Mechanistic Insights into WO3 Sensing and Related Perspectives
Abstract
:1. Introduction
2. Sensing Capabilities of WO3
2.1. NO2
2.2. Acetone
2.3. Ammonia and Related Gases
3. WO3 Structures and Surfaces
3.1. Structural Complexity of WO3 and Relevant Hints for the Field of Gas-Sensing
3.2. Oxygen Vacancies in WO3
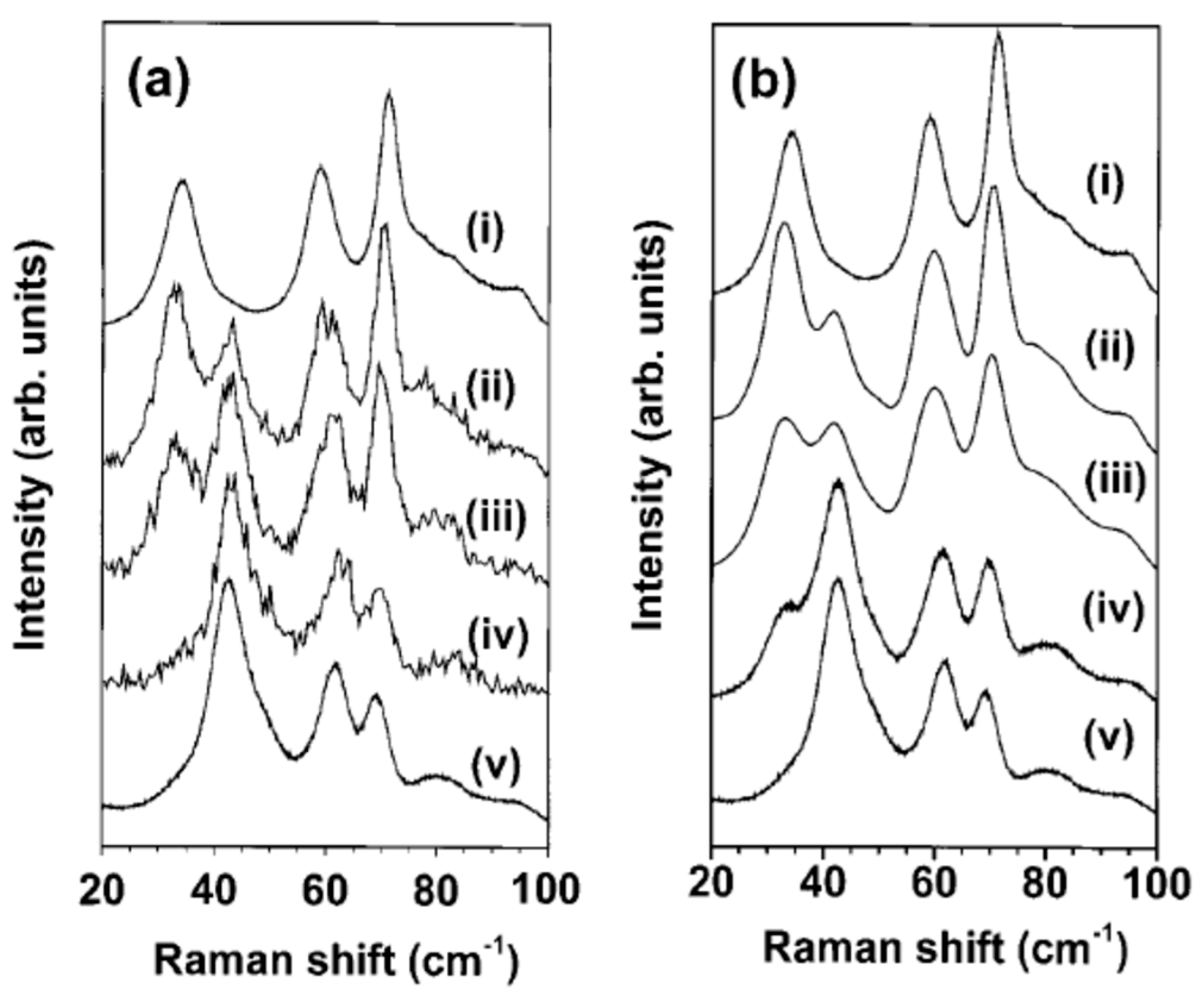
3.2.1. Experimental Findings: Surface Oxygen Vacancies
Electrical Properties
Spectroscopic Studies
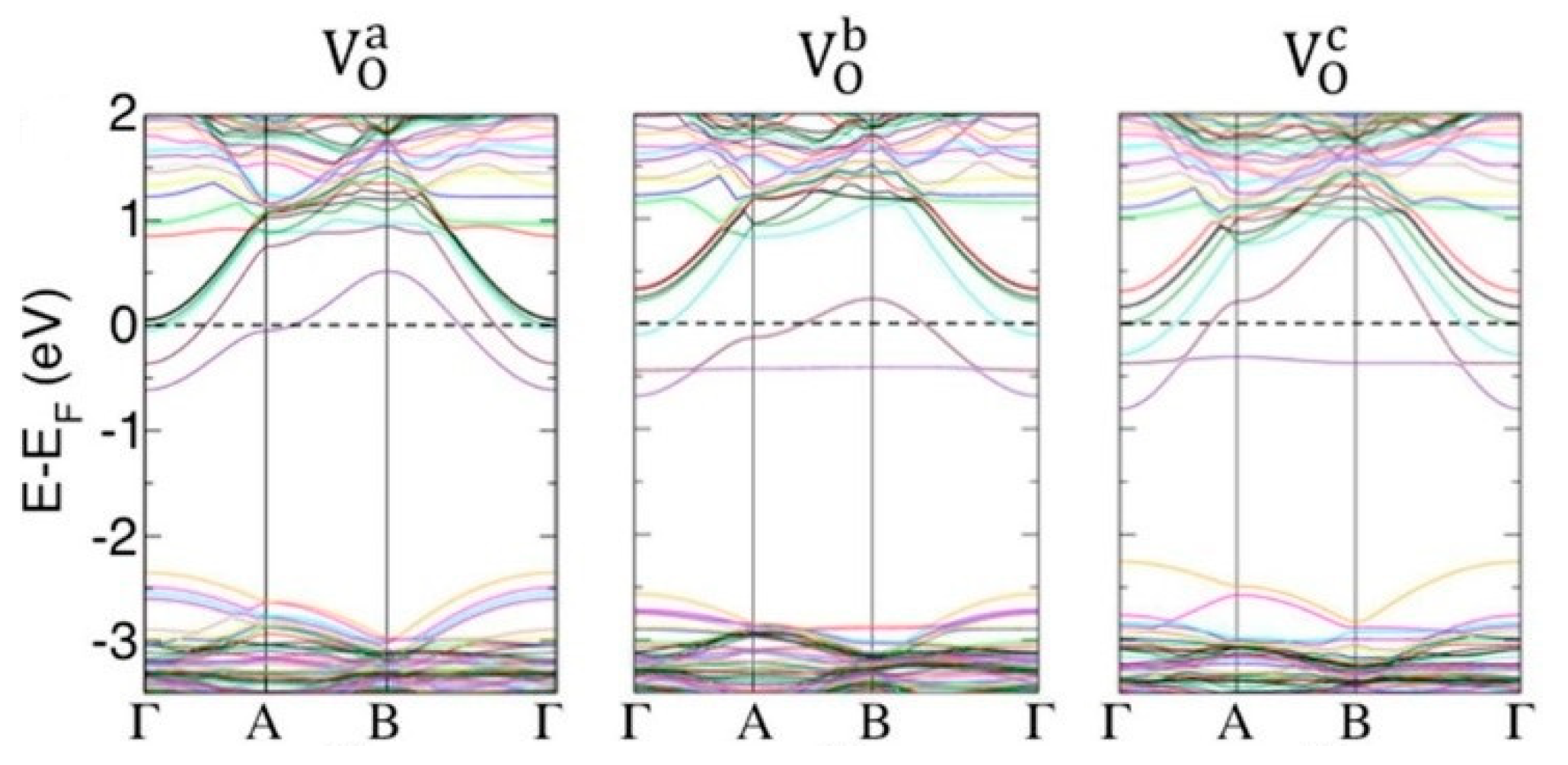
3.2.2. First Principles Modeling of Oxygen Vacancies in WO3
- Oxygen vacancies (both in bulk and on the surface) in WO3 are ubiquitous and favorably formed during the heat-treatment. Re-healing is not a favorable process, with WO3 tending to remain oxygen-defective;
- There is theoretical evidence that oxygen vacancies are anisotropic, with the formation energy depending on the particular crystallographic axis. However, a distribution of all the kinds of vacancies can be expected. The vacancies confer electrical conductivity to WO3. The most energetically favorable surface of monoclinic WO3 is the (001).
3.2.3. Surface Oxygen Vacancies in WO3 and Adsorption Properties
Experimental Studies
First Principles Modeling of Adsorption onto WO3 Surfaces
- Experimentally, the surface of monoclinic WO3 contains strongly acidic W sites; the reduction of the surface strongly favors adsorption of methanol and water. Methanol dissociation takes place on reduced surfaces, which is not the case with water.
- Theoretically speaking, water adsorption is generally calculated to be more favorable than dissociation. However, water desorption resulting from hydrogen dissociation results in surface reduction. We can take this result as an indication that, reciprocally, water dissociation on reduced WO3 surfaces may result in the healing of oxygen vacancies. While apparently unfavorable in the DFT scenario, water dissociation cannot be neglected from experimental studies where an electrical bias is also applied, such as in the case of chemoresistive sensors.
4. Mechanistic Studies of WO3 Sensor Properties
5. Conclusions
- The complex crystallographic phase diagram of WO3 must be taken into account when determining the phase involved in the sensing activity. At room temperature, the as-prepared samples may be constituted by a mixture of γ-monoclinic and δ-triclinic phases, whose speciation can be facilitated by combining XRD and Raman spectroscopy. The investigation of the sensing behavior cannot disregard that at typical operating temperatures, such as 300 °C, the active phase may be the orthogonal one. Therefore, determining the phase composition of the as-prepared samples can be insufficient for studying sensing properties.
- The oxygen vacancies dominate the physical properties of WO3. From first principles modeling, it appears that both bulk and surface vacancies share some features: the formation energy and the electronic effect of the vacancies depend on the crystallographic direction along which they are formed. However, an increasingly accepted view is that at least some of the vacancy levels merge with the Fermi level, explaining the electrical conductivity of oxygen-deficient WO3, in agreement with experimental findings. From an experimental point of view, XPS analysis is extremely useful in distinguishing the various W oxidation states connected to the presence of oxygen vacancies. However, such a technique must be used carefully. For instance, the fitting of the W4f region cannot exclude a W5p3/2 component. Analysis of the valence band spectra may provide useful information about the presence of oxygen vacancies.
- Experimental studies into the sensing mechanisms of WO3 have radically modified the common view of chemoresistive sensing. In the case of CO sensing, the direct generation of oxygen vacancies onto the WO3 surface has been solidly supported. In the case of NO2, the healing of surface vacancies with NO by-product has been backed up by considerable evidence. These conclusions deeply question the usual hypothesis regarding the charge depletion layer by oxygen adsorption and its subsequent modulation by the gas analyte. This is a general indication to carefully check for the applicability of widespread models.
- This final conclusion is of paramount importance: if oxygen vacancies display such a dominant role and they are present in every oxide, even though to different extents and with different concentrations and structures, the achievability of selectivity by chemoresisistive oxide sensors is deeply questionable.
- 5.
- If direct extraction of oxygen from the WO3 surface by CO (Section 4) was a surprising result, even direct vacancy healing by NO2 remarkably differs from the widespread mechanism based on NO2 adsorption/desorption. It seems that such a mechanism still has to be reinforced by suitable measurements of the sensor exhaust during the NO2 tests.
- 6.
- In turn, such mechanistic conclusions indicate the need for a deep revision of the currently accepted models. This can only be done by intensifying the operando investigation of the sensor operation and the effective identification of the evolved species.
- 7.
- DFT modeling has been demonstrated as a powerful tool for verifying hypotheses about the surfaces and structures of WO3. The most commonly considered surface is the (001) plane in the γ-monoclinic phase, which is the most favorable and the most immediately formed upon cleaving single crystals. However, in a polycrystalline nanopowder, several other exposed facets are commonly present. In this sense, there are not many studies of the energy ordering [131] of the exposed crystal planes and of the oxygen vacancy formation energy. This topic needs to be investigated in detail to complete our knowledge of the surface properties of WO3. Other crystallographic phases, such as the orthorhombic phase, should also be considered.
- 8.
- Moreover, still there are very few DFT studies directly related to WO3 sensing. It would be helpful to obtain a catalogue of the most favorable adsorption sites for several gaseous analytes of interest. This is more and more desirable given the remarkably improved computing capabilities currently available.
- 9.
- If oxygen vacancies are vitally important in establishing the sensing behavior of WO3, then any improvement of WO3 sensors must be based on understanding and controlling such vacancies. “Understanding” means that a suitable catalogue of characterization techniques must emerge as a standard toolbox for establishing the vacancy concentration, topology and electronic structure. Therefore, XPS should ideally be complemented by other techniques, such as electron paramagnetic resonance, cathodoluminescence, etc. [152].
- 10.
- “Controlling” the vacancies means that the synthesis should be tailored toward the achievement of a given distribution of oxygen vacancies. Currently, treatments in reduced atmospheres are employed for preparing oxygen-deficient WO3. However, such structures are naturally amenable to being reoxidized during the sensor operation. Another strategy could be the designed introduction of dopants into the structure of WO3. For instance, let A2O3 be the oxide of a trivalent cation. The incorporation equation for substitutional defects for such an oxide into WO3 is, in Kröger–Vink notation:
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeb, S.; Sun, G.X.; Nie, Y.; Xu, H.Y.; Cui, Y.; Jiang, X.C. Advanced developments in nonstoichiometric tungsten oxides for electrochromic applications. Mater. Adv. 2021, 2, 6839–6884. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic Oxides: A Unified View. Solid State Ionics 1994, 70, 678–685. [Google Scholar] [CrossRef]
- Wang, S.F.; Fan, W.R.; Liu, Z.C.; Yu, A.B.; Jiang, X.C. Advances on tungsten oxide based photochromic materials: Strategies to improve their photochromic properties. J. Mater. Chem. C 2018, 6, 191–212. [Google Scholar] [CrossRef]
- Janaky, C.; Rajeshwar, K.; de Tacconi, N.R.; Chanmanee, W.; Huda, M.N. Tungsten-based oxide semiconductors for solar hydrogen generation. Catal. Today 2013, 199, 53–64. [Google Scholar] [CrossRef]
- Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871. [Google Scholar] [CrossRef] [PubMed]
- Bignozzi, C.A.; Caramori, S.; Cristino, V.; Argazzi, R.; Meda, L.; Tacca, A. Nanostructured photoelectrodes based on WO3: Applications to photooxidation of aqueous electrolytes. Chem. Soc. Rev. 2013, 42, 2228–2246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Tian, W.; Chen, C.; Xu, W.W.; Li, L. Tungsten Trioxide Nanostructures for Photoelectrochemical Water Splitting: Material Engineering and Charge Carrier Dynamic Manipulation. Adv. Funct. Mater. 2019, 29, 1809036. [Google Scholar] [CrossRef]
- Dong, P.Y.; Hou, G.H.; Xi, X.U.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, X.T.; Cheng, G.; Chen, R.; Xiong, J.Y.; Li, W.J.; Wei, Y.C. Engineered tungsten oxide-based photocatalysts for CO2 reduction: Categories and roles. J. Mater. Chem. A 2021, 9, 22781–22809. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Porous nanoplate-like tungsten trioxide/reduced graphene oxide catalyst for sonocatalytic degradation and photocatalytic hydrogen production. Surf. Interfaces 2021, 24, 101075. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Viljoen, E.L. WO3-based catalysts for photocatalytic and photoelectrocatalytic removal of organic pollutants from water—A review. J. Water Process. Eng. 2021, 40, 101930. [Google Scholar] [CrossRef]
- Liao, M.J.; Su, L.; Deng, Y.C.; Xiong, S.; Tang, R.D.; Wu, Z.B.; Ding, C.X.; Yang, L.H.; Gong, D.X. Strategies to improve WO3-based photocatalysts for wastewater treatment: A review. J. Mater. Sci. 2021, 56, 14416–14447. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Shinde, P.A.; Jun, S.C. Review on Recent Progress in the Development of Tungsten Oxide Based Electrodes for Electrochemical Energy Storage. ChemSusChem 2020, 13, 11–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, D.; Wang, H.Y.; Lin, S.; Ou, G.; Wei, H.H.; Sun, S.Q.; Wu, H. Oxygen-deficient metal oxides: Synthesis routes and applications in energy and environment. Nano Res. 2019, 12, 2150–2163. [Google Scholar] [CrossRef]
- Shaver, P.J. Activated Tungsten Oxide Gas Detectors. Appl. Phys. Lett. 1967, 11, 255–257. [Google Scholar] [CrossRef]
- Frühberger, B.; Grunze, M.; Dwyer, D.J. Surface chemistry of H2S-sensitive tungsten oxide films. Sens. Actuators B Chem. 1996, 31, 167–174. [Google Scholar] [CrossRef]
- Dwyer, D.J. Surface chemistry of gas sensors: H2S on WO3 films. Sens. Actuators B Chem. 1991, 5, 155–159. [Google Scholar] [CrossRef]
- Sriram, S.R.; Parne, S.; Vaddadi, V.S.C.S.; Edla, D.; Nagaraju, P.; Avala, R.R.; Yelsani, V.; Sontu, U.B. Nanostructured WO3 based gas sensors: A short review. Sens. Rev. 2021, 41, 406–424. [Google Scholar] [CrossRef]
- Lei, G.L.; Lou, C.M.; Liu, X.H.; Xie, J.Y.; Li, Z.S.; Zheng, W.; Zhang, J. Thin films of tungsten oxide materials for advanced gas sensors. Sens. Actuators B Chem. 2021, 341, 129996. [Google Scholar] [CrossRef]
- Bonardo, D.; Septiani, N.L.W.; Amri, F.; Estananto; Humaidi, S.; Suyatman; Yuliarto, B. Review—Recent Development of WO3 for Toxic Gas Sensors Applications. J. Electrochem. Soc. 2021, 168, 107502. [Google Scholar] [CrossRef]
- Long, H.W.; Zeng, W.; Zhang, H. Synthesis of WO3 and its gas sensing: A review. J. Mater. Sci. Mater. Electron. 2015, 26, 4698–4707. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. Gasochromic WO3 Nanostructures for the Detection of Hydrogen Gas: An Overview. Appl. Sci. 2019, 9, 1775. [Google Scholar] [CrossRef] [Green Version]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Bouchikhi, B.; Chludziński, T.; Saidi, T.; Smulko, J.; Bari, N.E.; Wen, H.; Ionescu, R. Formaldehyde detection with chemical gas sensors based on WO3 nanowires decorated with metal nanoparticles under dark conditions and UV light irradiation. Sens. Actuators B Chem. 2020, 320, 128331. [Google Scholar] [CrossRef]
- Wu, R.; Tian, F.H.; Liu, Z.Z.; Xue, X.Y.; Zhang, J.; Zu, J.H. CH4 activation and sensing on hexagonal WO3 (001) and (110) surfaces. Appl. Surf. Sci. 2019, 481, 1154–1159. [Google Scholar] [CrossRef]
- Tian, F.H.; Gong, C.; Wu, R.; Liu, Z.Z. Oxygen density dominated gas sensing mechanism originated from CO adsorption on the hexagonal WO3 (001) surface. Mater. Today Chem. 2018, 9, 28–33. [Google Scholar] [CrossRef]
- Gerand, B.; Nowogrocki, G.; Guenot, J.; Figlarz, M. Structural study of a new hexagonal form of tungsten trioxide. J. Solid State Chem. 1979, 29, 429–434. [Google Scholar] [CrossRef]
- Tian, F.H.; Liu, Z.Z.; Tian, J.; Zhang, Y.F. Oxygen vacancy O-terminated surface: The most exposed surface of hexagonal WO3 (001) surface. Chin. Chem. Lett. 2020, 31, 2095–2098. [Google Scholar] [CrossRef]
- Hurtado-Aular, O.; Anez, R.; Sierraalta, A.; Calderon, J. Discovering the root of the stability of hexagonal WO3 surfaces from a periodic DFT perspective. Appl. Surf. Sci. 2020, 506, 144719. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Understanding the Potential of WO3 Based Sensors for Breath Analysis. Sensors 2016, 16, 1815. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Tamaki, J.; Miura, N.; Yamazoe, N. Tungsten Oxide-Based Semiconductor Sensor Highly Sensitive to NO and NO2. Chem. Lett. 1991, 20, 1611–1614. [Google Scholar] [CrossRef]
- Tamaki, J.; Zhang, Z.; Fujimori, K.; Akiyama, M.; Harada, T.; Miura, N.; Yamazoe, N. Grain-Size Effects in Tungsten Oxide-Based Sensor for Nitrogen-Oxides. J. Electrochem. Soc. 1994, 141, 2207–2210. [Google Scholar] [CrossRef]
- Kida, T.; Nishiyama, A.; Yuasa, M.; Shimanoe, K.; Yamazoe, N. Highly sensitive NO2 sensors using lamellar-structured WO3 particles prepared by an acidification method. Sens. Actuators B Chem. 2009, 135, 568–574. [Google Scholar] [CrossRef]
- Kim, S.-J.; Hwang, I.-S.; Choi, J.-K.; Lee, J.-H. Gas sensing characteristics of WO3 nanoplates prepared by acidification method. Thin Solid Film. 2011, 519, 2020–2024. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Sun, J. Controlled synthesis of defect-rich ultrathin two-dimensional WO3 nanosheets for NO2 gas detection. Sens. Actuators B Chem. 2017, 245, 828–834. [Google Scholar] [CrossRef]
- Wang, M.S.; Wang, Y.W.; Li, X.J.; Ge, C.X.; Hussain, S.; Liu, G.W.; Qiao, G.J. WO3 porous nanosheet arrays with enhanced sensing performance low temperature NO2 gas. Sens. Actuators B Chem. 2020, 316, 128050. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yoon, J.-W.; Hong, Y.J.; Kang, Y.C.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.-H. Highly sensitive and selective detection of ppb-level NO2 using multi-shelled WO3 yolk–shell spheres. Sens. Actuators B Chem. 2016, 229, 561–569. [Google Scholar] [CrossRef]
- You, L.; He, X.; Wang, D.; Sun, P.; Sun, Y.F.; Liang, X.S.; Du, Y.; Lu, G.Y. Ultrasensitive and low operating temperature NO2 gas sensor using nanosheets assembled hierarchical WO3 hollow microspheres. Sens. Actuators B Chem. 2012, 173, 426–432. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Preparation of WO3 nanoplatelet-based microspheres and their NO2 gas-sensing properties. J. Ceram. Soc. Jpn. 2014, 122, 674–678. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Park, S.; Ko, H.; Lee, C. Fabrication of WO3 nanotube sensors and their gas sensing properties. Ceram. Int. 2014, 40, 1423–1429. [Google Scholar] [CrossRef]
- Qi, J.J.; Gao, S.; Chen, K.; Yang, J.; Zhao, H.W.; Guo, L.; Yang, S.H. Vertically aligned, double-sided, and self-supported 3D WO3 nanocolumn bundles for low-temperature gas sensing. J. Mater. Chem. A 2015, 3, 18019–18026. [Google Scholar] [CrossRef]
- Wang, C.; Ding, M.D.; Kou, X.Y.; Guo, L.L.; Feng, C.H.; Li, X.; Zhang, H.; Sun, P.; Sun, Y.F.; Lu, G.Y. Detection of nitrogen dioxide down to ppb levels using flower-like tungsten oxide nanostructures under different annealing temperatures. J. Colloid Interface Sci. 2016, 483, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Zavabeti, A.; Wang, Y.C.; Harrison, C.J.; Carey, B.J.; Mohiuddin, M.; Chrimes, A.F.; De Castro, I.A.; Zhang, B.Y.; Sabri, Y.M.; et al. Quasi physisorptive two dimensional tungsten oxide nanosheets with extraordinary sensitivity and selectivity to NO2. Nanoscale 2017, 9, 19162–19175. [Google Scholar] [CrossRef] [PubMed]
- Harale, N.S.; Dalavi, D.S.; Mali, S.S.; Tarwal, N.L.; Vanalakar, S.A.; Rao, V.K.; Hong, C.K.; Kim, J.H.; Patil, P.S. Single-step hydrothermally grown nanosheet-assembled tungsten oxide thin films for sensitive and selective NO2 gas detection. J. Mater. Sci. 2018, 53, 6094–6105. [Google Scholar] [CrossRef]
- Hua, Z.Q.; Tian, C.; Qiu, Z.L.; Li, Y.; Tian, X.M.; Wang, M.J.; Li, E.P. An investigation on NO2 sensing mechanism and shielding behavior of WO3 nanosheets. Sens. Actuators B Chem. 2018, 259, 250–257. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Patil, V.L.; Harale, N.S.; Suryawanshi, M.P.; Patil, A.P.; Patil, V.B.; Kim, J.H.; Patil, P.S. 2-D to 3-D conversion of WO3 nanostructures using structure directing agent for enhanced NO2 gas sensing performance. Sens. Actuators A Phys. 2020, 304, 111882. [Google Scholar] [CrossRef]
- Song, W.Q.; Zhang, R.; Bai, X.; Jia, Q.Q.; Ji, H.M. Exposed crystal facets of WO3 nanosheets by phase control on NO2-sensing performance. J. Mater. Sci. Mater. Electron. 2020, 31, 610–620. [Google Scholar] [CrossRef]
- Morais, P.V.; Suman, P.H.; Silva, R.A.; Orlandi, M.O. High gas sensor performance of WO3 nanofibers prepared by electrospinning. J. Alloys Compd. 2021, 864, 158745. [Google Scholar] [CrossRef]
- Zhang, J.N.; Leng, D.Y.; Zhang, L.Z.; Li, G.; Ma, F.; Gao, J.Z.; Lu, H.B.; Zhu, B.P. Porosity and oxygen vacancy engineering of mesoporous WO3 nanofibers for fast and sensitive low-temperature NO2 sensing. J. Alloys Compd. 2021, 853, 157339. [Google Scholar] [CrossRef]
- Masikini, M.; Chowdhury, M.; Nemraoui, O. Review-Metal Oxides: Application in Exhaled Breath Acetone Chemiresistive Sensors. J. Electrochem. Soc. 2020, 167, 037537. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, N.; Jamalabadi, H.; Tavoli, F. Breath Acetone Sensors as Non-Invasive Health Monitoring Systems: A Review. IEEE Sens. J. 2020, 20, 5–31. [Google Scholar] [CrossRef]
- Li, X.L.; Lou, T.J.; Sun, X.M.; Li, Y.D. Highly sensitive WO3 hollow-sphere gas sensors. Inorg. Chem. 2004, 43, 5442–5449. [Google Scholar] [CrossRef]
- Khadayate, R.S.; Sali, V.; Patil, P.P. Acetone vapor sensing properties of screen printed WO3 thick films. Talanta 2007, 72, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Hou, X.X.; Li, T.; Yin, L.; Fan, B.B.; Wang, H.L.; Li, X.J.; Xu, H.L.; Lu, H.X.; Zhang, R.; et al. Effects of morphologies on acetone-sensing properties of tungsten trioxide nanocrystals. Sens. Actuators B Chem. 2011, 153, 373–381. [Google Scholar] [CrossRef]
- Shi, J.C.; Hu, G.J.; Sun, Y.; Geng, M.; Wu, J.; Liu, Y.F.; Ge, M.Y.; Tao, J.C.; Cao, M.; Dai, N. WO3 nanocrystals: Synthesis and application in highly sensitive detection of acetone. Sens. Actuators B Chem. 2011, 156, 820–824. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, F.; Li, H.; Chen, T.; Wang, Y.D. Acetone detection properties of single crystalline tungsten oxide plates synthesized by hydrothermal method using cetyltrimethyl ammonium bromide supermolecular template. Sens. Actuators B Chem. 2012, 162, 259–268. [Google Scholar] [CrossRef]
- Zou, X.X.; Li, G.D.; Wang, P.P.; Su, J.; Zhao, J.; Zhou, L.J.; Wang, Y.N.; Chen, J.S. A precursor route to single-crystalline WO3 nanoplates with an uneven surface and enhanced sensing properties. Dalton Trans. 2012, 41, 9773–9780. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Sun, P.; Yang, T.L.; Gao, Y.; Li, X.W.; Lu, G.Y.; Du, Y. Flower-like WO3 architectures synthesized via a microwave-assisted method and their gas sensing properties. Sens. Actuators B Chem. 2013, 186, 734–740. [Google Scholar] [CrossRef]
- Chi, X.; Liu, C.B.; Liu, L.; Li, Y.; Wang, Z.J.; Bo, X.Q.; Liu, L.L.; Su, C. Tungsten trioxide nanotubes with high sensitive and selective properties to acetone. Sens. Actuators B Chem. 2014, 194, 33–37. [Google Scholar] [CrossRef]
- Chen, D.L.; Ge, L.F.; Yin, L.; Shi, H.Y.; Yang, D.W.; Yang, J.; Zhang, R.; Shao, G.S. Solvent-regulated solvothermal synthesis and morphology-dependent gas-sensing performance of low-dimensional tungsten oxide nanocrystals. Sens. Actuators B Chem. 2014, 205, 391–400. [Google Scholar] [CrossRef]
- Zhang, H.L.; Liu, Z.F.; Yang, J.Q.; Guo, W.; Zhu, L.J.; Zheng, W.J. Temperature and acidity effects on WO3 nanostructures and gas-sensing properties of WO3 nanoplates. Mater. Res. Bull. 2014, 57, 260–267. [Google Scholar] [CrossRef]
- Yin, M.L.; Yu, L.M.; Liu, S.Z. Synthesis of thickness-controlled cuboid WO3 nanosheets and their exposed facets-dependent acetone sensing properties. J. Alloys Compd. 2017, 696, 490–497. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Ji, H.M.; Bai, X. Selective sensing property of triclinic WO3 nanosheets towards ultra-low concentration of acetone. J. Mater. Sci. Mater. Electron. 2019, 30, 7824–7833. [Google Scholar] [CrossRef]
- Xu, H.Y.; Gao, J.; Li, M.H.; Zhao, Y.Y.; Zhang, M.; Zhao, T.; Wang, L.J.; Jiang, W.; Zhu, G.J.; Qian, X.Y.; et al. Mesoporous WO3 Nanofibers with Crystalline Framework for High-Performance Acetone Sensing. Front. Chem. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.T.; Xu, S.; Liu, S.; Wang, N.N.; Sun, S.B.; Zhu, X.J.; Li, J.F.; Ola, O.; Zhu, Y.Q. Highly sensitive acetone sensor based on WO3 nanosheets derived from WS2 nanoparticles with inorganic fullerene-like structures. Sens. Actuators B Chem. 2021, 343, 130135. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, X.; Wang, Y.R.; Yang, Y.F.; Su, Q.; Li, J.P.; An, B.X.; Luo, Y.B.; Wu, Z.K.; Xie, E.Q. Sea urchins-like WO3 as a material for resistive acetone gas sensors. Sens. Actuators B Chem. 2022, 355, 131262. [Google Scholar] [CrossRef]
- Tomoki, M.; Jun, T.; Norio, M.; Noboru, Y. Gold-Loaded Tungsten Oxide Sensor for Detection of Ammonia in Air. Chem. Lett. 1992, 21, 639–642. [Google Scholar] [CrossRef]
- Jimenez, I.; Centeno, M.A.; Scotti, R.; Morazzoni, F.; Arbiol, J.; Cornet, A.; Morante, J.R. NH3 interaction with chromium-doped WO3 nanocrystalline powders for gas sensing applications. J. Mater. Chem. 2004, 14, 2412–2420. [Google Scholar] [CrossRef]
- Jimenez, I.; Centeno, M.A.; Scotti, R.; Morazzoni, F.; Cornet, A.; Morante, J.R. NH3 interaction with catalytically modified nano-WO3 powders for gas sensing applications. J. Electrochem. Soc. 2003, 150, H72–H80. [Google Scholar] [CrossRef]
- Epifani, M.; Andreu, T.; Arbiol, J.; Diaz, R.; Siciliano, P.; Morante, J.R. Chloro-Alkoxide Route to Transition Metal Oxides. Synthesis of WO3 Thin Films and Powders from a Tungsten Chloro-Methoxide. Chem. Mater. 2009, 21, 5215–5221. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Vu, V.Q.; Nguyen, D.H.; Kim, D. Preparing large-scale WO3 nanowire-like structure for high sensitivity NH3 gas sensor through a simple route. Curr. Appl. Phys. 2011, 11, 657–661. [Google Scholar]
- Wang, G.; Ji, Y.; Huang, X.; Yang, X.; Gouma, P.-I.; Dudley, M. Fabrication and Characterization of Polycrystalline WO3 Nanofibers and Their Application for Ammonia Sensing. J. Phys. Chem. B 2006, 110, 23777–23782. [Google Scholar] [CrossRef] [PubMed]
- Epifani, M.; Andreu, T.; Magaña, C.R.; Díaz, R.; Arbiol, J.; Siciliano, P.; Morante, J.R. From doping to phase transformation: Ammonia sensing performances of chloroalkoxide-derived WO3 powders modified with chromium. Sens. Actuators B Chem. 2010, 148, 200–206. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Xu, Y.; Li, Y. Nanosheet-assembled hierarchical WO3 flower-like nanostructures: Hydrothermal synthesis and NH3-sensing properties. Mater. Lett. 2019, 250, 155–158. [Google Scholar] [CrossRef]
- Liu, G.K.; Zhu, L.J.; Yu, Y.M.; Qiu, M.; Gao, H.J.; Chen, D.Y. WO3 nanoplates for sensitive and selective detections of both acetone and NH3 gases at different operating temperatures. J. Alloys Compd. 2021, 858, 157638. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kang, Y.C.; Lee, J.-H. Highly selective and sensitive detection of trimethylamine using WO3 hollow spheres prepared by ultrasonic spray pyrolysis. Sens. Actuators B Chem. 2013, 176, 971–977. [Google Scholar] [CrossRef]
- Zhai, C.; Zhu, M.; Jiang, L.; Yang, T.; Zhao, Q.; Luo, Y.; Zhang, M. Fast triethylamine gas sensing response properties of nanosheets assembled WO3 hollow microspheres. Appl. Surf. Sci. 2019, 463, 1078–1084. [Google Scholar] [CrossRef]
- Han, Y.T.; Liu, Y.; Su, C.; Chen, X.W.; Zeng, M.; Hu, N.T.; Su, Y.J.; Zhou, Z.H.; Wei, H.; Yang, Z. Sonochemical synthesis of hierarchical WO3 flower-like spheres for highly efficient triethylamine detection. Sens. Actuators B Chem. 2020, 306, 127536. [Google Scholar] [CrossRef]
- Hu, Q.; He, J.Q.; Chang, J.Y.; Gao, J.M.; Huang, J.H.; Feng, L. Needle-Shaped WO3 Nanorods for Triethylamine Gas Sensing. ACS Appl. Nano Mater. 2020, 3, 9046–9054. [Google Scholar] [CrossRef]
- Hamdi, H.; Salje, E.K.H.; Ghosez, P.; Bousquet, E. First-principles reinvestigation of bulk WO3. Phys. Rev. B 2016, 94, 245124. [Google Scholar] [CrossRef] [Green Version]
- Vogt, T.; Woodward, P.M.; Hunter, B.A. The High-Temperature Phases of WO3. J. Solid State Chem. 1999, 144, 209–215. [Google Scholar] [CrossRef]
- Woodward, P.M.; Sleight, A.W.; Vogt, T. Structure refinement of triclinic tungsten trioxide. J. Phys. Chem. Solids 1995, 56, 1305–1315. [Google Scholar] [CrossRef]
- Woodward, P.M.; Sleight, A.W.; Vogt, T. Ferroelectric Tungsten Trioxide. J. Solid State Chem. 1997, 131, 9–17. [Google Scholar] [CrossRef]
- Salje, E.K.H.; Rehmann, S.; Pobell, F.; Morris, D.; Knight, K.S.; Herrmannsdörfer, T.; Dove, M.T. Crystal structure and paramagnetic behaviour of ε−WO3−x. J. Phys. Condens. Matter 1997, 9, 6563–6577. [Google Scholar] [CrossRef]
- Salje, E. Structural phase transitions in the system WO3-NaWO3. Ferroelectrics 1976, 12, 215–217. [Google Scholar] [CrossRef]
- Diehl, R.; Brandt, G.; Saije, E. The crystal structure of triclinic WO3. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 1105–1111. [Google Scholar] [CrossRef]
- Salje, E.; Viswanathan, K. Physical properties and phase transitions in WO3. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1975, 31, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Tanisaki, S. On the Phase Transition of Tungsten Trioxide below Room Temperature. J. Phys. Soc. Jpn. 1960, 15, 566–573. [Google Scholar] [CrossRef]
- Loopstra, B.O.; Rietveld, H.M. Further refinement of the structure of WO3. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1969, 25, 1420–1421. [Google Scholar] [CrossRef] [Green Version]
- Loopstra, B.O.; Boldrini, P. Neutron diffraction investigation of WO3. Acta Crystallogr. 1966, 21, 158–162. [Google Scholar] [CrossRef]
- Cazzanelli, E.; Vinegoni, C.; Mariotto, G.; Kuzmin, A.; Purans, J. Raman study of the phase transitions sequence in pure WO3 at high temperature and in HxWO3 with variable hydrogen content. Solid State Ion. 1999, 123, 67–74. [Google Scholar] [CrossRef]
- Salje, E. The orthorhombic phase of WO3. Acta Crystallogr. 1977, 33, 574–577. [Google Scholar] [CrossRef]
- Kehl, W.L.; Hay, R.G.; Wahl, D. The Structure of Tetragonal Tungsten Trioxide. J. Appl. Phys. 1952, 23, 212–215. [Google Scholar] [CrossRef]
- Howard, C.J.; Luca, V.; Knight, K.S. High-temperature phase transitions in tungsten trioxide-the last word? J. Phys. Condens. Matter 2001, 14, 377–387. [Google Scholar] [CrossRef]
- Hassani, H.; Partoens, B.; Bousquet, E.; Ghosez, P. First-principles study of lattice dynamical properties of the room-temperature P21/n and ground-state P21/c phases of WO3. Phys. Rev. B 2022, 105, 014107. [Google Scholar] [CrossRef]
- Berak, J.M.; Sienko, M.J. Effect of oxygen-deficiency on electrical transport properties of tungsten trioxide crystals. J. Solid State Chem. 1970, 2, 109–133. [Google Scholar] [CrossRef]
- Stachiotti, M.G.; Corà, F.; Catlow, C.R.A.; Rodriguez, C.O. First-principles investigation of ReO3 and related oxides. Phys. Rev. B 1997, 55, 7508–7514. [Google Scholar] [CrossRef]
- Dickens, P.G.; Whittingham, M.S. The tungsten bronzes and related compounds. Q. Rev. Chem. Soc. 1968, 22, 30–44. [Google Scholar] [CrossRef]
- Bullett, D.W. Bulk and surface electron states in WO3 and tungsten bronzes. J. Phys. C Solid State Phys. 1983, 16, 2197–2207. [Google Scholar] [CrossRef]
- Kopp, L.; Harmon, B.N.; Liu, S.H. Band structure of cubic NaxWO3. Solid State Commun. 1977, 22, 677–679. [Google Scholar] [CrossRef]
- Bersuker, I.B. Pseudo-Jahn–Teller Effect—A Two-State Paradigm in Formation, Deformation, and Transformation of Molecular Systems and Solids. Chem. Rev. 2013, 113, 1351–1390. [Google Scholar] [CrossRef] [PubMed]
- De Wijs, G.A.; De Boer, P.K.; De Groot, R.A.; Kresse, G. Anomalous behavior of the semiconducting gap in WO3 from first-principles calculations. Phys. Rev. B 1999, 59, 2684–2693. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.G.; Di Valentin, C.; Pacchioni, G. Electronic and Structural Properties of WO3: A Systematic Hybrid DFT Study. J. Phys. Chem. C 2011, 115, 8345–8353. [Google Scholar] [CrossRef]
- Cazzanelli, E.; Vinegoni, C.; Mariotto, G.; Kuzmin, A.; Purans, J. Low-Temperature Polymorphism in Tungsten Trioxide Powders and Its Dependence on Mechanical Treatments. J. Solid State Chem. 1999, 143, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Souza-Filho, A.G.; Freire, V.N.; Sasaki, J.M.; Mendes Filho, J.; Julião, J.F.; Gomes, U.U. Coexistence of triclinic and monoclinic phases in WO3 ceramics. J. Raman Spectrosc. 2000, 31, 451–454. [Google Scholar] [CrossRef]
- Kuzmin, A.; Purans, J.; Cazzanelli, E.; Vinegoni, C.; Mariotto, G. X-ray diffraction, extended x-ray absorption fine structure and Raman spectroscopy studies of WO3 powders and (1−x)WO3−y⋅xReO2 mixtures. J. Appl. Phys. 1998, 84, 5515–5524. [Google Scholar] [CrossRef]
- Jimenez, I.; Arbiol, J.; Dezanneau, G.; Cornet, A.; Morante, J.R. Crystalline structure, defects and gas sensor response to NO2 and H2S of tungsten trioxide nanopowders. Sens. Actuators B Chem. 2003, 93, 475–485. [Google Scholar] [CrossRef]
- Kaneko, H.; Miyake, K.; Teramoto, Y. Preparation and properties of reactively sputtered tungsten oxide films. J. Appl. Phys. 1982, 53, 3070–3075. [Google Scholar] [CrossRef]
- Gillet, M.; Lemire, C.; Gillet, E.; Aguir, K. The role of surface oxygen vacancies upon WO3 conductivity. Surf. Sci. 2003, 532, 519–525. [Google Scholar] [CrossRef]
- Li, W.; Sasaki, A.; Oozu, H.; Aoki, K.; Kakushima, K.; Kataoka, Y.; Nishiyama, A.; Sugii, N.; Wakabayashi, H.; Tsutsui, K.; et al. Electron transport mechanism of tungsten trioxide powder thin film studied by investigating effect of annealing on resistivity. Microelectron. Reliab. 2015, 55, 407–410. [Google Scholar] [CrossRef]
- Hollinger, G.; Minh Duc, T.; Deneuville, A. Charge Transfer in Amorphous Colored WO3 Films Observed by X-Ray Photoelectron Spectroscopy. Phys. Rev. Lett. 1976, 37, 1564–1567. [Google Scholar] [CrossRef]
- De Angelis, B.A.; Schiavello, M. X-ray photoelectron spectroscopy study of nonstoichiometric tungsten oxides. J. Solid State Chem. 1977, 21, 67–72. [Google Scholar] [CrossRef]
- Colton, R.J.; Guzman, A.M.; Rabalais, J.W. Photochromism and electrochromism in amorphous transition metal oxide films. Acc. Chem. Res. 1978, 11, 170–176. [Google Scholar] [CrossRef]
- Salje, E.; Carley, A.F.; Roberts, M.W. The effect of reduction and temperature on the electronic core levels of tungsten and molybdenum in WO3 and WxMo1−xO3—A photoelectron spectroscopic study. J. Solid State Chem. 1979, 29, 237–251. [Google Scholar] [CrossRef]
- Bringans, R.D.; Höchst, H.; Shanks, H.R. Defect states in WO3 studied with photoelectron spectroscopy. Phys. Rev. B 1981, 24, 3481–3489. [Google Scholar] [CrossRef]
- Höchst, H.; Bringans, R.D. Electronic structure of evaporated and annealed tungsten oxide films studied with UPS. Appl. Surf. Sci. 1982, 11–12, 768–773. [Google Scholar] [CrossRef]
- Dixon, R.A.; Williams, J.J.; Morris, D.; Rebane, J.; Jones, F.H.; Egdell, R.G.; Downes, S.W. Electronic states at oxygen deficient WO3(001) surfaces: A study by resonant photoemission. Surf. Sci. 1998, 399, 199–211. [Google Scholar] [CrossRef]
- Bussolotti, F.; Lozzi, L.; Passacantando, M.; La Rosa, S.; Santucci, S.; Ottaviano, L. Surface electronic properties of polycrystalline WO3 thin films: A study by core level and valence band photoemission. Surf. Sci. 2003, 538, 113–123. [Google Scholar] [CrossRef]
- Jones, F.H.; Rawlings, K.; Foord, J.S.; Egdell, R.G.; Pethica, J.B.; Wanklyn, B.M.R.; Parker, S.C.; Oliver, P.M. An STM study of surface structures on WO3(001). Surf. Sci. 1996, 359, 107–121. [Google Scholar] [CrossRef]
- Karazhanov, S.Z.; Zhang, Y.; Wang, L.W.; Mascarenhas, A.; Deb, S. Resonant defect states and strong lattice relaxation of oxygen vacancies in WO3. Phys. Rev. B 2003, 68, 233204. [Google Scholar] [CrossRef] [Green Version]
- Karazhanov, S.Z.; Zhang, Y.; Mascarenhas, A.; Deb, S.; Wang, L.W. Oxygen vacancy in cubic WO3 studied by first-principles pseudopotential calculation. Solid State Ion. 2003, 165, 43–49. [Google Scholar] [CrossRef]
- Chatten, R.; Chadwick, A.V.; Rougier, A.; Lindan, P.J.D. The oxygen vacancy in crystal phases of WO3. J. Phys. Chem. B 2005, 109, 3146–3156. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Lambert-Mauriat, C.; Oison, V. Density-functional study of oxygen vacancies in monoclinic tungsten oxide. J. Phys. Condens. Matter 2006, 18, 7361–7371. [Google Scholar] [CrossRef]
- Wang, F.; Di Valentin, C.; Pacchioni, G. Semiconductor-to-metal transition in WO3-x: Nature of the oxygen vacancy. Phys. Rev. B 2011, 84, 073103. [Google Scholar] [CrossRef]
- Gerosa, M.; Di Valentin, C.; Onida, G.; Bottani, C.E.; Pacchioni, G. Anisotropic Effects of Oxygen Vacancies on Electrochromic Properties and Conductivity of γ-Monoclinic WO3. J. Phys. Chem. C 2016, 120, 11716–11726. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, F.; Pachter, R.; Murphy, N.R.; Johnson, W.E.; Ramana, C.V. Effect of oxygen vacancies on the electronic and optical properties of tungsten oxide from first principles calculations. J. Appl. Phys. 2016, 120, 233105. [Google Scholar] [CrossRef]
- Migas, D.B.; Shaposhnikov, V.L.; Rodin, V.N.; Borisenko, V.E. Tungsten oxides. I. Effects of oxygen vacancies and doping on electronic and optical properties of different phases of WO3. J. Appl. Phys. 2010, 108, 093713. [Google Scholar] [CrossRef]
- Lambert-Mauriat, C.; Oison, V.; Saadi, L.; Aguir, K. Ab initio study of oxygen point defects on tungsten trioxide surface. Surf. Sci. 2012, 606, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Ping, Y.; Goddard, W.A.; Galli, G.A. Energetics and Solvation Effects at the Photoanode/Catalyst Interface: Ohmic Contact versus Schottky Barrier. J. Am. Chem. Soc. 2015, 137, 5264–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, E.; Di Valentin, C.; Pacchioni, G. H2O Adsorption on WO3 and WO3-x (001) Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 23212–23221. [Google Scholar] [CrossRef]
- Haber, J.; Janas, J.; Schiavello, M.; Tilley, R.J.D. Tungsten oxides as catalysts in selective oxidation. J. Catal. 1983, 82, 395–403. [Google Scholar] [CrossRef]
- Monter-Guzmán, J.Y.M.; Chu, X.; Comini, E.; Epifani, M.; Zanella, R. How Chemoresistive Sensors Can Learn from Heterogeneous Catalysis. Hints, Issues, and Perspectives. Chemosensors 2021, 9, 193. [Google Scholar] [CrossRef]
- Ramis, G.; Cristiani, C.; Elmi, A.S.; Villa, P.; Busca, G. Characterization of the Surface-Properties of Polycrystalline WO3. J. Mol. Catal. 1990, 61, 319–331. [Google Scholar] [CrossRef]
- Kanan, S.M.; Lu, Z.X.; Cox, J.K.; Bernhardt, G.; Tripp, C.P. Identification of surface sites on monoclinic WO3 powders by infrared spectroscopy. Langmuir 2002, 18, 1707–1712. [Google Scholar] [CrossRef]
- Ma, S.; Amar, F.G.; Frederick, B.G. Surface Heterogeneity and Diffusion in the Desorption of Methanol from WO3(001) Surfaces. J. Phys. Chem. A 2003, 107, 1413–1423. [Google Scholar] [CrossRef]
- Ma, S.; Frederick, B.G. Reactions of aliphatic alcohols on WO3(001) surfaces. J. Phys. Chem. B 2003, 107, 11960–11969. [Google Scholar] [CrossRef]
- Ling, S.; Mei, D.; Gutowski, M. Reactivity of hydrogen and methanol on (001) surfaces of WO3, ReO3, WO3/ReO3 and ReO3/WO3. Catal. Today 2011, 165, 41–48. [Google Scholar] [CrossRef]
- Ge, Q.F.; Gutowski, M. A Comparative Study of Methanol Adsorption and Dissociation over WO3(001) and ReO3(001). Top. Catal. 2015, 58, 655–664. [Google Scholar] [CrossRef]
- Wang, F.G.; Di Valentin, C.; Pacchioni, G. DFT Study of Hydrogen Adsorption On the Monoclinic WO3 (001) Surface. J. Phys. Chem. C 2012, 116, 10672–10679. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, B.; Zhu, Y.-N.; Chai, Z.; Chen, X.; Chen, M. First-principles calculations of water adsorption on perfect and defect WO3(001). Comput. Mater. Sci. 2018, 150, 484–490. [Google Scholar] [CrossRef]
- Hurtado-Aular, O.; Vidal, A.B.; Sierraalta, A.; Anez, R. Periodic DFT study of water adsorption on m-WO3(001), m-WO3(100), h-WO3(001) and h-WO3(100). Role of hydroxyl groups on the stability of polar. Surf. Sci. 2020, 694, 121558. [Google Scholar] [CrossRef]
- Hubner, M.; Simion, C.E.; Haensch, A.; Barsan, N.; Weimar, U. CO sensing mechanism with WO3 based gas sensors. Sens. Actuators B Chem. 2010, 151, 103–106. [Google Scholar] [CrossRef]
- Staerz, A.; Berthold, C.; Russ, T.; Wicker, S.; Weimar, U.; Barsan, N. The oxidizing effect of humidity on WO3 based sensors. Sens. Actuators B Chem. 2016, 237, 54–58. [Google Scholar] [CrossRef]
- Kohl, D. Surface processes in the detection of reducing gases with SnO2-based devices. Sens. Actuators 1989, 18, 71–113. [Google Scholar] [CrossRef]
- Pokhrel, S.; Simion, C.E.; Teodorescu, V.S.; Barsan, N.; Weimar, U. Synthesis, Mechanism, and Gas-Sensing Application of Surfactant Tailored Tungsten Oxide Nanostructures. Adv. Funct. Mater. 2009, 19, 1767–1774. [Google Scholar] [CrossRef]
- Staerz, A.; Kobald, A.; Russ, T.; Weimar, U.; Hemeryck, A.; Barsan, N. Thermal Water Splitting on the WO3 Surface: Experimental Proof. ACS Appl. Electron. Mater. 2020, 2, 3254–3262. [Google Scholar] [CrossRef]
- Staerz, A.; Somacescu, S.; Epifani, M.; Kida, T.; Weimar, U.; Barsan, N. WO3-Based Gas Sensors: Identifying Inherent Qualities and Understanding the Sensing Mechanism. ACS Sens. 2020, 5, 1624–1633. [Google Scholar] [CrossRef]
- Oison, V.; Saadi, L.; Lambert-Mauriat, C.; Hayn, R. Mechanism of CO and O3 sensing on WO3 surfaces: First principle study. Sens. Actuators B Chem. 2011, 160, 505–510. [Google Scholar] [CrossRef]
- Saadi, L.; Lambert-Mauriat, C.; Oison, V.; Ouali, H.; Hayn, R. Mechanism of NOx sensing on WO3 surface: First principle calculations. Appl. Surf. Sci. 2014, 293, 76–79. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]





| Sample Morphology | Phase Composition | Gas Concentration | Response | Operating Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Sintered powder | n/d | 80 ppm | 97 | 300 | [32] |
| Lamellae | Monoclinic | 500 ppb | >150 | 200 | [34] |
| Nanoplates | Monoclinic | 5 ppm | 960 | 200 | [35] |
| 2D nanosheets | Monoclinic | 50 ppb | ~6 | 140 | [36] |
| Porous nanosheets | Monoclinic | 10 ppm | >450 | 100 | [37] |
| Yolk–shell spheres | Monoclinic | 50 ppb | ~100 | 100 | [38] |
| Nanosheets | Monoclinic | 100 ppb | ~50 | 75 | [39] |
| Nanoplatelets | Monoclinic | 1 ppm | ~80 | 200 | [40] |
| Nanotubes | Monoclinic | 5 ppm | 7 | 300 | [41] |
| Nanoflowers | Monoclinic | 50 ppb | >30 | 100 | [43] |
| Nanocolumns | Monoclinic | 10 ppm | 22 | 110 | [42] |
| Nanosheets | Monoclinic | 40 ppb | 30 | 150 | [44] |
| Nanobricks | Monoclinic | 100 ppm | 12 | 300 | [45] |
| Nanosheets | Monoclinic | 4 ppm | >32 | 300 | [46] |
| Nanoflowers | Monoclinic | 100 ppm | >50 | 100 | [47] |
| Nanosheets | Triclinic | 300 ppb | 18.8 | 100 | [48] |
| Nanofibers | Monoclinic | 50 ppm | >104 | 150 | [49] |
| Sample Morphology | Phase Composition | Gas Concentration (ppm) | Response | Operating Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Hollow spheres | Monoclinic | 50 | 3.5 | 400 | [53] |
| Thick films | Triclinic | 50 | 4.56 | 300 | [54] |
| Nanoplates | Triclinic | 2 | 5 | 300 | [55] |
| Nanoparticles | Monoclinic | 10 | 10 | 350 | [56] |
| Nanoplates | Monoclinic | 300 | 50 | 307 | [57] |
| Nanoplates | Monoclinic | 500 | 40 | 200 | [58] |
| Flower-like | Monoclinic | 100 | 7 | 300 | [59] |
| Nanotubes | Monoclinic | 100 | 42.5 | 250 | [60] |
| Urchin-like | Monoclinic | 25 | 15 | 300 | [61] |
| Nanoplates | Monoclinic | 100 | 8 | 300 | [62] |
| Nanosheets | Monoclinic | 100 | 50 | 340 | [63] |
| Nanosheets | Triclinic | 1 | 2.04 | 230 | [64] |
| Mesoporous nanofibers | Monoclinic | 50 | 22 | 300 | [65] |
| Nanosheets | Monoclinic | 50 | 15 | 300 | [66] |
| Urchin-like | Monoclinic | 100 | 30 | 200 | [67] |
| Sample Morphology | Phase Composition | Gas Concentration (ppm) | Response | Operating Temperature (°C) | Reference |
|---|---|---|---|---|---|
| AMMONIA | |||||
| Sintered powders | Probably monoclinic | 50 | <5 | 200–600 | [68] |
| Nanopowders | Monoclinic + triclinic | 500 | <5 | 350 | [69] |
| Nanopowders | Monoclinic + triclinic | 50 | <6 | 250 | [71] |
| Nanowires | Monoclinic | 1500 | 9.7 | 250 | [72] |
| Nanofibers | Orthorhombic | 100 | <5 | 300 | [73] |
| Nanosheets | Hexagonal | 100 | 35 | 350 | [75] |
| Nanoplates | Monoclinic | 100 | <20 | 300 | [76] |
| ALKYLAMINES (TEA/TMA) | |||||
| Hollow spheres | Orthorhombic | 5 | 56.9 | 450 | [77] |
| Nanosheets assembled in microspheres | Monoclinic | 50 | 16 | 220 | [78] |
| Hierarchical spheres | Monoclinic | 10 | 35.3 | 150 | [79] |
| Nanorods | Monoclinic | 50 | 50 | 250 | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epifani, M. Mechanistic Insights into WO3 Sensing and Related Perspectives. Sensors 2022, 22, 2247. https://doi.org/10.3390/s22062247
Epifani M. Mechanistic Insights into WO3 Sensing and Related Perspectives. Sensors. 2022; 22(6):2247. https://doi.org/10.3390/s22062247
Chicago/Turabian StyleEpifani, Mauro. 2022. "Mechanistic Insights into WO3 Sensing and Related Perspectives" Sensors 22, no. 6: 2247. https://doi.org/10.3390/s22062247
APA StyleEpifani, M. (2022). Mechanistic Insights into WO3 Sensing and Related Perspectives. Sensors, 22(6), 2247. https://doi.org/10.3390/s22062247






