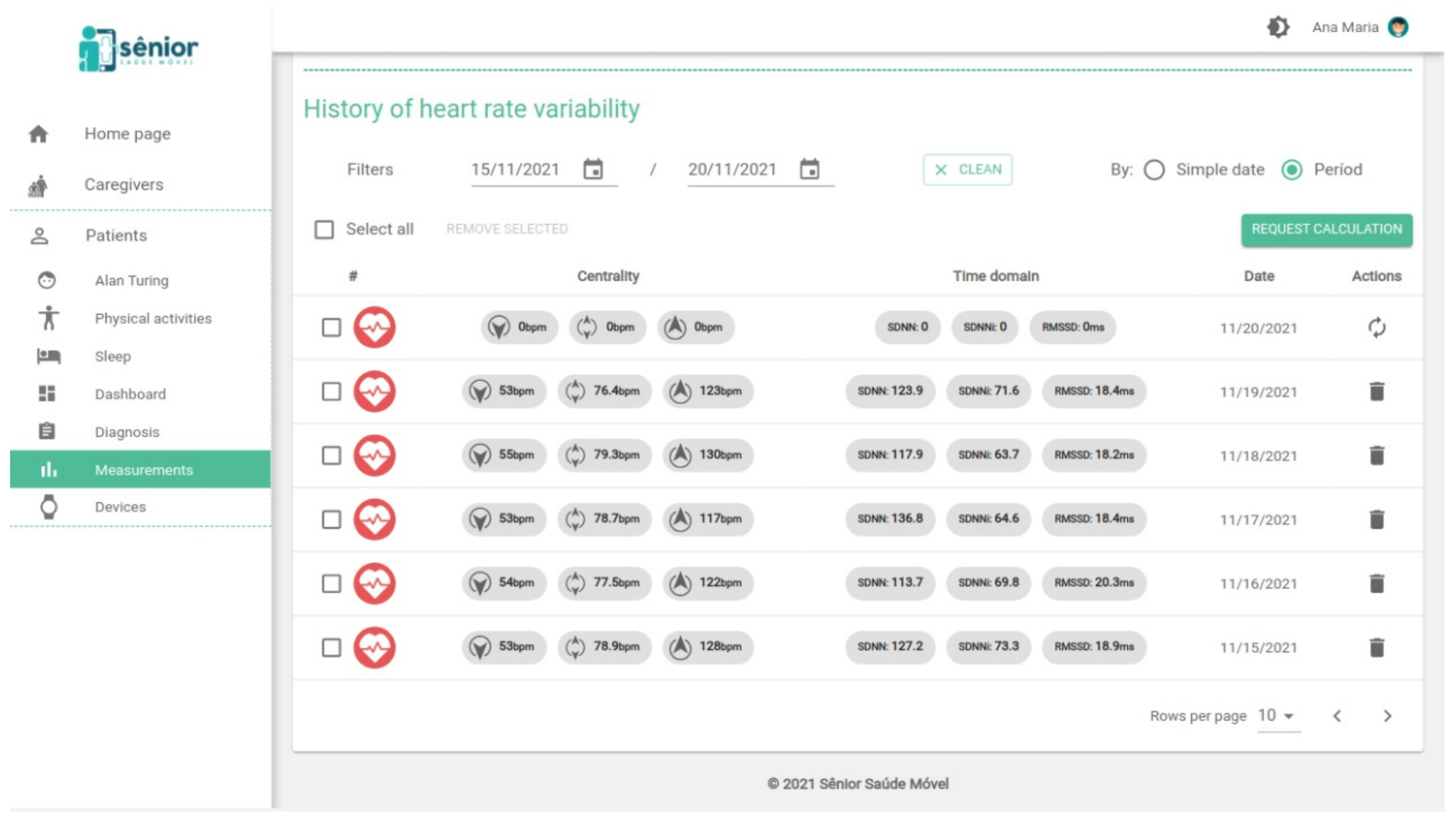
2.2. HRV Metrics
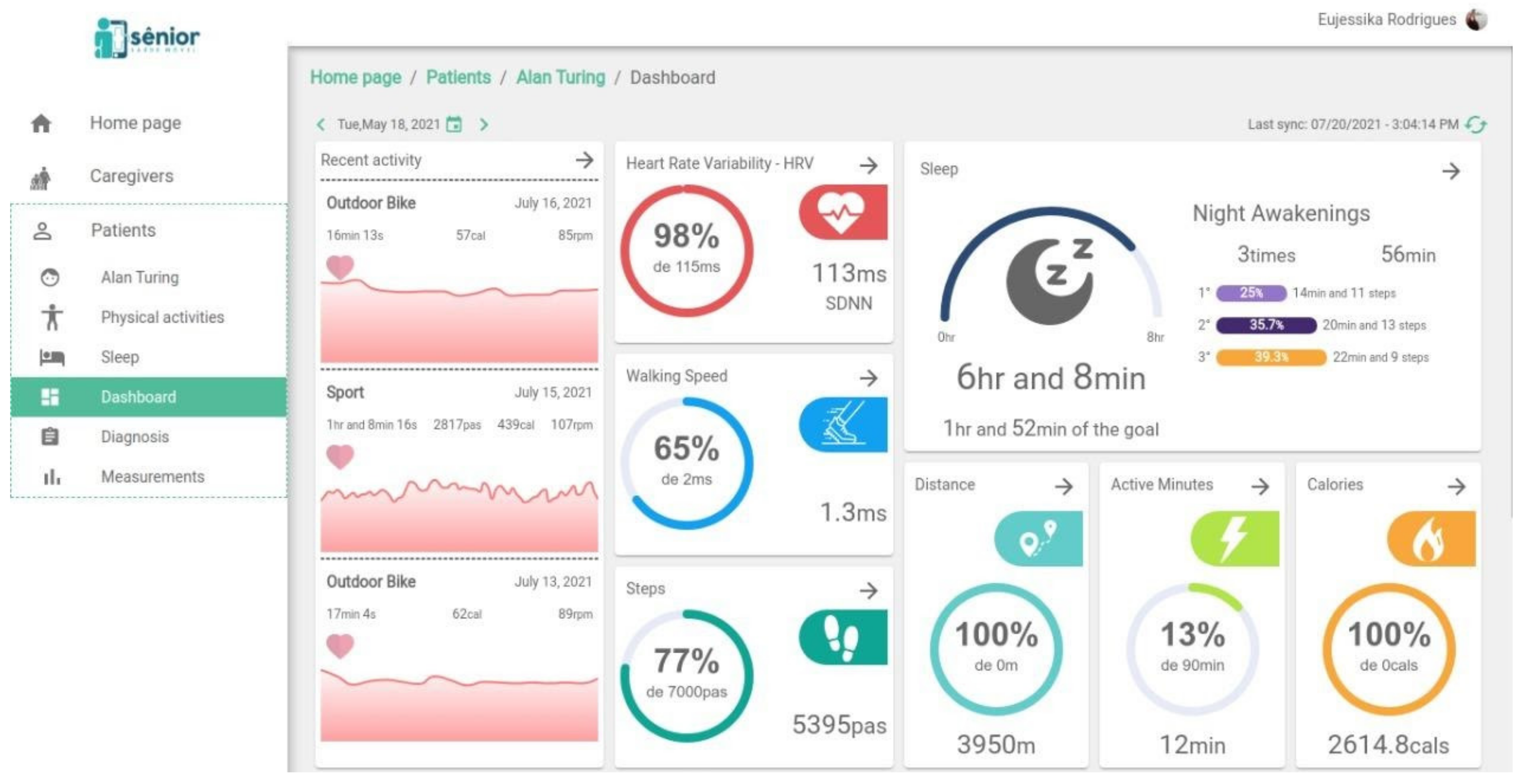
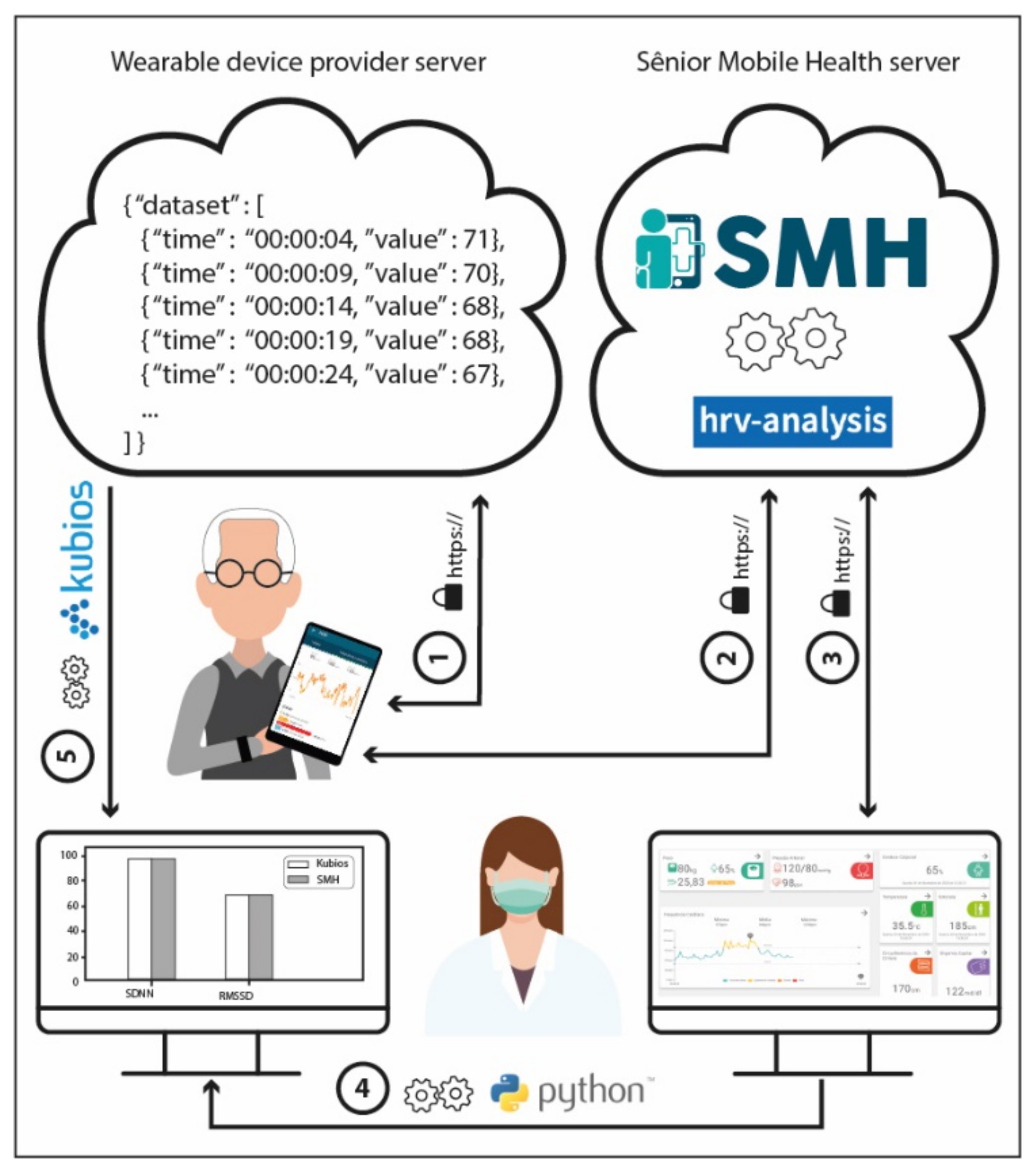
2.3. The SMH Platform
2.4. Sample Description and Solution Application
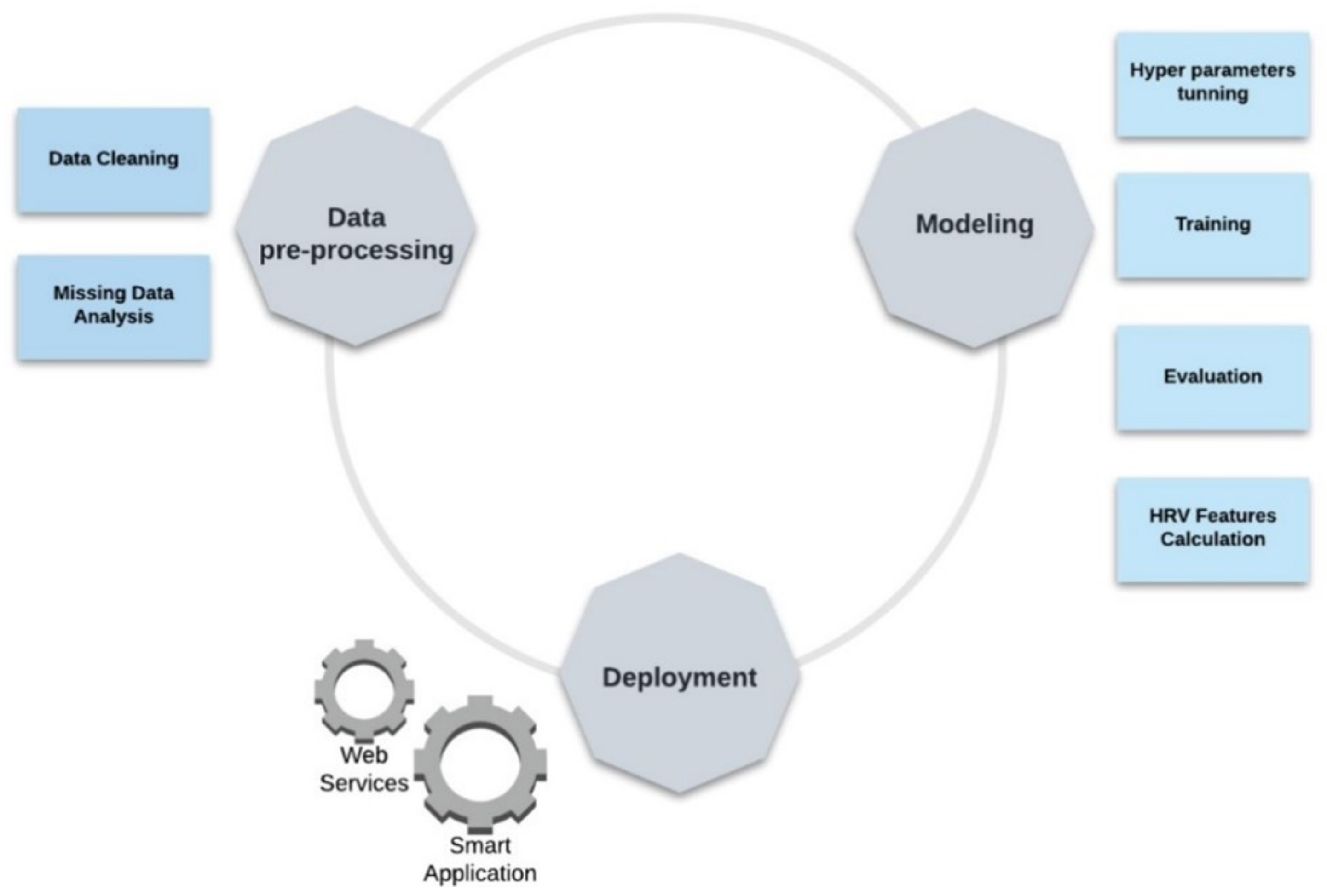
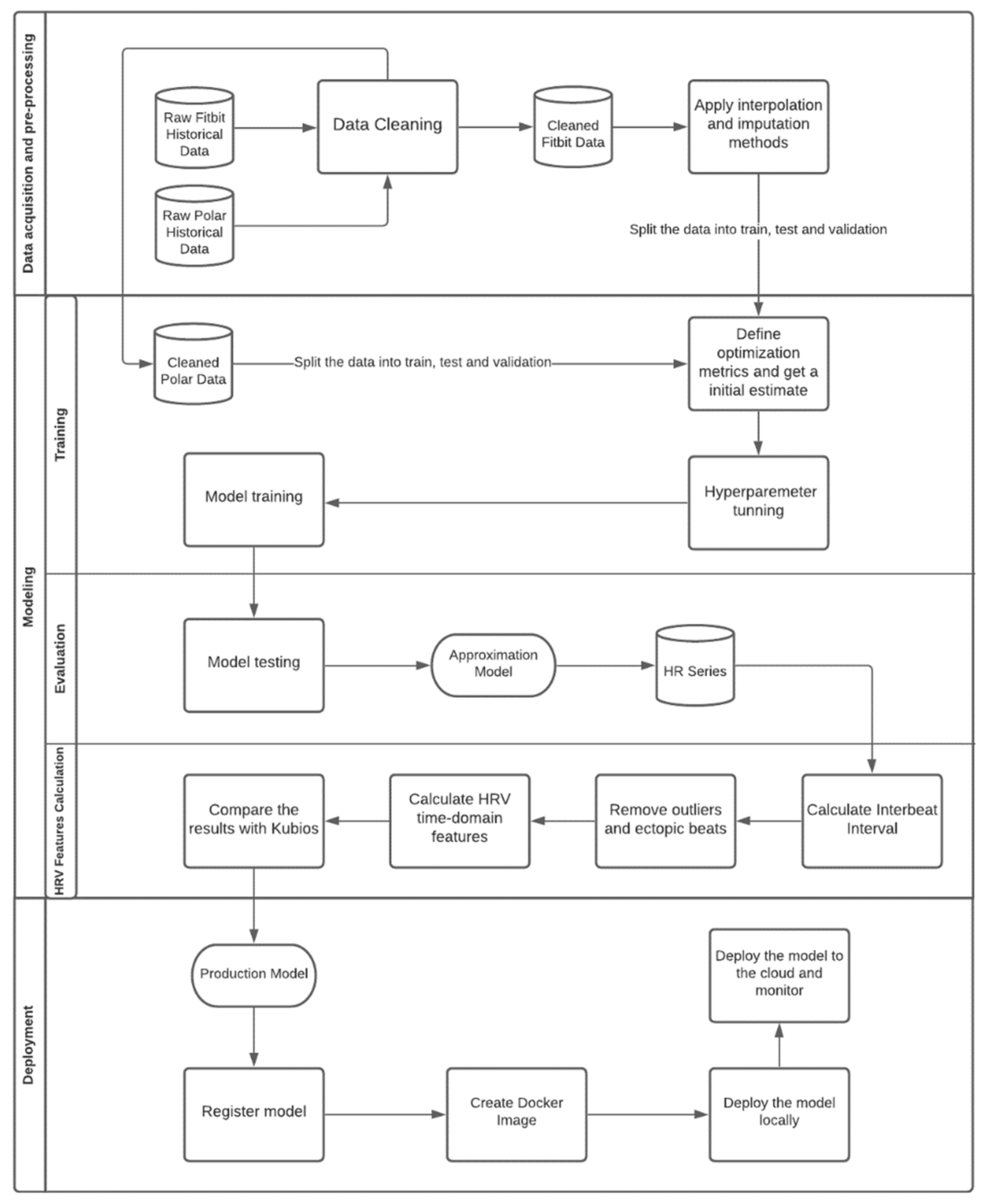
2.5. Development Process Workflow
2.5.1. Data Pre-Processing
- Mean filling: mean filling replaces the gaps with the mean value of the series;
- Forward fill: this method fills in the missing values by propagating the last valid observation forward;
- Backward fill: this approach fills the missing values of the data frame backwards;
- Linear interpolation: the linear interpolation method fits a line of polynomials to the end points of the gap using a linear formula to estimate the missing values;
- Quadratic and cubic spline interpolation: Type of interpolation in which the interpolant is a special type of piecewise polynomial named spline. This method fits lower degree polynomials, in this case quadratic or cubic, to a small subset of values rather than fitting only one polynomial with high degree to all the values;
- Piecewise cubic Hermite interpolating polynomial (PCHIP): PCHIP consists of a spline third-degree piecewise polynomial function specified by its values and first derivatives at the end points of the corresponding domain interval;
- Akima interpolation: The Akima interpolation is a continuously differentiable spline interpolation, formed from piecewise third order polynomials, in which data from the next neighbor points is used to determine the coefficients of the polynomial;
- Exponentially weighted moving average (EWMA): EWMA estimates the missing values as a weighted average of the historical observations, giving older observations lower weights that decrease exponentially as the data points age;
- Interpolation by nearest neighbor: in this method, the imputed value is equal to that of the closest known point;
- Mean of the nearest neighbors: This approach fills in the missing points with the average value for the k nearest neighbors. To select the optimum K, which corresponds to the number of neighbors considered, we run the algorithm several times with different values of K, choosing the value that reduces the number of errors committed.
2.5.2. Modeling
- Multinomial logistic regression: A logistic regression that admits for more than two categories for the dependent variable. Here, the discrete numerical values of our dependent variable were considered as categories;
- K nearest neighbors: a method that assigns a predicted value to a new observation based on the mean of its nearest neighbors;
- Decision tree: a tree structure in which the data are continuously split according to a certain parameter, where the internal nodes represent the features of the dataset, the branches represent the decision rules, and the leaf nodes represent the outcome;
- Random forest: a method for classification and regression that works by constructing a large amount of decision trees;
- AdaBoost: an ensemble machine learning algorithm that combines multiple predictions from many weak learners into a single stronger prediction;
- Linear regression: an algorithm that assumes a linear relationship between the input variables and the output, attempting to model the relationship between the two variables by fitting a linear equation to the observed data;
- Neural Networks: Neural Networks are a supervised learning algorithms that seek to approximate a function which is represented by the input data. The type of artificial neural network to handle with sequential data or time series data are a recurrent neural network (RNN). Here we compared traditional single-layer RNN with a Long Short-Term Memory network (LSTM), a special kind of RNN which is capable of learning long-term dependencies.
- Malik Rule: IBIs differing by more than 20% from the one preceding it are removed;
- Karlsson Rule: IBIs diverging by more than 20% of the mean of previous and next IBI are removed;
- Kamath Rule: this method considers a heartbeat abnormal whenever the IBI increased by more than 32.5% or decreased by more than 24.5% when compared with the previous IBI;
- Acar Rule: IBIs differing by more than the 20% of the mean of last nine IBIs are removed.
2.5.3. Model’s Execution
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pletscha, A.H.M.; Júnior, A.; Silveira, N.; Bittencourt, W.; Osórioe, R. Analysis of Spectral Indexes of Variability of Cardiac Frequency during the Postural Change of Hypertensive Elderly. J. Health Sci. 2018, 20, 146-0. [Google Scholar]
- De Oliveira Matos, F.; Vido, A.; Garcia, W.F.; Lopes, W.A.; Pereira, A. A Neurovisceral Integrative Study on Cognition, Heart Rate Variability, and Fitness in the Elderly. Front. Aging Neurosci. 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marães, V.R.F.S. Heart rate and its variability: Analysis and applications. Rev. Andal. Med. Deporte 2010, 3, 33–42. [Google Scholar]
- Vanderlei, L.C.M.; Pastre, C.M.; Hoshi, R.A.; Carvalho, T.D.; Godoy, M.F. Basic notions of heart rate variability and its clinical applicability. Rev. Bras. Cir. Cardiovasc. 2009, 24, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2020, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vicente, A.; Hernando, D.; Marín-Puyalto, J.; Vicente-Rodríguez, G.; Garatachea, N.; Pueyo, E.; Bailón, R. Validity of the Polar H7 Heart Rate Sensor for Heart Rate Variability Analysis during Exercise in Different Age, Body Composition and Fitness Level Groups. Sensors 2021, 21, 902. [Google Scholar] [CrossRef] [PubMed]
- Hinde, K.; White, G.; Armstrong, N. Wearable Devices Suitable for Monitoring Twenty Four Hour Heart Rate Variability in Military Populations. Sensors 2021, 21, 1061. [Google Scholar] [CrossRef]
- Jeyhani, V.; Mahdiani, S.; Peltokangas, M.; Vehkaoja, A. Comparison of HRV Parameters Derived from photoplethysmography and Electrocardiography Signals. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 5952–5955. [Google Scholar]
- Brothers, M.C.; Debrosse, M.; Grigsby, C.C.; Naik, R.R.; Hussain, S.M.; Heikenfeld, J.; Kim, S.S. Achievements and Challenges for Real-Time Sensing of Analytes in Sweat within Wearable Platforms. Acc. Chem. Res. 2019, 52, 297–306. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, L.; Jiang, K.; Wei, Z.; Shen, G. Reviews of wearable healthcare systems: Materials, devices and system integration. Mater. Sci. Eng. 2020, 140, 100523. [Google Scholar] [CrossRef]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A Critical Review of Consumer Wearables, Mobile Applications, and Equipment for Providing Biofeedback, Monitoring Stress, and Sleep in Physically Active Populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef]
- Pernice, R.; Javorka, M.; Krohova, J.; Czippelova, B.; Turianikova, Z.; Busacca, A.; Faes, L. Reliability of Short-Term Heart Rate Variability Indexes Assessed through Photoplethysmography. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5610–5613. [Google Scholar]
- Rassi, A. Understanding of measures to analyze heart rate variability. J. Diag. Cardiol. 2014, 8, 1–10. [Google Scholar]
- Pinheiro, N.; Couceiro, R.; Henriques, J.; Muehlsteff, J.; Quintal, I.; Goncalves, L.; Carvalho, P. Can PPG be used for HRV analysis? In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 16–20 August 2016; pp. 2945–2949. [Google Scholar]
- Chen, Y.-C.; Hsiao, C.-C.; Zheng, W.-D.; Lee, R.-G.; Lin, R. Artificial neural networks-based classification of emotions using wristband heart rate monitor data. Medicine 2019, 98, e16863. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Yang, F.; Taylor, J.A.; Stein, J.F. A comparison of photoplethysmography and ECG recording to analyse heart rate variability in healthy subjects. J. Med. Eng. Technol. 2009, 33, 634–641. [Google Scholar] [CrossRef]
- Macfarlane, D.J.; Phill, D.; Fogarty, B.A.; Hopkins, W.G. The accuracy and variability of commercially available heart rate monitors. N. Z. J. Sports Med. 1989, 17, 51–53. [Google Scholar]
- Kingsley, M.; Lewis, M.J.; Marson, R.E. Comparison of polar 810s and an ambulatory ECG system for RR interval measurement during progressive exercise. Int. J. Sports Med. 2005, 26, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Polar Research and Technology. Polar H10 Heart Rate Sensor System. 2019. Available online: https://www.polar.com/sites/default/files/static/science/white-papers/polar-h10-heart-rate-sensor-white-paper.pdf (accessed on 24 January 2022).
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-Ananta, T.; Ajmal; Ramella-Roman, J.C.; McShane, M.J.; Coté, G.L. Sources of Inaccuracy in Photoplethysmography for Continuous Cardiovascular Monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef]
- Lepot, M.; Aubin, J.-B.; Clemens, F.H. Interpolation in Time Series: An Introductive Overview of Existing Methods, Their Performance Criteria and Uncertainty Assessment. Water 2017, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Chabot, D.; Bayer, M.; de Roos, A. Instantaneous heart rates and other techniques introducing errors in the calculation of heart rate. Can. J. Zool. 1991, 69, 1117–1120. [Google Scholar] [CrossRef]
- Perrotta, A.; Jeklin, A.; Hives, B.; Meanwell, L.; Warburton, D. Validity of the Elite HRV Smartphone Application for Examining Heart Rate Variability in a Field-Based Setting. J. Strength Cond. Res. 2017, 31, 2296–2302. [Google Scholar] [CrossRef]
- Tarvainen, M.; Lipponen, J.; Niskanen, J.; Ranta-Aho, P. Kubios HRV Software: User’s Guide, Version 3. 5; Kubios Oy: Kuopio, Finland, 2021.
- Kamath, M.; Fallen, E. Correction of the heart rate variability signal for ectopics and missing beats. Heart Rate Var. 1995, 75–85. [Google Scholar]
- Kleiger, R.; Stein, P., Jr.; Bigger, J.T. Heart Rate Variability: Measurement and Clinical Utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.; Jeste, D.; Lee, E.; Wu, T.; Tu, X.; Kim, H.; Depp, C. Associations Between Heart Rate Variability Measured With a Wrist-Worn Sensor and Older Adults’ Physical Function: Observational Study. JMIR mHealth uHealth 2019, 7, e13757. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Pantelopoulos, A.; Emir-Farinas, H.; Natarajan, P. Heart rate variability with photoplethysmography in 8 million individuals: A cross-sectional study. Lancet Digit. Health 2020, 2, 650–657. [Google Scholar] [CrossRef]
- Tsou, M.-C.M.; Lung, S.-C.C.; Cheng, C.-H. Demonstrating the Applicability of Smartwatches in PM2.5 Health Impact Assessment. Sensors 2021, 21, 4585. [Google Scholar] [CrossRef]
- Anthony, B. Use of Telemedicine and Virtual Care for Remote Treatment in Response to COVID-19 Pandemic. J. Med. Syst. 2020, 44, 132. [Google Scholar]
- Caetano, R.; Silva, A.B.; Guedes, A.C.C.M.; Paiva, C.C.N.D.; Ribeiro, G.D.R.; Santos, D.L.; Silva, R.M.D. Challenges and opportunities for telehealth during the COVID-19 pandemic: Ideas on spaces and initiatives in the Brazilian context. Rep. Public Health 2020, 36, e00088920. [Google Scholar]
- Mann, D.M.; Chen, J.; Chunara, R.; Testa, P.; Nov, O. COVID-19 transforms health care through telemedicine: Evidence from the field. J. Am. Med. Inform. Assoc. 2020, 27, 1132–1135. [Google Scholar] [CrossRef]






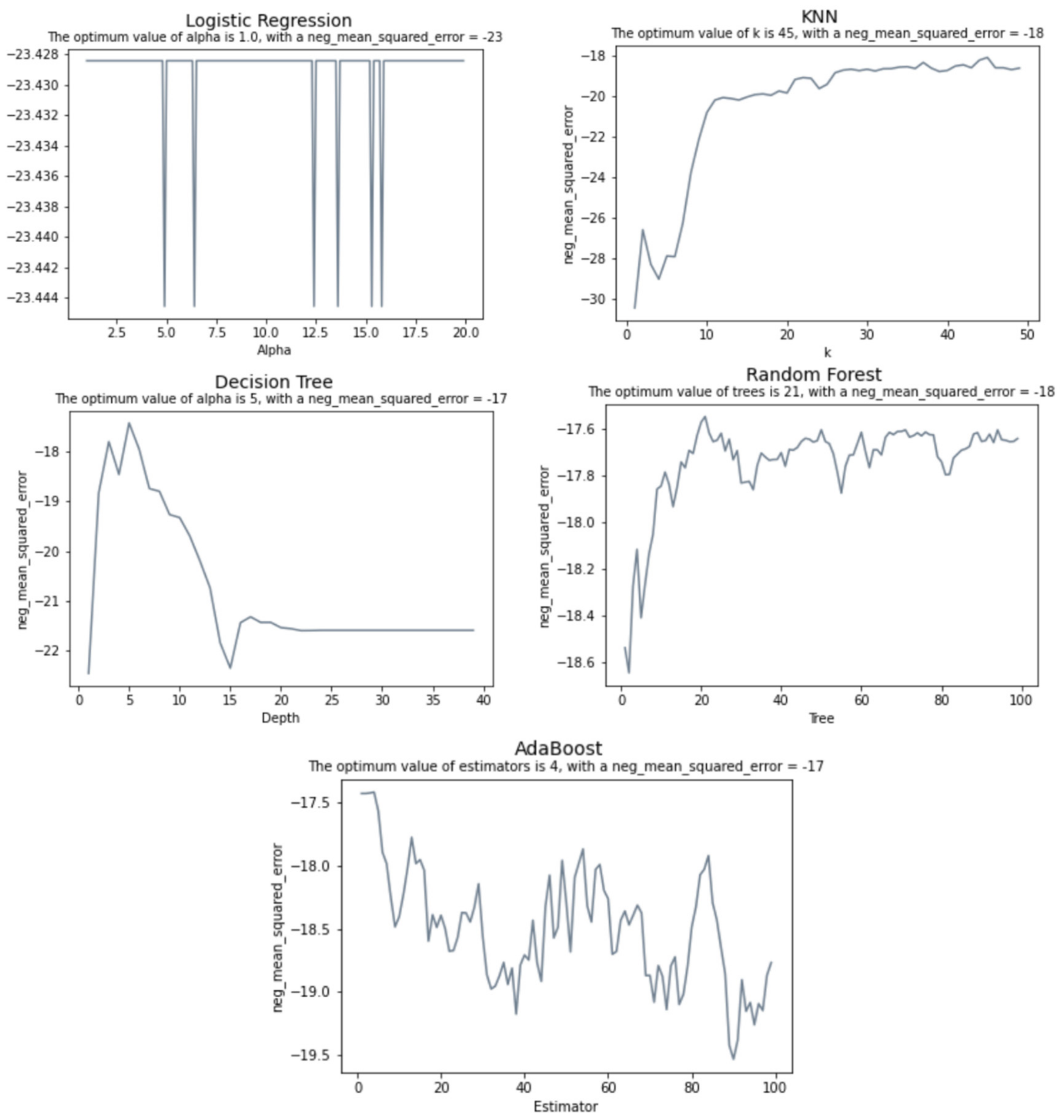






| Algorithm | RMSE |
|---|---|
| Logistic Regression | 4.95 |
| KNN | 4.01 |
| Decision Tree | 4.07 |
| Random Forest | 3.83 |
| AdaBoost | 3.91 |
| Linear Regression | 2.80 |
| RNN | 2.30 |
| LSTM | 2.23 |
| Null Hypothesis: There is no difference in the means of the metric calculated by SMH | |||
| Alternate Hypothesis: There is a difference in the means of the metric calculated by SMH | |||
| Metric | t-value | p-value | |
| SDNN | 1.772 | 0.110 | |
| RMSSD | 1.150 | 0.280 | |
| pNN50 | 0.025 | 0.980 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, E.; Lima, D.; Barbosa, P.; Gonzaga, K.; Guerra, R.O.; Pimentel, M.; Barbosa, H.; Maciel, Á. HRV Monitoring Using Commercial Wearable Devices as a Health Indicator for Older Persons during the Pandemic. Sensors 2022, 22, 2001. https://doi.org/10.3390/s22052001
Rodrigues E, Lima D, Barbosa P, Gonzaga K, Guerra RO, Pimentel M, Barbosa H, Maciel Á. HRV Monitoring Using Commercial Wearable Devices as a Health Indicator for Older Persons during the Pandemic. Sensors. 2022; 22(5):2001. https://doi.org/10.3390/s22052001
Chicago/Turabian StyleRodrigues, Eujessika, Daniella Lima, Paulo Barbosa, Karoline Gonzaga, Ricardo Oliveira Guerra, Marcela Pimentel, Humberto Barbosa, and Álvaro Maciel. 2022. "HRV Monitoring Using Commercial Wearable Devices as a Health Indicator for Older Persons during the Pandemic" Sensors 22, no. 5: 2001. https://doi.org/10.3390/s22052001
APA StyleRodrigues, E., Lima, D., Barbosa, P., Gonzaga, K., Guerra, R. O., Pimentel, M., Barbosa, H., & Maciel, Á. (2022). HRV Monitoring Using Commercial Wearable Devices as a Health Indicator for Older Persons during the Pandemic. Sensors, 22(5), 2001. https://doi.org/10.3390/s22052001









