Identification of Lower-Limb Motor Tasks via Brain–Computer Interfaces: A Topical Overview
Abstract
:1. Introduction
2. Related Works
3. Background Information
3.1. Electroencephalography
3.2. Frequency Bands
- Delta wave: frequencies below 4 Hz. It has been detected in infants or adults during deep sleep [46];
- Theta wave: frequencies between 4 and 7 Hz. It is detected in youngsters and adults in stages of drowsiness [47].
- Alpha wave: frequencies between 8 and 12 Hz. It is detected in young people and adults during low brain activity or rest [46].
- Mu wave: 7.5–12.5 (Performs a motor action):
- Unlike the alpha wave, which occurs at a similar frequency over the resting visual cortex at the back of the scalp, the mu wave is found over the motor cortex [47].
- The mu wave is even suppressed when observing another person performing a motor or abstract motion with biological characteristics. Researchers such as V. S. Ramachandran and colleagues have suggested that this is a sign that the mirror neuron system is involved in mu wave suppression, [48] although others disagree.
- Beta wave: 13–25/12.5–30 Hz (Alertness). This band is divided into three sub-bands: Low Beta Waves (12.5–16 Hz, “Beta 1 power”), Beta Waves (16.5–20 Hz, “Beta 2 power”), and High Beta Waves (20.5–28 Hz, “Beta 3 power”) [49].
- Gamma wave: >25/25–140 Hz (Awareness). They correlate with large-scale brain network activity and cognitive phenomena, such as working memory, attention and perceptual grouping [50].
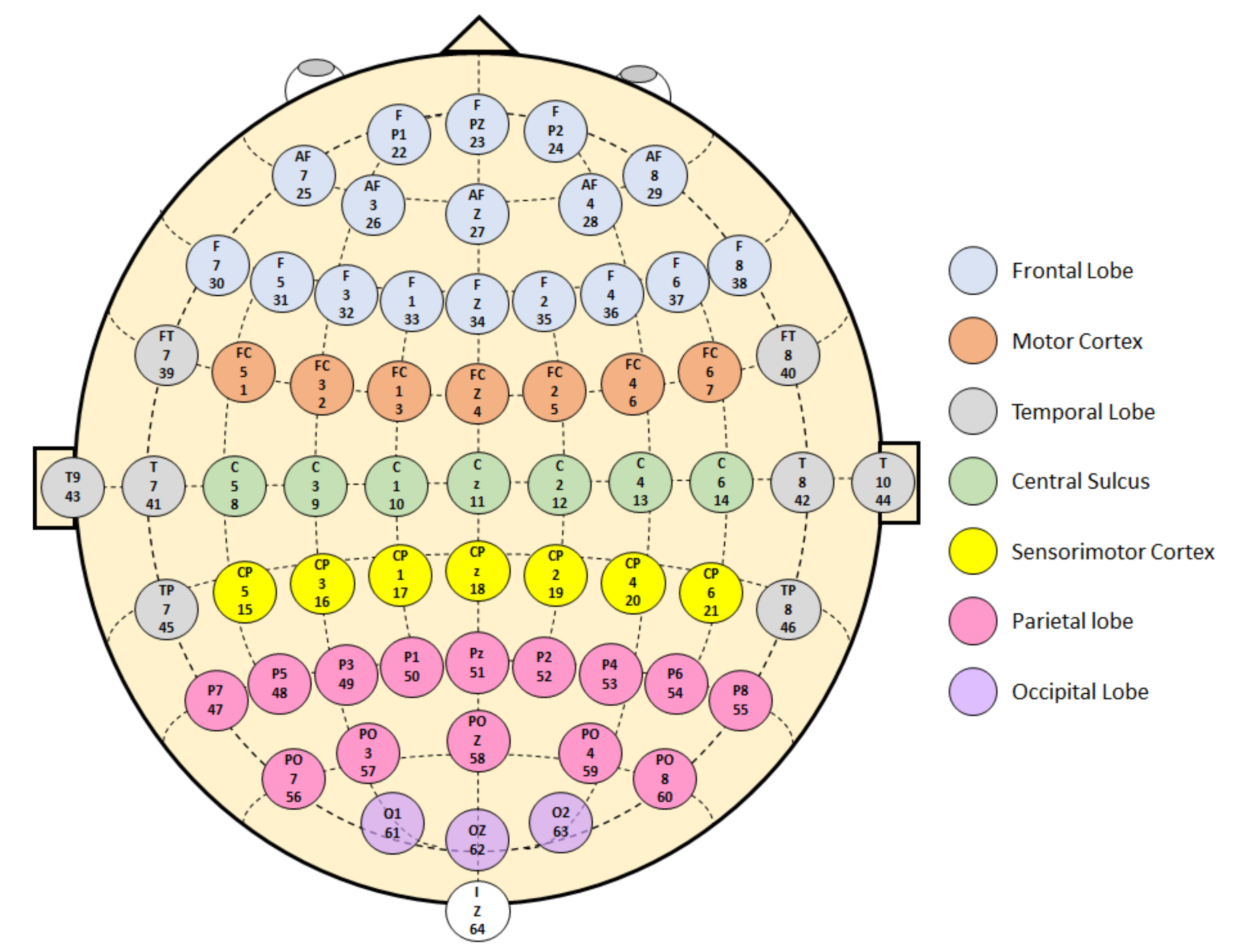
3.3. Brain Areas
4. Research Methodology
4.1. Scope and Research Questions
- What are the experimental methodologies used during data acquisition?
- What is the preprocessing technique used on EEG signals?
- What are the techniques used in feature extraction?
- What are the classification algorithms used in the detection of lower-limb movement intentions?
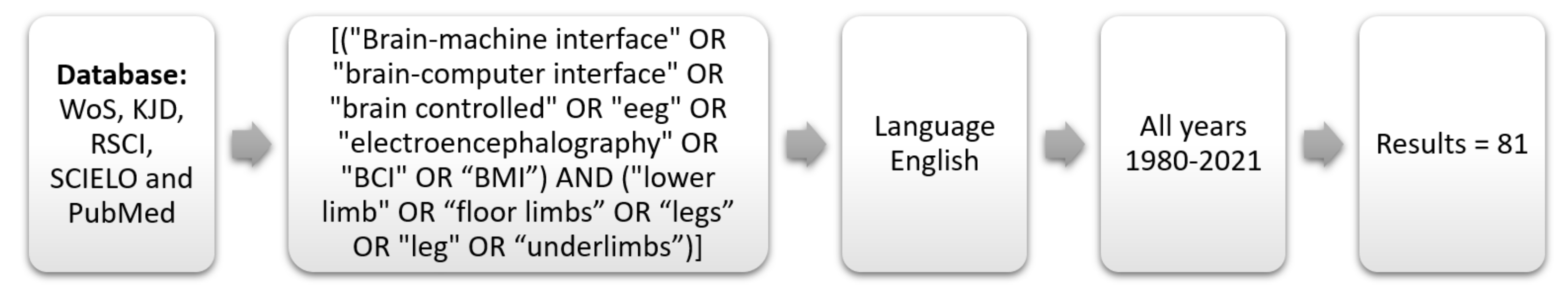
4.2. Search Method
4.3. Data Extraction
5. Results
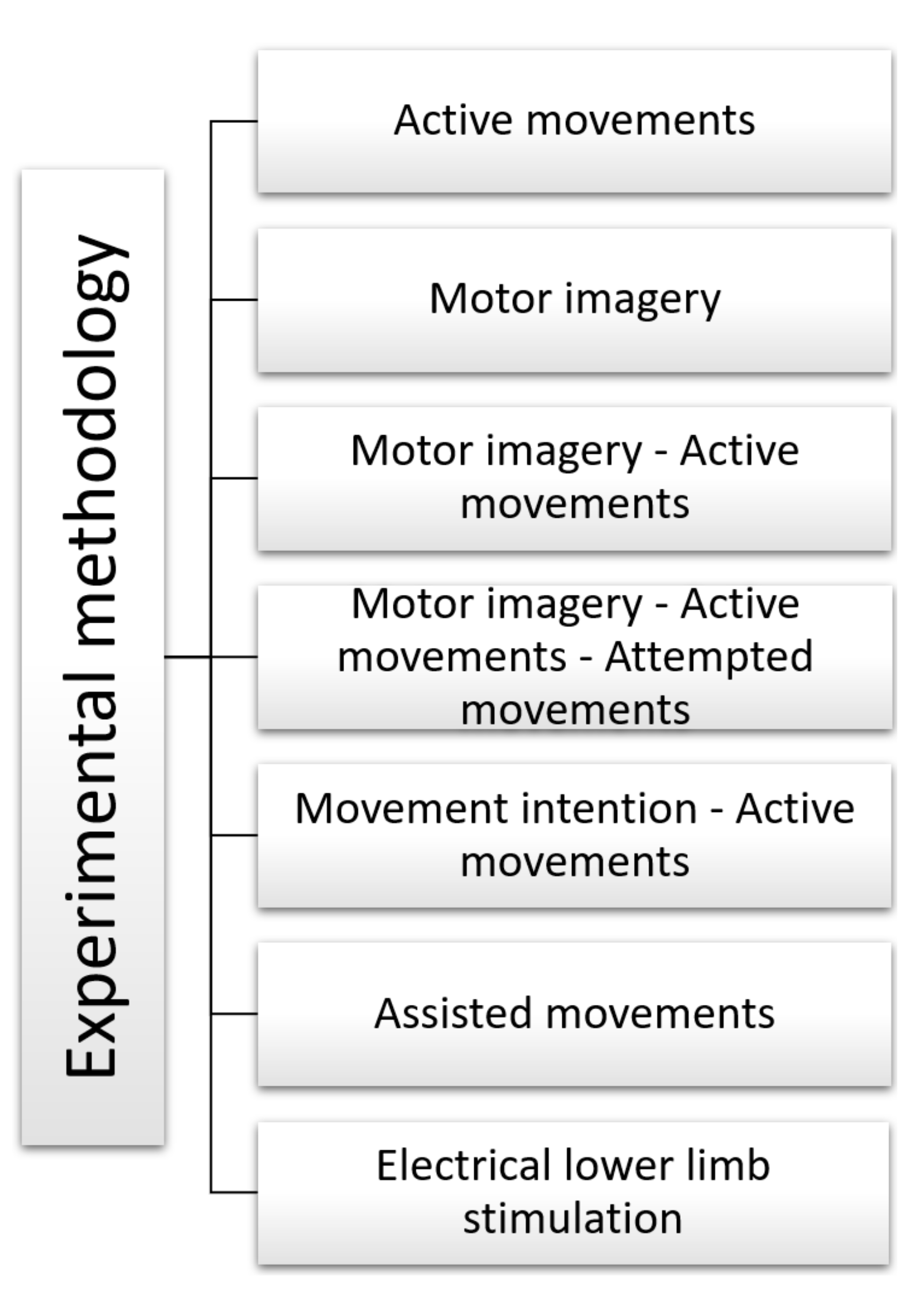
5.1. Experimental Methodology
- Active Movements
- -
- In their work on cortical activity tracking, Gwin, J. T., and Ferris, D.P. [59] recorded EEG signals in the 8–30 Hz frequency band from eight healthy right-handed subjects (seven men and one woman) between 21 and 31 years old. The volunteers were seated and performed movements using a knee device that assisted involuntary movements during the experiment. The tasks they performed were: isometric and isotonic ankle and knee movements. The physical tasks were performed with the dominant limb. As additional sensors, Gwin and Ferris used a load cell to measure the force and a goniometer to measure the flexion angle. No visual or audible stimulation was used to indicate to the volunteers when to execute the movement.
- -
- Chou et al. [67] recorded EEG signals from five volunteers with a Spinal Cord Injury (SCI). The volunteers stood facing a monitor during the experiment, and an avatar told them when to perform the movement. With the help of an exoskeleton, they performed left and right stepping movements. No additional sensors were used.
- -
- Chang et al. [72] recorded EEG signals in the 0.5–25 Hz frequency band from three healthy volunteers in the first experiment and two post-stroke patients in the second (two trials, one with and one without the music rehabilitation system). The volunteers were standing during the experiment and used mixed and augmented reality as a visual stimulus to indicate when to execute the movement. The task performed was walking. Motion capture sensors (Notch-knee joint angle) were used as additional sensors to obtain the knee flexion angle of the volunteers.
- -
- Hoshino et al. [73] recorded EEG signals in the alpha (8–12 Hz), beta (13–30 Hz), low-beta (13–19 Hz), and high-beta (20–30 Hz) frequency band from 24 post-stroke patients. Patient selection criteria were: first-ever stroke (ischemic or hemorrhagic), supratentorial lesion, between 20 and 85 years old, independently active before the stroke, and right hand dominant. They included patients within four weeks of the event who did not lose all of their motor function. As a result, 24 participants with an average age of 62 years were chosen. Patients were lying on a bed with their eyes closed during the experiment. The tasks performed were ankle movements, dorsiflexion, and plantar flexion. No additional sensors were used. No visual or audible stimulation was used to indicate to the patients when to perform the movement.
- -
- Choi et al. [74] recorded EEG signals in the 7–34 Hz frequency band from 10 healthy volunteers. All volunteers were right-handed males with an average age of 26.6 years and no history of neurological disorders. The volunteers performed active movements, and the task was gait and sit. Visual stimulation was used to indicate to the patients when to execute the movement. No additional sensors were used.
- Motor Imagery
- -
- Tariq et al. [61] recorded Event-Related Desynchronization and Event-Related Synchronization (ERD/ERS) EEG signals from 14 healthy volunteers. Participants were seated during the experiment and performed motor imagery tasks. No additional sensors were used. A monitor was used as visual stimulation.
- -
- Hsu et al. [63] recorded EEG signals in the 8–30 Hz frequency band from eight healthy volunteers aged 20–25 years. The tasks performed by the volunteers were left and right stepping. Electrooculography (EOG) was used as an additional sensor because a screen was used for visual stimulation.
- -
- Al-Quraishi et al. [64] recorded Event-Related Desynchronization (ERD) EEG signals from three healthy volunteers and four Spinal Cord Injury (SCI) patients. The participants were seated during the experiment and performed motor imagery with the aid of a prosthetic knee. The task performed was walking and idling. A screen was used for visual stimulation. No additional sensors were used during the experiment.
- -
- In their work on implementing a BCI system, Gu et al. [70] recorded EEG signals in the 1–30 Hz frequency band from 11 healthy right-handed volunteers (4 males and 7 females) aged 22–27 years with no history of neuromuscular disorders. The subjects were seated during the experiment and performed motor imagery. The task performed was foot dorsiflexing. Vertical and horizontal electrooculography (EOG) was used as an additional sensor. A screen was used as a means of visual stimulation to perform the timed tasks.
- -
- Ortiz et al. [75] recorded EEG signals in the 2–60 Hz frequency band from three adult volunteers without physical impairments. Participants were seated during the experiment and performed motor imagery. The task performed was walking. No additional sensors were used. Auditory stimulation was used to indicate the execution of the task while the participant was thinking about the action.
- -
- Do et al. [78] recorded EEG signals at a sampling rate of 256 Hz from two subjects (one able-bodied and one with paraplegia due to Spinal Cord Injury (SCI)). The task performed was kinesthetic motor imagery (KMI). The task consisted of walking using BCI-edRoGO along a linear trajectory. Electromyography (EMG) signals were measured to rule out BCI control by voluntary leg movements in the healthy subject.
- Motor imagery—Active Movements
- -
- Gordleeva et al. [71] recorded EEG signals in the 8–15 Hz frequency band from eight healthy volunteers aged 20–27 years. EEG and EMG signals to perform a leg lift movement were obtained using an HMI. The tasks performed were motor imagery and active movement. EMG sensors were also used for feedback of the lower limb exoskeleton control system.
- -
- Kline et al. [76] recorded EEG signals in the 8–45 Hz frequency band from sixteen healthy male volunteers with an average age of 24.7 years. EEG and fMRI data were collected during executed and imagined movements of the lower limbs. The tasks performed were motor imagery and active movement. Participants observed the Computer-Generated Image (CGI) of a walking human being and performed a lower limb movement or imagined it following the CGI rhythm.
- -
- Murphy et al. [77] recorded EEG signals in the 1–100 Hz frequency band from a 36-year-old male that underwent a right transfemoral amputation. Two additional Gyro + Accelerometer sensors were used. The subject performed ten visits of two test sessions using a lower limb prosthesis. A conductive gel was used to fill the space between the electrodes and the scalp to ensure good conductivity and minimize noise artifacts. At the first visit, the subject was trained to use the BCI system to control a switch on a lower limb prosthesis. Each training visit had two sessions. In the first session, training ensued. EEG signals were recorded while the subject performed motor imagery tasks of the amputated limb. These data were used to determine the parameters needed to predict movement intention. In the second session, these parameters were used to control a knee locking mechanism in the prosthesis in real-time while walking on parallel bars. No additional sensors were used. Auditory stimulation was used to indicate the execution of the task while the participant was thinking about the action.
- -
- Asanza et al. [79] used a database of 64-channel EEG signals recorded using the so-called BCI2000 system. Both the acquisition system and the data are widely described in [81]. EEG signals were recorded at 160 samples per second from eight healthy subjects. The tasks used for this study were motor activity and motor imagery of dorsi and plantar flexion of both feet. No additional sensors were used.
- Motor imagery—Active Movements—Attempted movements
- -
- Jochumsen et al. [80] recorded EEG signals in the 0.05–10 Hz frequency band from twelve healthy subjects (two females and ten males: 28 ± 4 years old) and six stroke patients with lower limb paresis. The subject was seated in a comfortable chair with the right foot (or the affected foot) attached to a foot pedal where a force transducer was set up. The tasks performed were executed and attempted movements and motor imagery kinetics. The healthy subjects performed the two tasks with Motor Execution (ME) and Motor Imagery (MI), while the stroke patients were asked to attempt the movements.
- Movement intention—Active Movements
- -
- Rea et al. [60] recorded EEG signals from seven right-handed patients (four men and three women) with chronic stroke and an average age of 54.7 years. The requirements for participation in the study were: interval since the stroke of at least 12 months, no psychiatric or neurological condition other than stroke, no cerebellar lesion or bilateral motor deficit, and ability to understand and follow instructions. The subjects were seated during the experiment and performed movements with a foot pedal. The tasks performed were hip movements with a knee and ankle constraint. The authors employed additional EMG sensors during the tasks.
- -
- Liu et al. [66] recorded EEG signals in the 0.1–1 Hz and 0.05–2 Hz frequency bands from ten healthy volunteers (seven males and three females) with an average age of 26.1 years. The subjects used a customize leg press as a gait trainer during the experiment. EMG sensors and a force pedal were used. In addition, an EOG sensor was employed as the subjects were in front of a monitor with visual stimulation to indicate the execution of plantar flexion.
- -
- Delisle-Rodriguez et al. [68] and Gurve, D. et al. [69] used the same data. They recorded EEG signals in the 8–24 Hz [68] and 0.1–30 Hz [69] frequency bands from ten healthy volunteers (three women and seven men) between 21 and 36 years old. The volunteers had to perform motor imagery and active movement. The task performed by the volunteers was to think about pedaling for five seconds and then actually pedal. sEMG signals were captured to verify the absence of muscle contractions. A screen with visual stimulation was used to perform the series of pedaling and gait movements [68,69].
- Assisted movements
- -
- Qiu et al. [62] recorded Event-Related Desynchronization (ERD) EEG signals from 12 healthy volunteers (five women and seven men) aged 20–26 years and a 56-year-old stroke patient with hemiplegia. The requirements for enrollment were: a minimum of 2.5 years since the last stroke, severe hemiparesis, and difficulty in extending the right knee. The tasks performed were right-leg lifts. A screen with visual stimulation was used to perform the series of movements. No additional sensors were used.
- Electrical lower limb stimulation
- -
- Hauck et al. [65] recorded EEG signals from six healthy right-handed volunteers with an average age of 24.5 years. In addition, Magnetic Resonance Imaging (MRI) was obtained from five volunteers for data recording. Subjects were lying down, and low amperage electrical stimulation was applied to the peroneal, proximal tibial, and distal tibial nerves. Electrooculography (EOG) sensors were also used.
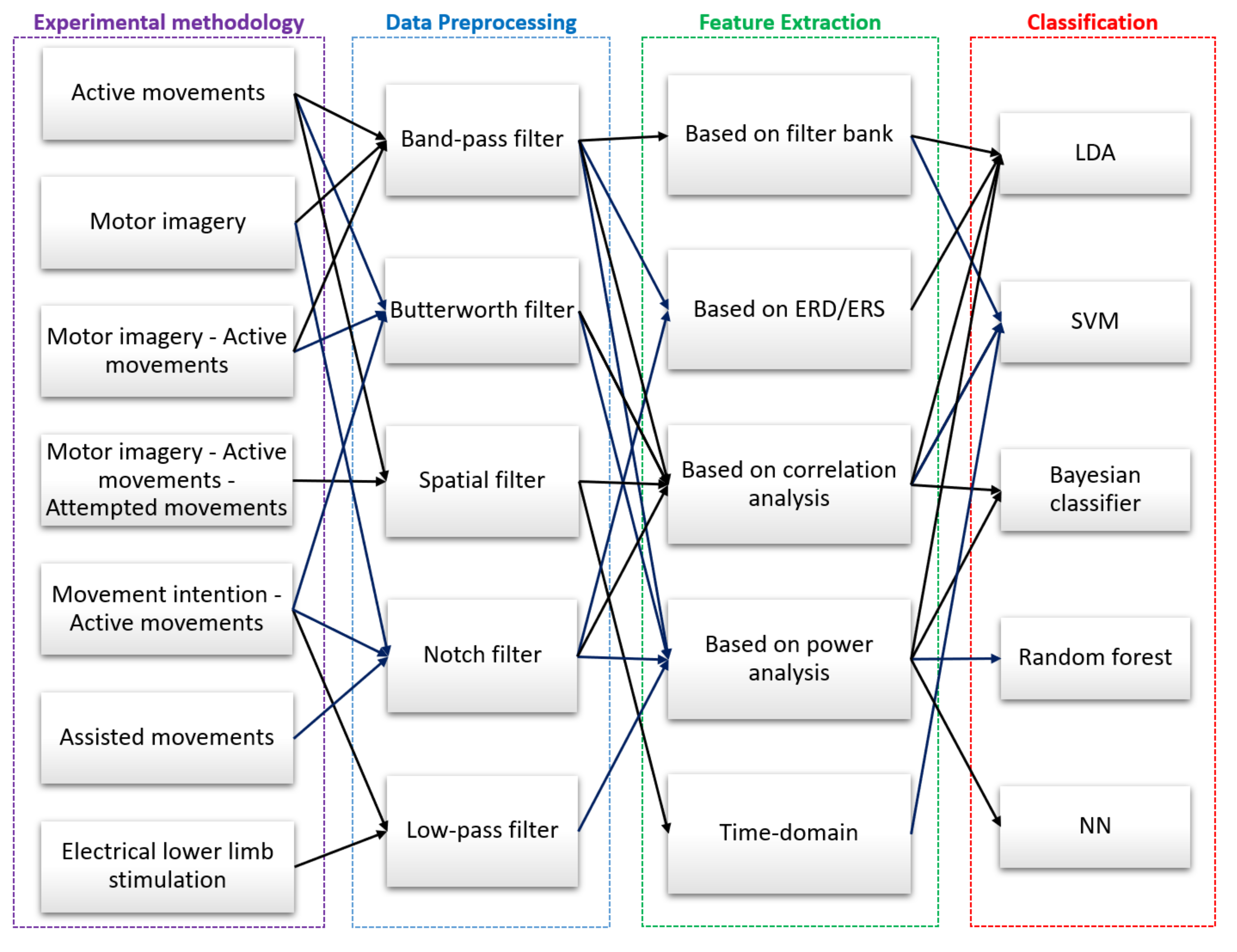
5.2. Data Preprocessing
- Butterworth filter
- -
- Gwin and Ferris [59] used a Butterworth 1 Hz High-Pass filter to remove noise from active movement EEG signals. Channels with a standard deviation greater than or equal to 1 mV were removed; channels whose kurtosis was higher than three standard deviations from the mean were removed; uncorrelated channels ( 0.4) with nearby channels for more than 0.1% of the time-samples were removed.
- -
- Liu et al. [66] removed noise from EEG signals of movement intention and active movement using a sixth-order non-causal Butterworth filter for the 30–300 Hz frequency bands. In addition, they used Teager-Kaiser Energy Operator (TKEO) to condition signals, minimize background noise, and reduce movement artifacts. Conditioning also included 2nd order non-causal Low pass Butterworth filter 50 Hz.
- -
- Gurve et al. [69] eliminated noise from EEG signals of motor imagery and active movement using a second-order Butterworth filter from 0.1 to 30 Hz and Riemann geometry Non-negative Matrix Factorization (NMF).
- -
- Asanza et al. [79] eliminated noise from EEG signals of motor activity and imaginary motor using a two hundred-order Butterworth Infinite Impulse Response (IIR) filter from 8 to 30 Hz.
- Low-pass filter
- -
- Rea et al. [60] eliminated noise from EEG signals of movement intention and active movement using a low-pass filter Wavelet-minimum description length Gaussian low-pass filter with 4-second Fullwidth-Half-Maximum (FWHM).
- -
- Hauck et al. [65] used a low-pass filter below 100 Hz to remove noise from EEG signals of induced movements.
- Notch filter
- -
- Qiu et al. [62] used a notch filter at 50 Hz and Downsampling at 200 Hz to remove noise from EEG signals of movement intention and active movement.
- -
- Delisle-Rodriguez et al. [68] removed noise from EEG signals of movement intention and active movement using a notch filter at 60 Hz, Spectrogram based on Short-Time Fourier Transform (SSTFT), and Riemann geometry.
- -
- Ortiz et al. [75] removed EEG signal noise from movement intention and active movement using a notch filter at 60 Hz.
- Band-pass Filter
- -
- Hsu et al. [63] removed noise from EEG signals of movement intention and active movement using a band-pass filter in 4–40 Hz frequency bands.
- -
- Gu et al. [70] removed noise from EEG signals of movement intention and active movement using a band-pass filter in 1–30 Hz frequency bands and Independent Component Analysis (ICA).
- -
- Gordleeva et al. [71] used a band-pass filter in 8–15 Hz frequency bands to remove noise from EEG signals of movement intention and active movement.
- -
- Chang et al. [72] used a band-pass filter in 1–50 Hz frequency bands to remove noise from EEG signals of movement intention and active movement.
- -
- Hoshino et al. [73] removed noise from EEG signals of movement intention and active movement using a band-pass filter in the 0.5–100 Hz frequency bands and multiple linear regression analysis.
- -
- Kline et al. [76] removed EEG signal noise from DC offset and noise associated with blinking using a bandpass filter between 5 and 55 Hz with a roll-off of 20 dB/decade.
- -
- Murphy et al. [77] used non-stimulus BCI signal event-related desynchronization EEG (ERD). For this, he employs a bandpass filter for the beta band frequencies (1–100 Hz) along with a custom-made MATLAB toolbox (BCI2VR).
- Spatial Filter
- -
- Choi et al. [74] removed EEG signal noise from movement intention and active movement using a Filter Bank Common Spatial Pattern (FBCSP).
- -
- Jochumsen et al. [80] used an Optimized Spatial Filter (OSF). The output of the OSF (one channel) was bandpass filtered from 0.05 to 10 Hz with a second-order Butterworth filter and downsampled to 20 Hz.
5.3. Feature Extraction
- Time-domain
- -
- Jochumsen et al. [80] used six time-domain features extracted from the 2-second data segment before movement detection. The features were: (i+ii) slope and intercept of a linear regression of the entire data segment, (iii+iv) slope and intercept of a linear regression of the data segment from the point of detection and 0.5 s prior to this point, (v) average amplitude of the entire data segment, and (vi) the peak of maximum negativity.
- Based on ERD/ERS
- -
- Qiu et al. [62] used Event-Related Spectral Perturbation (ERSP) and Event-Related Desynchronization (ERD) for feature extraction from highly event-related EEG signals in right leg lifting tasks.
- -
- Murphy et al. [77] used Event-Related Desynchronization (ERD) for feature extraction from the beta band (16–24 Hz). It was calculated in real-time against baseline activity when the subject was relaxed.
- Based on Filter bank
- -
- Hsu et al. [63] used the Filter-bank CSP (FB-CSP) for feature extraction from highly event-related EEG signals in left-and-right stepping and motor imagery tasks.
- -
- Gordleeva et al. [71] used the Common Spatial Pattern Filter (CSP) for feature extraction from highly event-related EEG signal characteristics in motor imagery and active movement leg lifting tasks.
- Based on Power Analysis
- -
- Rea et al. [60] used T-value for feature extraction of EEG signals with high temporal resolution in movement intention and active movement tasks of hip movements with a knee and ankle constraint.
- -
- Hauck et al. [65] used the mean global field power signal-to-noise ratio (SNR) for feature extraction of EEG signals of induced movements.
- -
- Liu et al. [66] used Gini index scores of tree nodes for feature extraction of EEG signals related to movement intention and active movement.
- -
- Chang et al. [72] used the Power Spectrum over the main channel for feature extraction of EEG signals of walking active movement.
- -
- Ortiz et al. [75] used Empirical Mode Decomposition (EMD) for Intrinsic Mode Functions (IMFs) and Variation of Power for IMFs for feature extraction of EEG signals from motor imagery of walking.
- -
- Kline et al. [76] used the power spectrum value of all the studied frequencies (alpha, beta, and gamma) for each EEG electrode.
- -
- Do et al. [78] used spatio-spectral features from the 8–10 Hz frequency band for able-bodied subjects and the 10–12 Hz frequency band for SCI subjects.
- -
- Asanza et al. [79] used Power Spectral Density (PSD) from 8 to 30 Hz, calculated at 10 s of sampling of each EEG electrode.
- Based on Correlation Analysis
- -
- Gwin and Ferris [59] used an Adaptive Mixture Independent Component Analysis (AMICA) for feature extraction of EEG signals with a high temporal resolution in isometric and isotonic ankle and knee movements.
- -
- -
- Gu et al. [70] used Sparse Multinomial Logistic Regression for feature extraction of EEG signals from motor imagery during the foot dorsiflexing task.
- -
- Hoshino et al. [73] used Amplitude Envelope Correlation (AEC) for feature extraction of EEG signals from active movement during ankle movements, dorsiflexion, and plantarflexion.
- -
- Choi et al. [74] used Mutual Information-based Best Individual Feature (MIBIF) for feature extraction of EEG signals from active movement during the gait and sit task.
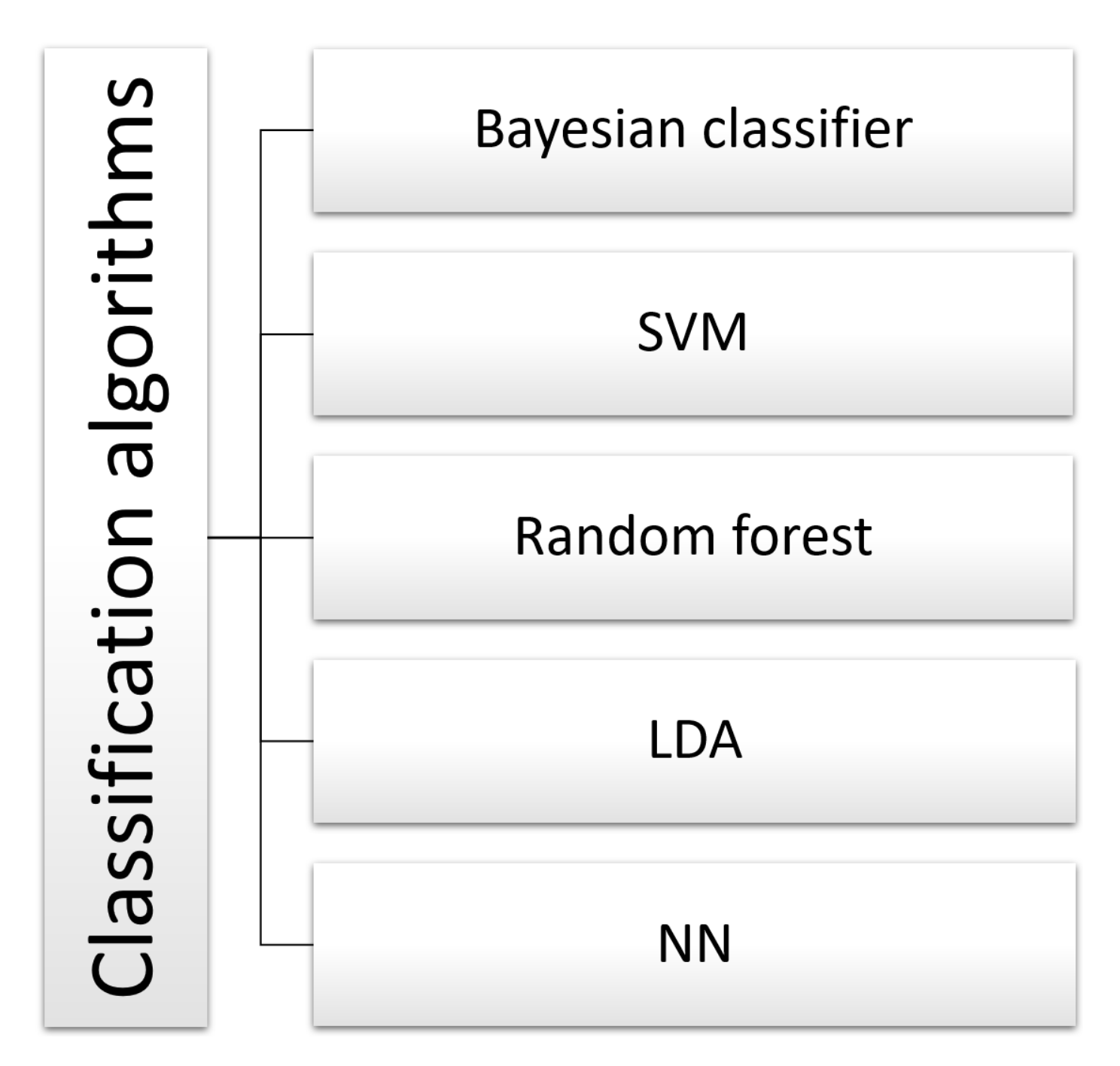
5.4. Classification Algorithms
- Bayesian Classifier
- -
- Gwin and Ferris [59] used a four-way linear naïve Bayesian Classifier to classify isometric and isotonic ankle and knee movements with an accuracy of 87%.
- -
- Do et al. [78] used a Bayesian Classifier, performing stratified 10-fold cross-validation and used 90% of the EEG data to train. This offline analysis resulted in a model classification accuracy of 94.8 ± 0.8% and 77.8 ± 2.0% for able-bodied and SCI subjects, respectively.
- Support Vector Machine (SVM)
- -
- Hsu et al. [63] used Fuzzy SVM to classify EEG signals in left-and-right stepping motor imagery tasks with an accuracy of 86.25%.
- -
- Gu et al. [70] used SVM to classify EEG signals in motor imagery during the foot dorsiflexing task with an accuracy of 67.13%.
- -
- Choi et al. [74] used SVM to classify the EEG signals of active movement during the gait and sit task, achieving 80% accuracy.
- -
- Jochumsen et al. [80] classified EEG signals from executed, imaginary, and attempted movement tasks using SVM with accuracy of 57 ± 3%, 53 ± 6%, and 47 ± 7%, respectively.
- Liu et al. [66] used Random Forest to classify the EEG signals related to movement intention and active movement with an accuracy of 85%.
- Linear discriminant analysis (LDA)
- -
- Rea et al. [60] used LDA with linear kernel to classify the EEG signals in movement intention and active movement tasks of hip movements with a knee and ankle constraint. They achieved an accuracy of 67.77%.
- -
- -
- Gordleeva et al. [71] classified EEG signals in motor imagery and active movement leg lifting tasks with LDA and achieved an accuracy of 65.7%.
- -
- Murphy et al. [77] classified EEG signals from motor imagery with an offline LDA model. This model was made for online detection of the subject’s intention to activate the switch by imaging lower-limb movement. The algorithms used the BCI2VR toolbox.
- Neural Network (NN)
- -
- Kline et al. [76] used a neural network (NN) implemented in Python 3.7 to classify right and left lower limb movement. The model used the Keras toolbox, achieving greater than 66% accuracy.
- -
- Asanza et al. [79] used a neural network (NN) trained in Matlab and then implemented it on Field-Programmable Gate Arrays (FPGAs). The model classified motor activity and motor imagery of both feet, with accuracies of 92.1% and 93.8%, respectively.
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dollar, A.M.; Herr, H. Lower extremity exoskeletons and active orthoses: Challenges and state-of-the-art. IEEE Trans. Robot. 2008, 24, 144–158. [Google Scholar] [CrossRef]
- Tucker, M.R.; Olivier, J.; Pagel, A.; Bleuler, H.; Bouri, M.; Lambercy, O.; del R Millán, J.; Riener, R.; Vallery, H.; Gassert, R. Control strategies for active lower extremity prosthetics and orthotics: A review. J. Neuroeng. Rehabil. 2015, 12, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann, D.; Adamovich, S.; Aghagolzadeh, M.; Agrawal, S.; Allemand, Y.; Andrysek, J.; Ashe, J.; Astolfi, L.; Azevedo-Coste, C.; Babiloni, F.; et al. 2009 Index IEEE Transactions on Neural Systems and Rehabilitation Engineering Vol. 17. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 609. [Google Scholar]
- Pennycott, A.; Wyss, D.; Vallery, H.; Klamroth-Marganska, V.; Riener, R. Towards more effective robotic gait training for stroke rehabilitation: A review. J. Neuroeng. Rehabil. 2012, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Nazmi, N.; Rahman, M.A.A.; Yamamoto, S.I.; Ahmad, S.A. Walking gait event detection based on electromyography signals using artificial neural network. Biomed. Signal Process. Control 2019, 47, 334–343. [Google Scholar] [CrossRef]
- Al-Quraishi, M.S.; Ishak, A.J.; Ahmad, S.A.; Hasan, M.K.; Al-Qurishi, M.; Ghapanchizadeh, H.; Alamri, A. Classification of ankle joint movements based on surface electromyography signals for rehabilitation robot applications. Med. Biol. Eng. Comput. 2017, 55, 747–758. [Google Scholar] [CrossRef]
- de Freitas, R.C.; Alves, R.; da Silva Filho, A.G.; de Souza, R.E.; Bezerra, B.L.; dos Santos, W.P. Electromyography-controlled car: A proof of concept based on surface electromyography, Extreme Learning Machines and low-cost open hardware. Comput. Electr. Eng. 2019, 73, 167–179. [Google Scholar] [CrossRef]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: Emerging avenues and challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef]
- Barsotti, M.; Dupan, S.; Vujaklija, I.; Došen, S.; Frisoli, A.; Farina, D. Online finger control using high-density EMG and minimal training data for robotic applications. IEEE Robot. Autom. Lett. 2018, 4, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Fang, P.; Geng, Y.; Wei, Z.; Zhou, P.; Tian, L.; Li, G. New control strategies for multifunctional prostheses that combine electromyographic and speech signals. IEEE Intell. Syst. 2015, 30, 47–53. [Google Scholar] [CrossRef]
- Meier, J.D.; Aflalo, T.N.; Kastner, S.; Graziano, M.S. Complex organization of human primary motor cortex: A high-resolution fMRI study. J. Neurophysiol. 2008, 100, 1800–1812. [Google Scholar] [CrossRef] [Green Version]
- Pfurtscheller, G.; Neuper, C.; Krausz, G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin. Neurophysiol. 2000, 111, 1873–1879. [Google Scholar] [CrossRef]
- Constantine, A.; Asanza, V.; Loayza, F.R.; Peláez, E.; Peluffo-Ordóñez, D. BCI System using a Novel Processing Technique Based on Electrodes Selection for Hand Prosthesis Control. IFAC-Papers OnLine 2021, 54, 364–369. [Google Scholar] [CrossRef]
- He, Y.; Luu, T.P.; Nathan, K.; Nakagome, S.; Contreras-Vidal, J.L. A mobile brain-body imaging dataset recorded during treadmill walking with a brain-computer interface. Sci. Data 2018, 5, 180074. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Chen, W.; Pei, Z.; Wang, J. A brain-controlled lower-limb exoskeleton for human gait training. Rev. Sci. Instrum. 2017, 88, 104302. [Google Scholar] [CrossRef]
- Alturki, F.A.; AlSharabi, K.; Abdurraqeeb, A.M.; Aljalal, M. EEG signal analysis for diagnosing neurological disorders using discrete wavelet transform and intelligent techniques. Sensors 2020, 20, 2505. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Wang, H. Early Alzheimer’s disease diagnosis based on EEG spectral images using deep learning. Neural Netw. 2019, 114, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Di Iorio, R.; Vecchio, F.; Anfossi, M.; Babiloni, C.; Bozzali, M.; Bruni, A.C.; Cappa, S.F.; Escudero, J.; Fraga, F.J.; et al. Early diagnosis of Alzheimer’s disease: The role of biomarkers including advanced EEG signal analysis. Report from the IFCN-sponsored panel of experts. Clin. Neurophysiol. 2020, 131, 1287–1310. [Google Scholar] [CrossRef] [PubMed]
- Cassani, R.; Estarellas, M.; San-Martin, R.; Fraga, F.J.; Falk, T.H. Systematic review on resting-state EEG for Alzheimer’s disease diagnosis and progression assessment. Dis. Markers 2018, 2018, 5174815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussen, D.F.; Hussein, A.A.F.; Monzer, M.A.M.; Hammad, S.A. Combined markers for predicting cognitive deficit in patients with Alzheimer’s disease. Egypt. J. Med. Hum. Genet. 2021, 22, 63. [Google Scholar] [CrossRef]
- Naghsh, E.; Sabahi, M.F.; Beheshti, S. Spatial analysis of EEG signals for Parkinson’s disease stage detection. Signal Image Video Process. 2020, 14, 397–405. [Google Scholar] [CrossRef]
- Oh, S.L.; Hagiwara, Y.; Raghavendra, U.; Yuvaraj, R.; Arunkumar, N.; Murugappan, M.; Acharya, U.R. A deep learning approach for Parkinson’s disease diagnosis from EEG signals. Neural Comput. Appl. 2018, 32, 10927–10933. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.; Sun, J.; Zhang, Z.; Wu, Z.; Yang, T.; Xue, G.; Cheng, C. Using a deep recurrent neural network with EEG signal to detect Parkinson’s disease. Ann. Transl. Med. 2020, 8, 874. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Behari, M.; Goyal, V.; Sharma, R. Study of EEG microstates in Parkinson’s disease: A potential biomarker? Cogn. Neurodyn. 2021, 15, 463–471. [Google Scholar] [CrossRef]
- Gordleeva, S.Y.; Lukoyanov, M.; Mineev, S.; Khoruzhko, M.; Mironov, V.; Kaplan, A.Y.; Kazantsev, V. Exoskeleton control system based on motor-imaginary brain–computer interface. Sovrem. Tehnol. Med. 2017, 9, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Duda, R.O.; Stork, D.G.; Hart, P.E. Pattern Classification, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Amin, H.U.; Mumtaz, W.; Subhani, A.R.; Saad, M.N.M.; Malik, A.S. Classification of EEG Signals Based on Pattern Recognition Approach. Front. Comput. Neurosci. 2017, 11, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain–computer interface paradigms. J. Neural Eng. 2019, 16, 011001. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, Y.; Qian, I.; Qi, Y.; Wang, Y.; Guan, C.; Sun, Y. Design a novel BCI for neurorehabilitation using concurrent LFP and EEG features: A case study. IEEE Trans. Biomed. Eng. 2021. [Google Scholar] [CrossRef]
- Bressan, G.; Cisotto, G.; Müller-Putz, G.R.; Wriessnegger, S.C. Deep learning-based classification of fine hand movements from low frequency EEG. Future Internet 2021, 13, 103. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, L.; Kong, W.; Peng, Y.; Hu, H.; Cao, J. A novel classification method for EEG-based motor imagery with narrow band spatial filters and deep convolutional neural network. Cogn. Neurody. 2021. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, M.; Wei, C.; Mantini, D.; Li, Z.; Liu, Q. Eegdenoisenet: A benchmark dataset for deep learning solutions of eeg denoising. J. Neural Eng. 2021, 18, 056057. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, X.; Wang, Z.; Ma, Y. Implementation of a brain-computer interface on a lower-limb exoskeleton. IEEE Access 2018, 6, 38524–38534. [Google Scholar] [CrossRef]
- Vinoj, P.G.; Jacob, S.; Menon, V.G.; Rajesh, S.; Khosravi, M.R. Brain-Controlled Adaptive Lower Limb Exoskeleton for Rehabilitation of Post-Stroke Paralyzed. IEEE Access 2019, 7, 132628–132648. [Google Scholar] [CrossRef]
- Vouga, T.; Zhuang, K.Z.; Olivier, J.; Lebedev, M.A.; Nicolelis, M.A.; Bouri, M.; Bleuler, H. EXiO—A brain-controlled lower limb exoskeleton for rhesus macaques. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Lennon, O.; Tonellato, M.; Del Felice, A.; Di Marco, R.; Fingleton, C.; Korik, A.; Guanziroli, E.; Molteni, F.; Guger, C.; Otner, R.; et al. A systematic review establishing the current state-of-the-art, the limitations, and the DESIRED checklist in studies of direct neural interfacing with robotic gait devices in stroke rehabilitation. Front. Neurosci. 2020, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- Comani, S.; Velluto, L.; Schinaia, L.; Cerroni, G.; Serio, A.; Buzzelli, S.; Sorbi, S.; Guarnieri, B. Monitoring neuro-motor recovery from stroke with high-resolution EEG, robotics and virtual reality: A proof of concept. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Guger, C.; Allison, B.Z.; Mrachacz-Kersting, N. Recent Advances in Brain-Computer Interface Research—A Summary of the 2017 BCI Award and BCI Research Trends. In Brain-Computer Interface Research; Springer: Cham, Switzerland, 2019; pp. 115–127. [Google Scholar]
- Rosati, S.; Agostini, V.; Knaflitz, M.; Balestra, G. Muscle activation patterns during gait: A hierarchical clustering analysis. Biomed. Signal Process. Control 2017, 31, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y. Brain-Computer Interface for Cyberpsychology. In Analyzing Human Behavior in Cyberspace; IGI Global: Hershey, PA, USA, 2019; pp. 102–122. [Google Scholar]
- Minguillon, J.; Lopez-Gordo, M.A.; Pelayo, F. Trends in EEG-BCI for daily-life: Requirements for artifact removal. Biomed. Signal Process. Control 2017, 31, 407–418. [Google Scholar] [CrossRef]
- Lee, J.J.; Lee, D.R.; Shin, Y.K.; Lee, N.G.; Han, B.S.; You, S.J.H. Comparative neuroimaging in children with cerebral palsy using fMRI and a novel EEG-based brain mapping during a motor task—A preliminary investigation. NeuroRehabilitation 2013, 32, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Lee, D.R.; Hwang, H.J.; You, S.J.H.; Im, C.H. A novel EEG-based brain mapping to determine cortical activation patterns in normal children and children with cerebral palsy during motor imagery tasks. NeuroRehabilitation 2012, 31, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, J.; Chen, L. Event-related desynchronization and synchronization quantification in motor-related EEG by Kolmogorov entropy. J. Neural Eng. 2013, 10, 036023. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.J.; Sutterer, D.W.; Serences, J.T.; Vogel, E.K.; Awh, E. Alpha-band oscillations enable spatially and temporally resolved tracking of covert spatial attention. Psychol. Sci. 2017, 28, 929–941. [Google Scholar] [CrossRef]
- Niedermeyer, E.; Da Silva, F.L. Electroencephalography–Basic Principles, Clinical Applications, and Related Fields; Urban & Schwarzenberg: Munich, Germany, 2020. [Google Scholar]
- Pineda, J.A. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Res. Rev. 2005, 50, 57–68. [Google Scholar] [CrossRef]
- Rangaswamy, M.; Porjesz, B.; Chorlian, D.B.; Wang, K.; Jones, K.A.; Bauer, L.O.; Rohrbaugh, J.; O’connor, S.J.; Kuperman, S.; Reich, T.; et al. Beta power in the EEG of alcoholics. Biol. Psychiatry 2002, 52, 831–842. [Google Scholar] [CrossRef]
- McDermott, B.; Porter, E.; Hughes, D.; McGinley, B.; Lang, M.; O’Halloran, M.; Jones, M. Gamma band neural stimulation in humans and the promise of a new modality to prevent and treat Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 65, 363–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bascil, M.S.; Tesneli, A.Y.; Temurtas, F. Spectral feature extraction of EEG signals and pattern recognition during mental tasks of 2-D cursor movements for BCI using SVM and ANN. Australas. Phys. Eng. Sci. Med. 2016, 39, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Ji, X.; Huang, D. Classification of EEG signals based on autoregressive model and wavelet packet decomposition. Neural Process. Lett. 2017, 45, 365–378. [Google Scholar] [CrossRef]
- Chai, R.; Naik, G.R.; Nguyen, T.N.; Ling, S.H.; Tran, Y.; Craig, A.; Nguyen, H.T. Driver fatigue classification with independent component by entropy rate bound minimization analysis in an EEG-based system. IEEE J. Biomed. Health Inform. 2016, 21, 715–724. [Google Scholar] [CrossRef]
- Al-Qazzaz, N.K.; Hamid Bin Mohd Ali, S.; Ahmad, S.A.; Islam, M.S.; Escudero, J. Selection of Mother Wavelet Functions for Multi-Channel EEG Signal Analysis during a Working Memory Task. Sensors 2015, 15, 29015–29035. [Google Scholar] [CrossRef] [Green Version]
- Ghaderpour, E.; Pagiatakis, S.D.; Hassan, Q.K. A Survey on Change Detection and Time Series Analysis with Applications. Appl. Sci. 2021, 11, 6141. [Google Scholar] [CrossRef]
- Singla, R.; Haseena, B. Comparison of ssvep signal classification techniques using svm and ann models for bci applications. Int. J. Inf. Electron. Eng. 2014, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Gandhoke, G.S.; Belykh, E.; Zhao, X.; Leblanc, R.; Preul, M.C. Edwin Boldrey and Wilder Penfield’s Homunculus: A life given by Mrs. Cantlie (in and out of realism). World Neurosurg. 2019, 132, 377–388. [Google Scholar] [CrossRef]
- Condylis, C.; Lowet, E.; Ni, J.; Bistrong, K.; Ouellette, T.; Josephs, N.; Chen, J.L. Context-dependent sensory processing across primary and secondary somatosensory cortex. Neuron 2020, 106, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Ferris, D.P. An EEG-based study of discrete isometric and isotonic human lower limb muscle contractions. J. Neuroeng. Rehabil. 2012, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Rea, M.; Rana, M.; Lugato, N.; Terekhin, P.; Gizzi, L.; Brötz, D.; Fallgatter, A.; Birbaumer, N.; Sitaram, R.; Caria, A. Lower limb movement preparation in chronic stroke: A pilot study toward an fNIRS-BCI for gait rehabilitation. Neurorehabilit. Neural Repair 2014, 28, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Trivailo, P.M.; Simic, M. EEG-based BCI control schemes for lower-limb assistive-robots. Front. Hum. Neurosci. 2018, 12, 312. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Yi, W.; Xu, J.; Qi, H.; Du, J.; Wang, C.; He, F.; Ming, D. Event-related beta EEG changes during active, passive movement and functional electrical stimulation of the lower limb. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 283–290. [Google Scholar] [CrossRef]
- Hsu, W.C.; Lin, L.F.; Chou, C.W.; Hsiao, Y.T.; Liu, Y.H. EEG classification of imaginary lower limb stepping movements based on fuzzy support vector machine with kernel-induced membership function. Int. J. Fuzzy Syst. 2017, 19, 566–579. [Google Scholar] [CrossRef]
- Al-Quraishi, M.S.; Elamvazuthi, I.; Daud, S.A.; Parasuraman, S.; Borboni, A. EEG-based control for upper and lower limb exoskeletons and prostheses: A systematic review. Sensors 2018, 18, 3342. [Google Scholar] [CrossRef] [Green Version]
- Hauck, M.; Baumgärtner, U.; Hille, E.; Hille, S.; Lorenz, J.; Quante, M. Evidence for early activation of primary motor cortex and SMA after electrical lower limb stimulation using EEG source reconstruction. Brain Res. 2006, 1125, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, W.; Lee, K.; Chavarriaga, R.; Iwane, F.; Bouri, M.; Pei, Z.; del, R. Millán, J. EEG-based lower-limb movement onset decoding: Continuous classification and asynchronous detection. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.W.; Yu, H.Y.; Shih, Y.H.; Yiu, C.H.; Kwan, S.Y.; Yen, D.J.; Lin, Y.Y. Lateralisation value of lower limb behaviors in complex partial seizures of temporal lobe origin: A video-EEG analysis. Seizure 2004, 13, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Delisle-Rodriguez, D.; Cardoso, V.; Gurve, D.; Loterio, F.; Romero-Laiseca, M.A.; Krishnan, S.; Bastos-Filho, T. System based on subject-specific bands to recognize pedaling motor imagery: Towards a BCI for lower-limb rehabilitation. J. Neural Eng. 2019, 16, 056005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurve, D.; Delisle-Rodriguez, D.; Romero-Laiseca, M.; Cardoso, V.; Loterio, F.; Bastos, T.; Krishnan, S. Subject-specific EEG channel selection using non-negative matrix factorization for lower-limb motor imagery recognition. J. Neural Eng. 2020, 17, 026029. [Google Scholar] [CrossRef]
- Gu, L.; Yu, Z.; Ma, T.; Wang, H.; Li, Z.; Fan, H. EEG-based classification of lower limb motor imagery with brain network analysis. Neuroscience 2020, 436, 93–109. [Google Scholar] [CrossRef]
- Gordleeva, S.Y.; Lobov, S.A.; Grigorev, N.A.; Savosenkov, A.O.; Shamshin, M.O.; Lukoyanov, M.V.; Khoruzhko, M.A.; Kazantsev, V.B. Real-time EEG–EMG human–machine interface-based control system for a lower-limb exoskeleton. IEEE Access 2020, 8, 84070–84081. [Google Scholar] [CrossRef]
- Chang, W.C.; Ko, L.W.; Yu, K.H.; Ho, Y.C.; Chen, C.H.; Jong, Y.J.; Huang, Y.P. EEG analysis of mixed-reality music rehabilitation system for post-stroke lower limb therapy. J. Soc. Inf. Disp. 2019, 27, 372–380. [Google Scholar] [CrossRef]
- Hoshino, T.; Oguchi, K.; Inoue, K.; Hoshino, A.; Hoshiyama, M. Relationship between lower limb function and functional connectivity assessed by EEG among motor-related areas after stroke. Top. Stroke Rehabil. 2020, 28, 614–623. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.T.; Jeong, J.H.; Kim, L.; Lee, S.J.; Kim, H. Developing a motor imagery-based real-time asynchronous hybrid BCI controller for a lower-limb exoskeleton. Sensors 2020, 20, 7309. [Google Scholar] [CrossRef]
- Ortiz, M.; Iáñez, E.; Contreras-Vidal, J.L.; Azorín, J.M. Analysis of the EEG rhythms based on the empirical mode decomposition during motor imagery when using a lower-limb exoskeleton. A case of study. Front. Neurorobotics 2020, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Kline, A.; Forkert, N.D.; Felfeliyan, B.; Pittman, D.; Goodyear, B.; Ronsky, J. fMRI-Informed EEG for brain mapping of imagined lower limb movement: Feasibility of a brain computer interface. J. Neurosci. Methods 2021, 363, 109339. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.P.; Bai, O.; Gorgey, A.S.; Fox, J.; Lovegreen, W.T.; Burkhardt, B.W.; Atri, R.; Marquez, J.S.; Li, Q.; Fei, D.Y. Electroencephalogram-based brain–computer interface and lower-limb prosthesis control: A case study. Front. Neurol. 2017, 8, 696. [Google Scholar] [CrossRef] [Green Version]
- Do, A.H.; Wang, P.T.; King, C.E.; Chun, S.N.; Nenadic, Z. Brain-computer interface controlled robotic gait orthosis. J. Neuroeng. Rehabil. 2013, 10, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asanza, V.; Constantine, A.; Valarezo, S.; Peláez, E. Implementation of a Classification System of EEG Signals Based on FPGA. In Proceedings of the 2020 Seventh International Conference on eDemocracy eGovernment (ICEDEG), Buenos Aires, Argentina, 22–24 April 2020; pp. 87–92. [Google Scholar] [CrossRef]
- Jochumsen, M.; Khan Niazi, I.; Samran Navid, M.; Nabeel Anwar, M.; Farina, D.; Dremstrup, K. Online multi-class brain-computer interface for detection and classification of lower limb movement intentions and kinetics for stroke rehabilitation. Brain-Comput. Interfaces 2015, 2, 202–210. [Google Scholar] [CrossRef]
- Schalk, G.; McFarland, D.; Hinterberger, T.; Birbaumer, N.; Wolpaw, J. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans. Biomed. Eng. 2004, 51, 1034–1043. [Google Scholar] [CrossRef]
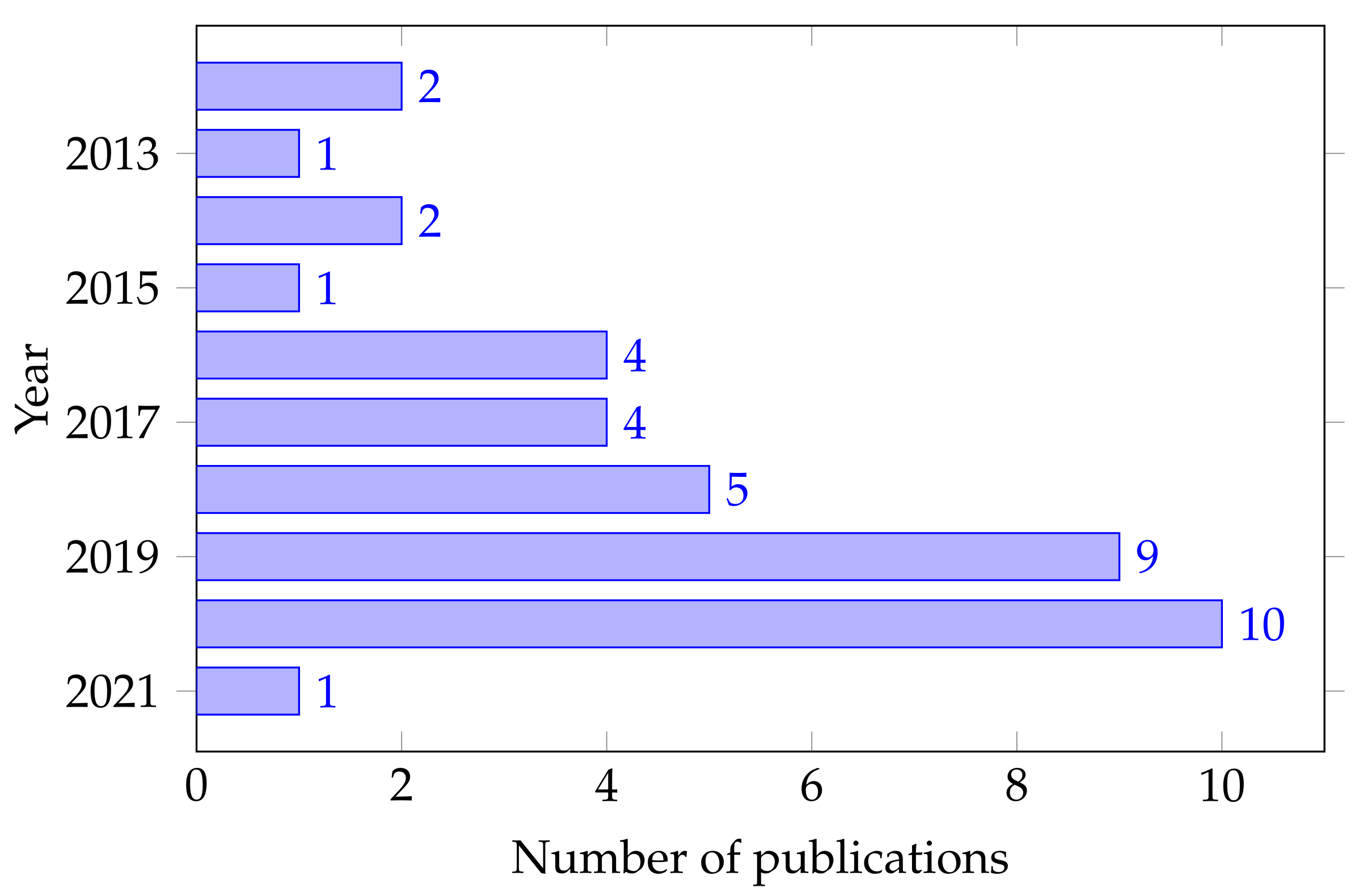




| Band | Frequency (Hz) | Mental State |
|---|---|---|
| <4 | Infants or Adults during Deep Sleep. | |
| 4–7 | Youngsters and adults in stages of drowsiness. | |
| 8–12 | Young people and adults during low brain activity or rest. | |
| 7.5–12.5 | Present in the motor cortex during the execution or thinking of motor activities. | |
| 16–31 | Present during active or busy thinking, state of concentration, high alertness, and anxiety. | |
| 32 | High Brain Activity. |
| Author [Ref] | Application | Subjects | Protocol | Task | EEG Signal | Other Input Signal |
|---|---|---|---|---|---|---|
| Gwin and Ferris [59] | Tracking cortical activity | 8 healthy subjects | Active Movements | Isometric and isotonic ankle and knee movement | 8–30 Hz | Load Cell |
| Rea et al. [60] | Custom pedal chair | 7 chronic stroke patients | Movemenet intention—Active movement | Hip movements—knee and ankle constrained | - | EMG |
| Tariq et al. [61] | RE lower limb Exoskeleton | 14 healthy subject | Motor imagery | Gait | ERD/ERS | - |
| Qiu et al. [62] | Personal assistance, VitalStim Therapy + visual coordination | 12 healthy subject + 1 hemiplegic stroke patient | Active, assisted, and FES-induced movements | Right leg raise | ERD | - |
| Hsu et al. [63] | Elevated platform + visual coordination | 8 healthy subject | Motor imagery | Left and right Stepping | 8–30 Hz | EOG |
| Al-Quraishi et al. [64] | Prosthetic Knee | 3 healthy and 4 SCI patients | Motor imagery | Walking and Idling | ERD | - |
| Hauck et al. [65] | sensory stimulation | 6 healthy subject | Electrical lower-limb stimulation | - | - | MRI/EOG |
| Liu et al. [66] | Customize leg Press—Gait trainer + visual coordination | 10 healthy subject | Movemenet intention—Active movement | Plantar flexion | (0.1–1 Hz) (0.05–2 Hz) | EOG, EMG, force on pedal |
| Chou et al. [67] | Avatar, BWS and Overground exoskeleton | 5 SCI subjects | Motor execution | Left and right Stepping | - | - |
| Delisle-Rodriguez et al. [68] | Motorized pedal + visual coordination | 10 healthy subject | Motor imagery—Active Movement | Pedaling | 8–24 Hz | sEMG |
| Gurve et al. [69] | Motorized pedal + visual coordination | 10 healthy subject | Motor imagery—Active Movement | Gait | 0.1–30 Hz | sEMG |
| Gu et al. [70] | BCI system | 11 healthy subject | Motor imagery | Foot dosiflexing | 1–30 Hz | Vertical and horizontal EOG |
| Gordleeva et al. [71] | MI-based BCI lower limb exoskeleton control system | 8 healthy subjects | Motor imagery—Active Movement | Leg lift | 8–15 Hz | EMG |
| Chang et al. [72] | Mixed Augmented Reality (Hololens) | 3 healthy subject + 2 stroke subjects | Active movements | Walking | 0.5–25 Hz | Motion capture sensors (Notch—knee joint angle) |
| Hoshino et al. [73] | - | 24 post-stroke subjects | Active movements | Ankle movements—Dorsiflexion and plantar flexion | alpha bands (8–12), beta (13–30), low beta (13–19), high beta (20–30) | - |
| Choi et al. [74] | MI-based BCI lower limb exoskeleton control system + visual coordination | 10 healthy subject | Active movements | Gait and sit | 7–34 Hz | - |
| Ortiz et al. [75] | MI-based BCI lower limb exoskeleton control system | 3 healthy subject | Motor imagery | Walking | 2–60 Hz | - |
| Kline et al. [76] | Mapping of spatial brain activity | 16 healthy subjects | Executed and imagined | lower limb movements | alpha (8–12 Hz), beta (13–30 Hz) and gamma (31–45 Hz) | fMRI |
| Murphy et al. [77] | Event-related desynchronization (ERD) for lower extremity prosthesis control system | A subjects male suffered a right transfemoral amputation | Imaging right lower-limb movement and walking | Motor imagery task | 16–24 Hz | Gyro + Accelerometer |
| Do et al. [78] | Brain-Controlled robotic gait orthosis | Two subjects (one able-bodied and one with paraplegia due to Spinal Cord Injury (SCI)) | Complete a goal-oriented task of Walking along a linear path | Kinesthetic motor imagery (KMI) | 8–10 Hz and 10–12 Hz | EMG electrodes and gyroscope |
| Asanza et al. [79] | BCI System | 8 healthy subjects | Dorsi and plantar flexion of both feet | Motor activity and imaginary motor | 8–30 Hz | - |
| Jochumsen et al. [80] | BCI for stroke rehabilitation | 12 healthy subjects and 6 stroke patients with lower limb paresis | movement kinetics | Executed, imaginary, and attempted movements | 0.1–10 Hz | Force transducer |
| Task | Algorithm | Accuracy | Reference |
|---|---|---|---|
| Active Movements | Naive Bayesian Classifier | 87% | [59] |
| Motor Imagery—Active Movements | LDA | 65.7% | [71] |
| Motor Imagery | SVM | 86.25% | [63] |
| Kinesthetic motor imagery (KMI) | Bayesian Classifier | 94.8% | [78] |
| Movement Intention—Active Movements | LDA | 96.66% | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asanza, V.; Peláez, E.; Loayza, F.; Lorente-Leyva, L.L.; Peluffo-Ordóñez, D.H. Identification of Lower-Limb Motor Tasks via Brain–Computer Interfaces: A Topical Overview. Sensors 2022, 22, 2028. https://doi.org/10.3390/s22052028
Asanza V, Peláez E, Loayza F, Lorente-Leyva LL, Peluffo-Ordóñez DH. Identification of Lower-Limb Motor Tasks via Brain–Computer Interfaces: A Topical Overview. Sensors. 2022; 22(5):2028. https://doi.org/10.3390/s22052028
Chicago/Turabian StyleAsanza, Víctor, Enrique Peláez, Francis Loayza, Leandro L. Lorente-Leyva, and Diego H. Peluffo-Ordóñez. 2022. "Identification of Lower-Limb Motor Tasks via Brain–Computer Interfaces: A Topical Overview" Sensors 22, no. 5: 2028. https://doi.org/10.3390/s22052028
APA StyleAsanza, V., Peláez, E., Loayza, F., Lorente-Leyva, L. L., & Peluffo-Ordóñez, D. H. (2022). Identification of Lower-Limb Motor Tasks via Brain–Computer Interfaces: A Topical Overview. Sensors, 22(5), 2028. https://doi.org/10.3390/s22052028