Scapular Dynamic Muscular Stiffness Assessed through Myotonometry: A Narrative Review
Abstract
:1. Introduction
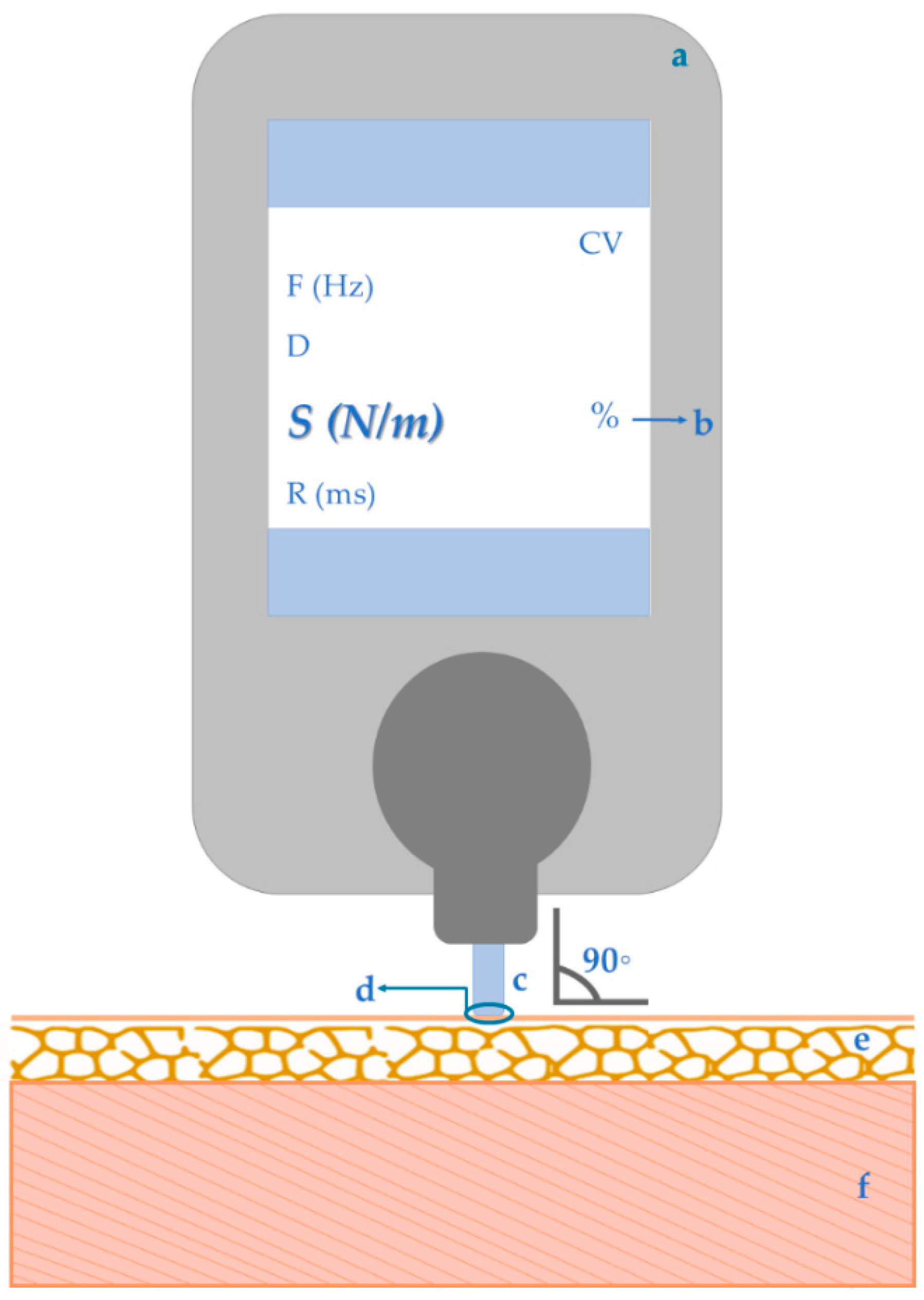
2. Guidelines to Myotonometry Measurements of Muscular Stiffness and Obtained Data
- Equipment:
- Programming the data acquisition:
- Introducing participant data (as weight, height, gender, and dominant side) [9]
- Planning a “pattern composer”, this is, defining an assessment protocol regarding the muscles to include and their condition of assessment (rest or contraction), the subject position and the measurements side, location and nº of repetitions [9]
- Uploading the participant and assessment data to the myotonometry tool
- The assessor should guarantee the equipment’s stability and avoid the contact with external factors (as clothes) to not influence the device’s impulses neither the tissues oscillations [9];
- Coefficient of variation (total measurements’ variability according to subject, assessor and device accuracy): should be lower than 3% [9];
- Measurement point: superficial reference of the muscles of interest, based not only in previous studies using myotonometry [48], but also researches using tools as algometer [25,48] and electromyography [4,48,49]. For repeated measurements, the same measuring points as well as same muscular and environmental conditions (as time of the day and subject’s position), must be kept [9];
3. Myotonometry Psychometric Properties Regarding the Measure of Muscular Stiffness
3.1. Validity
3.2. Reliability
3.3. Responsiveness
4. Applicability of Myotonometry for Assessing Scapular Muscles Stiffness
Myotonometry Ability for Measuring Differences or Changes in Muscular Stiffness in Pain Conditions Involving Scapular Muscles
5. Points That Need to Be Addressed in Future Studies
- Myotonometry assessment of serratus anterior and levator scapulae muscles are needed to validate the purposed assessment points, to define the myotonometry psychometric properties considering these muscles and to increase the knowledge about these muscles’ mechanical properties.
- The myotonometry psychometric properties should also be researched in subjects with different conditions, such as pain.
- The use of myotonometry not only at rest condition but also during contraction, could bring new information that could help to standout adaptations in muscle stiffness modulation, given the muscular activity required and variation in the range of motion used in this muscular condition [112,113,114].
- In studies with the intention to infer about intervention effects, the inclusion of follow-up moments could help to understand whether stiffness changes will be kept over time.
6. Study Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kisilewicz, A.; Madeleine, P.; Ignasiak, Z.; Ciszek, B.; Kawczynski, A.; Larsen, R.G. Eccentric Exercise Reduces Upper Trapezius Muscle Stiffness Assessed by Shear Wave Elastography and Myotonometry. Front. Bioeng. Biotechnol. 2020, 8, 928. [Google Scholar] [CrossRef] [PubMed]
- Bilston, L.E.; Bolsterlee, B.; Nordez, A.; Sinha, S. Contemporary image-based methods for measuring passive mechanical properties of skeletal muscles in vivo. J. Appl. Physiol. 2019, 126, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Sánchez, A.; Abián, P.; Sánchez-Infante, J.; Esteban-Gacía, P.; Jiménez, F.; Abián-Vicén, J. Objective Assessment of Regional Stiffness in Vastus Lateralis with Different Measurement Methods: A Reliability Study. Sensors 2021, 21, 3213. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Koppenhaver, S.L.; Michener, L.A.; Proulx, L.; Bisagni, F.; Cleland, J.A. Characterization of tissue stiffness of the infraspinatus, erector spinae, and gastrocnemius muscle using ultrasound shear wave elastography and superficial mechanical deformation. J. Electromyogr. Kinesiol. 2018, 38, 73–80. [Google Scholar] [CrossRef]
- Blackburn, J.T.; Padua, D.A.; Riemann, B.L.; Guskiewicz, K.M. The relationships between active extensibility, and passive and active stiffness of the knee flexors. J. Electromyogr. Kinesiol. 2004, 14, 683–691. [Google Scholar] [CrossRef]
- Schleip, R.; Naylor, I.L.; Ursu, D.; Melzer, W.; Zorn, A.; Wilke, H.-J.; Lehmann-Horn, F.; Klingler, W. Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med. Hypotheses. 2006, 66, 66–71. [Google Scholar] [CrossRef]
- Viir, R.; Laiho, K.; Kramarenko, J.; Mikkelsson, M. Repeatability of trapezius muscle tone assessment by a myometric method. J. Mech. Med. Biol. 2006, 6, 215–228. [Google Scholar] [CrossRef]
- Maïsetti, O.; Hug, F.; Bouillard, K.; Nordez, A. Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J. Biomech. 2012, 45, 978–984. [Google Scholar] [CrossRef]
- Myoton, A.S. MyotonPRO Digital Palpation—USER MANUAL, Desktop Software v 5.0.0.211. MYOTON AS; Tallinn, Estonia, 2020; pp. 1–115. Available online: www.myoton.com (accessed on 1 March 2022).
- Bravo-Sánchez, A.; Abián, P.; Jimenez, F.; Abián-Vicén, J. Structural and mechanical properties of the Achilles tendon in senior badminton players: Operated vs. non-injured tendons. Clin. Biomech. 2021, 85, 105366. [Google Scholar] [CrossRef]
- Bernabei, M.; Lee, S.S.M.; Perreault, E.J.; Sandercock, T.G. Shear wave velocity is sensitive to changes in muscle stiffness that occur independently from changes in force. J. Appl. Physiol. 2020, 128, 8–16. [Google Scholar] [CrossRef]
- Kocur, P.; Tomczak, M.; Wiernicka, M.; Goliwas, M.; Lewandowski, J.; Łochyński, D. Relationship between age, BMI, head posture and superficial neck muscle stiffness and elasticity in adult women. Sci. Rep. 2019, 9, 8515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxel, K.C.; Swanik, C.B.; Swanik, K.A.; Bartolozzi, A.R.; Hillstrom, H.J.; Sitler, M.R.; Moffit, D.M. Stiffness Regulation and Muscle-Recruitment Strategies of the Shoulder in Response to External Rotation Perturbations. J. Bone Jt. Surg. 2008, 90, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Rizo, A.M.; Petersen, K.K.; Arendt-Nielsen, L.; Madeleine, P. Eccentric Training Changes the Pressure Pain and Stiffness Maps of the Upper Trapezius in Females with Chronic Neck-Shoulder Pain: A Preliminary Study. Pain Med. 2020, 21, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Eby, S.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.-N. Validation of shear wave elastography in skeletal muscle. J. Biomech. 2013, 46, 2381–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marusiak, J.; Jarocka, E.; Jaskólska, A.; Jaskólski, A. Influence of number of records on reliability of myotonometric measurements of muscle stiffness at rest and contraction. Acta Bioeng. Biomech. 2018, 20, 123–131. [Google Scholar] [PubMed]
- Hodges, P.W.; Tucker, K. Moving differently in pain: A new theory to explain the adaptation to pain. Pain 2011, 152 (Suppl. 3), S90–S98. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8, 17064. [Google Scholar] [CrossRef]
- Ishikawa, H.; Muraki, T.; Morise, S.; Sekiguchi, Y.; Yamamoto, N.; Itoi, E.; Izumi, S.-I. Changes in stiffness of the dorsal scapular muscles before and after computer work: A comparison between individuals with and without neck and shoulder complaints. Eur. J. Appl. Physiol. 2017, 117, 179–187. [Google Scholar] [CrossRef]
- Leong, H.T.; Hug, F.; Fu, S.N. Increased Upper Trapezius Muscle Stiffness in Overhead Athletes with Rotator Cuff Tendinopathy. PLoS ONE 2016, 11, e0155187. [Google Scholar]
- Liu, C.; Feng, Y.; Zhang, H.; Li, Y.; Zhu, Y.; Zhang, Z. Assessing the viscoelastic properties of upper trapezius muscle: Intra- and inter-tester reliability and the effect of shoulder elevation. J. Electromyogr. Kinesiol. 2018, 43, 226–229. [Google Scholar] [CrossRef]
- Taş, S.; Korkusuz, F.; Erden, Z. Neck Muscle Stiffness in Participants With and Without Chronic Neck Pain: A Shear-Wave Elastography Study. J. Manip. Physiol. Ther. 2018, 41, 580–588. [Google Scholar] [CrossRef]
- Larkin-Kaiser, K.A.; Parr, J.J.; Borsa, P.A.; George, S.Z. Range of motion as a predictor of clinical shoulder pain during recovery from delayed-onset muscle soreness. J. Athl. Train. 2015, 50, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luime, J.J.; Koes, B.W.; Hendriksen, I.J.; Burdorf, A.; Verhagen, A.P.; Miedema, H.S.; Verhaar, J.A. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand. J. Rheumatol. 2004, 33, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kisilewicz, A.; Janusiak, M.; Szafraniec, R.; Smoter, M.; Ciszek, B.; Madeleine, P.; Fernández-de-Las-Peñas, C.; Kawczyński , A. Changes in Muscle Stiffness of the Trapezius Muscle After Application of Ischemic Compression into Myofascial Trigger Points in Professional Basketball Players. J. Hum. Kinet. 2018, 64, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Hyong, I. Study on Change of Muscle Tone and Stiffness According to upper trapezius Mild Pain tf young Adults. Indian J. Public Health Res. Dev. 2018, 9, 605. [Google Scholar] [CrossRef]
- Yang, J.L.; Jan, M.H.; Hung, C.J.; Yang, P.L.; Lin, J.J. Reduced scapular muscle control and impaired shoulder joint position sense in subjects with chronic shoulder stiffness. J. Electromyogr. Kinesiol. 2010, 20, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Moeller, C.R.; Bliven, K.C.; Valier, A.R. Scapular muscle-activation ratios in patients with shoulder injuries during functional shoulder exercises. J. Athl. Train. 2014, 49, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Nodehi Moghadam, A.; Rahnama, L.; Noorizadeh Dehkordi, S.; Abdollahi, S. Exercise therapy may affect scapular position and motion in individuals with scapular dyskinesis: A systematic review of clinical trials. J. Shoulder Elbow Surg. 2020, 29, e29–e36. [Google Scholar] [CrossRef] [Green Version]
- Kara, D.; Harput, G.; Duzgun, I. Trapezius muscle activation levels and ratios during scapular retraction exercises: A comparative study between patients with subacromial impingement syndrome and healthy controls. Clin. Biomech. 2019, 67, 119–126. [Google Scholar] [CrossRef]
- Hong, S.H.; Hong, S.; Yoon, J.; Oh, C.; Cha, J.G.; Kim, H.K.; Bolster, B., Jr. Magnetic resonance elastography (MRE) for measurement of muscle stiffness of the shoulder: Feasibility with a 3 T MRI system. Acta Radiol. 2016, 57, 1099–1106. [Google Scholar] [CrossRef]
- Brandenburg, J.E.; Eby, S.F.; Song, P.; Zhao, H.; Brault, J.S.; Chen, S.; An, K.-N. Ultrasound Elastography: The New Frontier in Direct Measurement of Muscle Stiffness. Arch. Phys. Med. Rehab. 2014, 95, 2207–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringleb, S.I.; Bensamoun, S.F.; Chen, Q.; Manduca, A.; An, K.-N.; Ehman, R.L. Applications of magnetic resonance elastography to healthy and pathologic skeletal muscle. J. Magn. Reson. Imaging 2007, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Taş, S.; Yaşar, Ü.; Kaynak, B.A. Interrater and Intrarater Reliability of a Handheld Myotonometer in Measuring Mechanical Properties of the Neck and Orofacial Muscles. J. Manip. Physiol. Ther. 2020, 44, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Infante, J.; Bravo-Sánchez, A.; Jiménez, F.; Abián-Vicén, J. Effects of dry needling on mechanical and contractile properties of the upper trapezius with latent myofascial trigger points: A randomized controlled trial. Musculoskelet. Sci. Pract. 2021, 56, 102456. [Google Scholar] [CrossRef]
- Lohr, C.; Schmidt, T.; Medina-Porqueres, I.; Braumann, K.-M.; Reer, R.; Porthun, J. Diagnostic accuracy, validity, and reliability of Tensiomyography to assess muscle function and exercise-induced fatigue in healthy participants. A systematic review with meta-analysis. J. Electromyogr. Kinesiol. 2019, 47, 65–87. [Google Scholar] [CrossRef]
- Park, S. Theory and usage of tensiomyography and the analysis method for the patient with low back pain. J. Exerc. Rehabil. 2020, 16, 325–331. [Google Scholar] [CrossRef]
- Cole, A.K.; McGrath, M.L.; Harrington, S.; Padua, D.; Rucinski, T.J.; Prentice, W.E. Scapular Bracing and Alteration of Posture and Muscle Activity in Overhead Athletes With Poor Posture. J. Athl. Train. 2013, 48, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Moezy, A.; Sepehrifar, S.; Dodaran, M.S. The effects of scapular stabilization based exercise therapy on pain, posture, flexibility and shoulder mobility in patients with shoulder impingement syndrome: A controlled randomized clinical trial. Med. J. Islam. Repub. Iran 2014, 28, 87. [Google Scholar]
- Tsuruike, M.; Ellenbecker, T.S. Serratus anterior and lower trapezius muscle activities during multi-joint isotonic scapular exercises and isometric contractions. J. Athl. Train. 2015, 50, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Magarey, M.E.; Jones, M.A. Dynamic evaluation and early management of altered motor control around the shoulder complex. Man. Ther. 2003, 8, 195–206. [Google Scholar] [CrossRef]
- Phadke, V.; Camargo, P.; Ludewig, P. Scapular and rotator cuff muscle activity during arm elevation: A review of normal function and alterations with shoulder impingement. Braz. J. Phys. Ther. 2009, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulla, S.Y.; Southerst, D.; Côté, P.; Shearer, H.M.; Sutton, D.; Randhawa, K.; Varatharajan, S.; Wong, J.J.; Yu, H.; Marchand, A.-A.; et al. Is exercise effective for the management of subacromial impingement syndrome and other soft tissue injuries of the shoulder? A systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Man. Ther. 2015, 20, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Bury, J.; West, M.; Chamorro-Moriana, G.; Littlewood, C. Effectiveness of scapula-focused approaches in patients with rotator cuff related shoulder pain: A systematic review and meta-analysis. Man Ther. 2016, 25, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Shire, A.R.; Stæhr, T.A.B.; Overby, J.B.; Dahl, M.B.; Jacobsen, J.S.; Christiansen, D.H. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet. Disord. 2017, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Reijneveld, E.A.; Noten, S.; Michener, L.A.; Cools, A.; Struyf, F. Clinical outcomes of a scapular-focused treatment in patients with subacromial pain syndrome: A systematic review. Br. J. Sports Med. 2017, 51, 436–441. [Google Scholar] [CrossRef] [PubMed]
- van den Dolder, P.A.; Ferreira, P.H.; Refshauge, K.M. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: A systematic review with meta-analysis. Br. J. Sports Med. 2014, 48, 1216–1226. [Google Scholar]
- Klich, S.; Pietraszewski, B.; Zago, M.; Galli, M.; Lovecchio, N.; Kawczyński, A. Ultrasonographic and Myotonometric Evaluation of the Shoulder Girdle After an Isokinetic Muscle Fatigue Protocol. J. Sport Rehabil. 2020, 29, 1047–1052. [Google Scholar] [CrossRef]
- Gözübüyük, Ö.B.; Tahirbegolli, B.; Isik, A. The correlation between myotonometer and elastography at measuring viscoelastic properties of muscle tissue. In Proceedings of the 10th European Sports Medicine Congress of EFSMA, Cascais, Portugal, 16–18 November 2017. [Google Scholar]
- Nair, K.; Masi, A.T.; Andonian, B.; Barry, A.J.; Coates, B.; Dougherty, J.; Schaefer, E.; Henderson, J.; Kelly, J. Stiffness of resting lumbar myofascia in healthy young subjects quantified using a handheld myotonometer and concurrently with surface electromyography monitoring. J. Bodyw. Mov. Ther. 2016, 20, 388–396. [Google Scholar] [CrossRef]
- Murphy, A.J.; Watsford, M.L.; Coutts, A.J.; Pine, M.J. Reliability of a test of musculotendinous stiffness for the triceps-surae. Phys. Ther. Sport 2003, 4, 175–181. [Google Scholar] [CrossRef]
- Zinder, S.M.; Padua, D.A. Reliability, validity, and precision of a handheld myometer for assessing in vivo muscle stiffness. J. Sport Rehabilitation 2011, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pruyn, E.C.; Watsford, M.L.; Murphy, A.J. Validity and reliability of three methods of stiffness assessment. J. Sport Health Sci. 2016, 5, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, L.-L.; Wu, C.-Y.; Lin, K.-C. Reliability, Validity, and Responsiveness of Myotonometric Measurement of Muscle Tone, Elasticity, and Stiffness in Patients with Stroke. Arch. Phys. Med. Rehabil. 2012, 93, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Zwahlen, A.; Casartelli, N.; Item-Glatthorn, J.; Maffiuletti, N. Validity of resting myotonometric assessment of lower extremity muscles in chronic stroke patients with limited hypertonia: A preliminary study. J. Electromyogr. Kinesiol. 2014, 24, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Lee, H. The Measurement of Stiffness for Major Muscles with Shear Wave Elastography and Myoton: A Quantitative Analysis Study. Diagnostics 2021, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.S.; Marsh, G.D.; Petrella, R.J.; Rice, C.L. Muscle fiber number in the biceps brachii muscle of young and old men. Muscle Nerve 2003, 28, 62–68. [Google Scholar] [CrossRef]
- Anderson, G.S.; Gaetz, M.; Holzmann, M.; Twist, P. Comparison of EMG activity during stable and unstable push-up protocols. Eur. J. Sport Sci. 2013, 13, 42–48. [Google Scholar] [CrossRef]
- Czaprowski, D.; Stoliński, Ł.; Tyrakowski, M.; Kozinoga, M.; Kotwicki, T. Non-structural misalignments of body posture in the sagittal plane. Scoliosis Spinal Disord. 2018, 13, 6. [Google Scholar] [CrossRef] [Green Version]
- Houmard, J.A.; Weidner, M.L.; Gavigan, K.E.; Tyndall, G.L.; Hickey, M.S.; Alshami, A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J. Appl. Physiol. 1998, 85, 1337–1341. [Google Scholar] [CrossRef]
- Reinold, M.M.; Wilk, K.E.; Fleisig, G.S.; Zheng, N.; Barrentine, S.W.; Chmielewski, T.; Cody, R.C.; Jameson, G.G.; Andrews, J.R. Electromyographic Analysis of the Rotator Cuff and Deltoid Musculature During Common Shoulder External Rotation Exercises. J. Orthop. Sports Phys. Ther. 2004, 34, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Ooi, C. Evaluation of Achilles Tendon (AT) Stiffness: Comparison between Shearwave elastography and MyotonPro in Healthy AT. In Proceedings of the European Congress of Radiology-ESSR, Amsterdam, The Netherlands, 13–16 June 2018. [Google Scholar]
- Kawczyński, A.; Mroczek, D.; Andersen, R.E.; Stefaniak, T.; Arendt-Nielsen, L.; Madeleine, P. Trapezius viscoelastic properties are heterogeneously affected by eccentric exercise. J. Sci. Med. Sport 2018, 21, 864–869. [Google Scholar] [CrossRef]
- Xie, Y.; Thomas, L.; Hug, F.; Johnston, V.; Coombes, B.K. Quantifying cervical and axioscapular muscle stiffness using shear wave elastography. J. Electromyogr. Kinesiol. 2019, 48, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, J.; Liu, C.; Tang, C.; Zhang, Z. Modulation in Elastic Properties of Upper Trapezius with Varying Neck Angle. Appl. Bionics Biomech. 2019, 2019, 6048562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokk, J.; Gapeyeva, H.; Ereline, J.; Merila, M.; Pääsuke, M. Shoulder muscle electromyographic activity and stiffness in patients with frozen shoulder syndrome: Six-month follow-up study. Acta Kinesiol. Univ. Tartu. 2013, 19, 73. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Krebs, H.I.; Bever, C.T.; Forrester, L.W.; Macko, R.F.; Hogan, N. Measurement of passive ankle stiffness in subjects with chronic hemiparesis using a novel ankle robot. J. Neurophysiol. 2011, 105, 2132–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, C.-M.; Andrasik, F.; Schleip, R.; Birbaumer, N.; Rea, M. Myofascial triggerpoint release (MTR) for treating chronic shoulder pain: A novel approach. J. Bodyw. Mov. Ther. 2016, 20, 614–622. [Google Scholar] [CrossRef]
- Vain, A.; Peipsi, A.; Mart, L. Device and Method for Real-Time Measurement of Parameters of Mechanical Stress State and Biomechanical Properties of Soft Biological Tissue (Patent No. US 2013/0289365 A1). Google Patent 2017. [Google Scholar]
- Hirata, K.; Kanehisa, H.; Miyamoto, N. Acute effect of static stretching on passive stiffness of the human gastrocnemius fascicle measured by ultrasound shear wave elastography. Eur. J. Appl. Physiol. 2017, 117, 493–499. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.; Tan, L.; Chen, Q. Quantitative assessment of normal middle deltoid muscle elasticity at various arm abduction using ultrasound shear wave elastography. Sci. Rep. 2021, 11, 112479. [Google Scholar] [CrossRef]
- Akagi, R.; Kusama, S. Comparison Between Neck and Shoulder Stiffness Determined by Shear Wave Ultrasound Elastography and a Muscle Hardness Meter. Ultrasound Med. Biol. 2015, 41, 2266–2271. [Google Scholar] [CrossRef]
- Sousa, A.S.P.; da Silva, C.I.C.; Mesquita, I.A.; Silva, A.; Macedo, R.; Imatz-Ojanguren, E. Optimal multi-field functional electrical stimulation parameters for the “drinking task—Reaching phase” and related upper limb kinematics repeatability in post stroke subjects. J. Hand Ther. 2021, 102, 1180–1190. [Google Scholar]
- Huang, J.; Qin, K.; Tang, C.; Zhu, Y.; Klein, C.S.; Zhang, Z.; Liu, C. Assessment of Passive Stiffness of Medial and Lateral Heads of Gastrocnemius Muscle, Achilles Tendon, and Plantar Fascia at Different Ankle and Knee Positions Using the MyotonPRO. Med Sci. Monit. 2018, 24, 7570–7576. [Google Scholar] [CrossRef]
- Lee, P.J.; Rogers, E.L.; Granata, K.P. Active trunk stiffness increases with co-contraction. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2006, 16, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokkink, L.B.; Terwee, C.B.; Knol, D.L.; Stratford, P.W.; Alonso, J.; Patrick, D.L.; Bouter, L.M.; De Vet, H.C. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: A clarification of its content. BMC Med. Res. Methodol. 2010, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Kocur, P.; Wilski, M.; Lewandowski, J.; Łochyński, D. Female Office Workers with Moderate Neck Pain Have Increased Anterior Positioning of the Cervical Spine and Stiffness of Upper Trapezius Myofascial Tissue in Sitting Posture. PM&R 2019, 11, 476–482. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, S.H.; Kim, S.H. Effects of Thoracic Mobilization and Extension Exercise on Thoracic Alignment and Shoulder Function in Patients with Subacromial Impingement Syndrome: A Randomized Controlled Pilot Study. Healthcare 2020, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.R.; Neumann, D.A. Kinesiologic considerations for targeting activation of scapulothoracic muscles—Part 2: Trapezius. Braz. J. Phys. Ther. 2019, 23, 467–475. [Google Scholar] [CrossRef]
- Ben Kibler, W.; McMullen, J.O.H.N.; Uhl, T.I.M. Shoulder rehabilitation strategies, guidelines, and practice. Orthop. Clin. N. Am. 2001, 32, 527–538. [Google Scholar] [CrossRef]
- Pizzari, T.; Wickham, J.; Balster, S.; Ganderton, C.; Watson, L. Modifying a shrug exercise can facilitate the upward rotator muscles of the scapula. Clin. Biomech. 2014, 29, 201–205. [Google Scholar] [CrossRef]
- Watson, L.; Pizzari, T.; Balster, S. Thoracic outlet syndrome Part 2: Conservative management of thoracic outlet. Man. Ther. 2010, 15, 305–314. [Google Scholar] [CrossRef]
- Castelein, B.; Cagnie, B.; Parlevliet, T.; Cools, A. Superficial and Deep Scapulothoracic Muscle Electromyographic Activity During Elevation Exercises in the Scapular Plane. J. Orthop. Sports Phys. Ther. 2016, 46, 184–193. [Google Scholar] [CrossRef]
- Ravichandran, H.; Janakiraman, B.; Gelaw, A.Y.; Fisseha, B.; Sundaram, S.; Sharma, H.R. Effect of scapular stabilization exercise program in patients with subacromial impingement syndrome: A systematic review. J. Exerc. Rehabil. 2020, 16, 216–226. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S.; Fernandez-Sanchez, M.; Struyf, F.; Martinez-Calderon, J.; Morales-Asencio, J.M.; Luque-Suarez, A. Differences in scapular upward rotation, pectoralis minor and levator scapulae muscle length between the symptomatic, the contralateral asymptomatic shoulder and control subjects: A cross-sectional study in a Spanish primary care setting. BMJ Open 2019, 9, e023020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borstad, J.D. Measurement of Pectoralis Minor Muscle Length: Validation and Clinical Application. J. Orthop. Sports Phys. Ther. 2008, 38, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, N.; Oliveira-Campelo, N.; Lopes, Â.; Torres, R.; Sousa, A.S.P.; Ribeiro, F. The Acute Effects of Manual and Instrument-Assisted Cervical Spine Manipulation on Pressure Pain Threshold, Pressure Pain Perception, and Muscle-Related Variables in Asymptomatic Subjects: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Jung, J.-H.; Hahm, S.-C.; Oh, H.-K.; Jung, K.-S.; Cho, H.-Y. Effects of lumbar lordosis assistive support on craniovertebral angle and mechanical properties of the upper trapezius muscle in subjects with forward head posture. J. Phys. Ther. Sci. 2018, 30, 457–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; An, S.Y.; Park, W.; Hwang, J.H. Detection of early changes in the muscle properties of the pectoralis major in breast cancer patients treated with radiotherapy using a handheld myotonometer. Support. Care Cancer 2020, 29, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Knapik, G.G.; Marras, W.S. Application of MR-derived cross-sectional guideline of cervical spine muscles to validate neck surface electromyography placement. J. Electromyogr. Kinesiol. 2018, 43, 127–139. [Google Scholar] [CrossRef]
- Johnson, G.; Bogduk, N.; Nowitzke, A.; House, D. Anatomy and actions of the trapezius muscle. Clin. Biomech. 1994, 9, 44–50. [Google Scholar] [CrossRef]
- Wallden, M. The trapezius—Clinical & conditioning controversies. J. Bodyw. Mov. Ther. 2014, 18, 282–291. [Google Scholar] [CrossRef]
- Ekstrom, R.A.; Bifulco, K.M.; Lopau, C.J.; Andersen, C.F.; Gough, J.R. Comparing the Function of the Upper and Lower Parts of the Serratus Anterior Muscle Using Surface Electromyography. J. Orthop. Sports Phys. Ther. 2004, 34, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Yoo, W.-G. Differential activation of parts of the serratus anterior muscle during push-up variations on stable and unstable bases of support. J. Electromyogr. Kinesiol. 2011, 21, 861–867. [Google Scholar] [CrossRef]
- Smith, R., Jr.; Nyquist-Battie, C.; Clark, M.; Rains, J. Anatomical Characteristics of the Upper Serratus Anterior: Cadaver Dissection. J. Orthop. Sports Phys. Ther. 2003, 33, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.S.; Campbell, B.M.; Swartz, E.E.; Armstrong, C.W. Electromyography of 3 Scapular Muscles: A Comparative Analysis of The Cuff Link Device and a Standard Push-Up. J. Athl. Train. 2008, 43, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, J.M.; Uhl, T. Thickness of the lower trapezius and serratus anterior using ultrasound imaging during a repeated arm lifting task. Man. Ther. 2013, 18, 588–593. [Google Scholar] [CrossRef]
- McKenna, L.J.; de Ronde, M.; Le, M.; Burke, W.; Graves, A.; Williams, S. Measurement of muscle thickness of the serratus anterior and lower trapezius using ultrasound imaging in competitive recreational adult swimmers, with and without current shoulder pain. J. Sci. Med. Sport 2018, 21, 129–133. [Google Scholar] [CrossRef]
- Owen, P.J.; Rantalainen, T.; Scheuring, R.A.; Belavy, D.L. Serratus Anterior Contraction During Resisted Arm Extension (GravityFit) Assessed by MRI. Front. Physiol. 2019, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Salavati, M.; Akhbari, B.; Ebrahimi-Takamjani, I.; Ezzati, K.; Haghighatkhah, H. Reliability of the Upper Trapezius Muscle and Fascia Thickness and Strain Ratio Measures by Ultrasonography and Sonoelastography in Participants With Myofascial Pain Syndrome. J. Chiropr. Med. 2017, 16, 316–323. [Google Scholar] [CrossRef]
- Talbott, N.R.; Smith, A.M.; Stith, T.; Witt, D. Ultrasound Measurement of Levator Scapulae and Upper Trapezius Thickness during Scapular Elevation. In Proceedings of the 2020 APTA Combined Sections Meeting (CSM), Denver, CO, USA, 12–15 February 2020. [Google Scholar]
- Fujimoto, H.; Yabumoto, T.; Sugimori, H.; Shin, S.; Watanabe, T.; Matsuoka, T. Muscular Contraction Ability Develops in the Lower Trapezius Muscle of the Dominant Arm in Team Hand-Ball Players. Adv. Biosci. Biotechnol. 2015, 6, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Hackett, L.; Reed, D.; Halaki, M.; Ginn, K.A. Assessing the validity of surface electromyography for recording muscle activation patterns from serratus anterior. J. Electromyogr. Kinesiol. 2014, 24, 221–227. [Google Scholar] [CrossRef]
- Sommerich, C.M.; Joines, S.; Hermans, V.; Moon, S.D. Use of surface electromyography to estimate neck muscle activity. J. Electromyogr. Kinesiol. 2000, 10, 377–398. [Google Scholar] [CrossRef]
- Ludewig, P.; Cook, T.M. The effect of head position on scapular orientation and muscle activity during shoulder elevation. J. Occup. Rehabil. 1996, 6, 147–158. [Google Scholar] [CrossRef]
- Choi, W.-J.; Cynn, H.-S.; Lee, C.-H.; Jeon, H.-S.; Lee, J.-H.; Jeong, H.-J.; Yoon, T.-L. Shrug exercises combined with shoulder abduction improve scapular upward rotator activity and scapular alignment in subjects with scapular downward rotation impairment. J. Electromyogr. Kinesiol. 2015, 25, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Cynn, H.-S.; Choi, W.-J.; Jeong, H.-J.; Yoon, T.-L. Various shrug exercises can change scapular kinematics and scapular rotator muscle activities in subjects with scapular downward rotation syndrome. Hum. Mov. Sci. 2016, 45, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Umehara, J.; Nakamura, M.; Nishishita, S.; Tanaka, H.; Kusano, K.; Ichihashi, N. Scapular kinematic alterations during arm elevation with decrease in pectoralis minor stiffness after stretching in healthy individuals. J. Shoulder Elb. Surg. 2018, 27, 1214–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umehara, J.; Nakamura, M.; Saeki, J.; Tanaka, H.; Yanase, K.; Fujita, K.; Yamagata, M.; Ichihashi, N. Acute and Prolonged Effects of Stretching on Shear Modulus of the Pectoralis Minor Muscle. J. Sports Sci. Med. 2021, 20, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Castelein, B.; Cagnie, B.; Parlevliet, T.; Danneels, L.; Cools, A. Optimal Normalization Tests for Muscle Activation of the Levator Scapulae, Pectoralis Minor, and Rhomboid Major: An Electromyography Study Using Maximum Voluntary Isometric Contractions. Arch. Phys. Med. Rehabil. 2015, 96, 1820–1827. [Google Scholar] [CrossRef]
- Khosravi, F.; Rahnama, M.; Karimi, N.; Amiri, M.; Geil, M.D.; Rahnama, L. Rehabilitative ultrasound imaging of the levator scapula muscle at rest and during contraction: Technical description and reliability. J. Bodyw. Mov. Ther. 2021, 28, 411–417. [Google Scholar] [CrossRef]
- Cloke, D.J.; Watson, H.; Purdy, S.; Steen, I.N.; Williams, J.R. A pilot randomized, controlled trial of treatment for painful arc of the shoulder. J. Shoulder Elb. Surg. 2008, 17, S17–S21. [Google Scholar] [CrossRef]
- Hermans, J.; Luime, J.J.; Meuffels, D.E.; Reijman, M.; Simel, D.L.; Bierma-Zeinstra, S.M. Does this patient with shoulder pain have rotator cuff disease? The Rational Clinical Examination systematic review. JAMA 2013, 310, 837–847. [Google Scholar] [CrossRef]
- Ludewig, P.; Cook, T.M. Alterations in Shoulder Kinematics and Associated Muscle Activity in People with Symptoms of Shoulder Impingement. Phys. Ther. 2000, 80, 276–291. [Google Scholar] [CrossRef]




| Muscle | Correlation Values | ||
|---|---|---|---|
| Values (p Value) | Classification | ||
| Upper trapezius | r = −0.25 to 0.50 (p > 0.05) [1] | Not statistically significant | |
| Infraspinatus | r = 0.35 to 0.37 (p < 0.05) [4] | Fair | |
| Rectus femoris | r = 0.398 to 0.416 (p < 0.05 and p < 0.01, respectively) [56] | ||
| Biceps brachii | r = 0.479 to 0.583 (p < 0.05) [49] | Fair to Moderate | |
| Gastrocnemius | r = 0.463 to 0.71 (p < 0.05 or p < 0.01) [4,18,56] | Fair to Good | |
| Erector spinae | r = 0.51 to 0.54 (p < 0.05) [4] | Moderate | |
| Biceps femoris | r = 0.594 to 0.652 (p < 0.01) [56] | ||
| Tibialis anterior | r = 0.540 to 0.561 (p < 0.01) [56] | ||
| Shear Wave Elastography | Myotonometry | |
|---|---|---|
| Instrument characteristics | • Objective [4,18] • Non-invasive [1,4,18] | |
| Real-time [1,3,64] Required technical expertise [18] | Less expensive [1,4] Handheld [1,3,4] Easy to use [1,3,4,66] | |
| Structures assessed | Deep [1,4] | Superficial [1,4] |
| Type of stiffness measured | Passive [1,3]: resistance to elongation or shortening or, in physical terms, the change in tension per unit change in length [67] | Dynamic [1,25,68]: resistance to a force that deforms muscle initial shape [3,25,68] |
| Measurement mode | Elastic [4]/shear [3] modulus, that uses ultrasound radiation forces [4] | Damped oscillation method following a dynamic transformation of the muscle in response to a short-term external mechanical impulse [69] |
| Measurement process |
| |
| Measurement Interpretation | Velocity of shear waves (proportional to shear modulus [64]) rise with increase in passive muscle stiffness [1,64] | Higher values of dynamic stiffness imply more energy to modify the shape of the tissue [3] |
| Scapular muscles Assessed | In healthy subjects:
| In healthy subjects:
|
| Results SWE vs. Myotonometry |
| |
| Muscle | Sample | Assessment Conditions | Reliability Values | |||
|---|---|---|---|---|---|---|
| Assessment Moment | Rater | Muscle Condition | ICC Values | Classification | ||
| Upper trapezius | Healthy and MSKd | IS | Inter-rater | Rest | 0.97 [7] | Very high |
| Healthy | IS | Intra-rater | Rest | 0.86 [48] | High | |
| BD | 0.229 [25] to 0.86 [63] | Low to very high | ||||
| IS | Inter-rater | Rest and contraction considered together | 0.97 [21] | Very high | ||
| BD | Intra-rater | 0.97 [21] | Very high | |||
| Middle trapezius | BD | Intra-rater | Rest | 0.813 to 0.963 [25] | High to very high | |
| Lower trapezius | BD | Intra-rater | Rest | 0.820 to 0.926 [25] | High to very high | |
| Infraspinatus | IS | Intra-rater | Rest | 0.98 [4] | High to very high | |
| Contraction | 0.98 [4] | High to very high | ||||
| Erector spinae | IS | Intra-rater | Rest | 1 [4] | High to very high | |
| Contraction | 0.99 to 1 [4] | High to very high | ||||
| Rectus femoris | IS | Intra-rater | Rest | 0.938 [56] | Very high | |
| Contraction | 0.872 [56] | High | ||||
| Vastus Lateralis | IS | Intra-rater | Rest | 0.97 [3] | Very high | |
| BD | 0.93 [3] | Very high | ||||
| Medial gastrocnemius | IS | Intra-rater | Rest | 0.904 [56] to 1 [4] | Very high | |
| Contraction | 0.856 [56] to 0.99 [4] | High to very high | ||||
| Biceps femoris | IS | Intra-rater | Rest | 0.884 [56] | High | |
| Contraction | 0.861 [56] | High | ||||
| Tibialis anterior | IS | Intra-rater | Rest | 0.880 [56] | High | |
| Contraction | 0.894 [56] | High | ||||
| Muscle of Interest | Measurement Points |
|---|---|
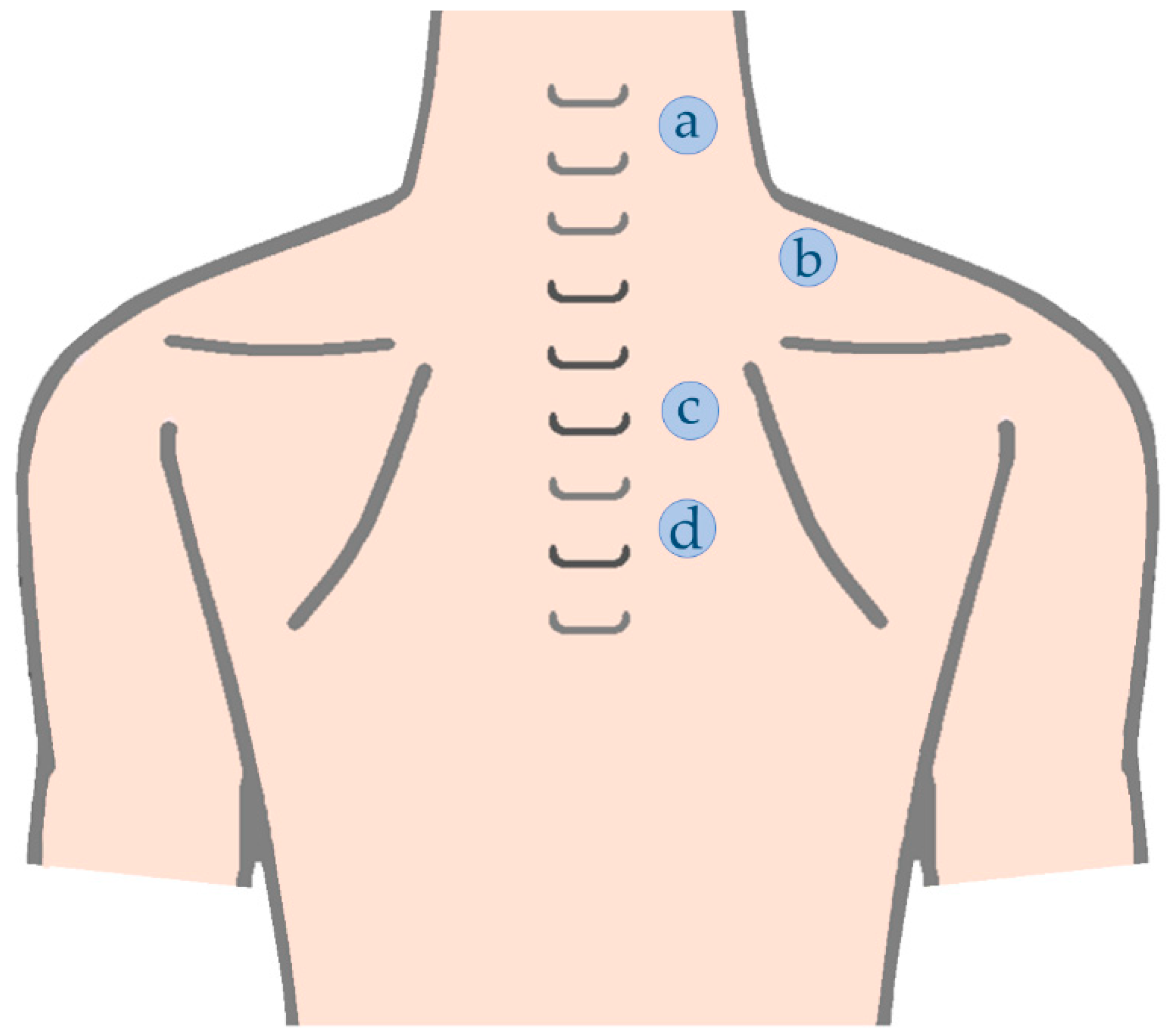
| Upper trapezius C5/6 level | At the level of C5/C6 about 2 cm lateral from the midline [90] |
| Upper trapezius C7 level | Mid-way between C7 spinous process and the angle of acromion [7,12,21,87,88,89] |
| Middle trapezius | Mid-way from T4 spinous process to the medial border of spine of the scapulae [25] |
| Lower Trapezius | Mid-way from T6 spinous process to the medial border of spine of the scapulae [25] OR Mid-point of the lateral border of the fibers of lower trapezius [25] |
| Muscle of Interest | Measurement Points |
|---|---|
| Levator scapulae | Between the posterior margin of sternocleidomastoid and anterior margin of the upper trapezius [104,105,106,107], at level of C4/5 [90] |
| SA upper/middle portion | Over the fourth rib, at the midpoint between the latissimus dorsi and the pectoralis major [93,94] |
| SA lower portion | Over the seventh rib, in the midline of the axilla [93], for SA lower portion (SAlow) [93] |
| Study Objective | Group | Muscle Assessed | SD (N/m) | p Value | |||
|---|---|---|---|---|---|---|---|
| Mild (until 3 in VAS) UT pain (20.83 ± 1.12 years old) [26] | BGc | ✓ | VAS 0 | UT (muscle belly) | 170.56 | 28.45 | p < 0.05 *, for VAS 3 in comparison with other 3 groups |
| VAS 1 | 161.67 | 16.59 | |||||
| VAS 2 | 160.48 | 20.72 | |||||
| VAS 3 | 191.50 | 25.74 | |||||
| IE | - | - | |||||
| Moderate work-related neck disorders (30–55 years old) [77] | BGc | ✓ | Pain | UT (C5/6 and C7 level) | 301.50 | 23.50 | p = 0.006 * |
| Control | 270.90 | 33.70 | |||||
| IE | - | - | |||||
| Unilateral chronic shoulder pain together with, at least, 2 sensitive sites (myofascial trigger points) (18–70 years old) [68] | BGc | X | Control (Us, before) | UT (trigger points) | 324.42 | 11.39 | p = 0.057 |
| Control (Us, after) | 334.68 | 11.10 | |||||
| Pain (before) | 332.32 | 10.97 | |||||
| Pain (after) | 300.66 | 9.43 | |||||
| IE | ✓ for Myofascial trigger-point Release | Pain (before vs. after) | p = 0.012 * | ||||
| Long-standing, nonspecific and nontraumatic neck-shoulder pain (20–61 years old) [14] | BGc | X | Control (MB sites) | UT (15 adjacent points) | 237.80 | 42.8 | p = 0.273, for comparison of both measurement sites |
| Control (Mt sites) | 327.50 | 55.9 | |||||
| Pain (MB sites before) | 258.70 | 41.10 | |||||
| Pain (Mt sites before) | 330.40 | 50.8 | |||||
| IE | ✓ for Eccentric Training | Pain (MB sites after) | 226.80 | 20.00 | p < 0.001 *, for comparison in both measurement sites | ||
| Pain (Mt sites after) | 287.30 | 47.80 | |||||
| Subacromial impingement syndrome (49.20 ± 9.48 to 50.90 ± 9.10 years old) [78] | BGc | - | - | ||||
| IE | ✓ for Thoracic mobilization and/or Extension exercise | Pain (TM before) | UT (center of muscle belly) | 257.90 | 29.03 | p = 0.001 * | |
| Pain (TM after) | 232.50 | 20.49 | |||||
| Pain (exercise before) | 257.70 | 19.33 | p = 0.001 * | ||||
| Pain (exercise after) | 236.10 | 27.27 | |||||
| Pain (TM plus exercise before) | 257.50 | 25.61 | p = 0.001 * | ||||
| Pain (TM plus exercise after) | 223.00 | 32.83 | |||||
| Stage II or III of unilateral frozen shoulder syndrome (38–74 years old) [66] | BGc | X | Control (Us, before) | UT (center of muscle belly) | ≈235 | _ | p > 0.05 |
| Control (Us, 1 m after) | ≈215 | _ | |||||
| Control (Us, 6 m after) | ≈200 | _ | |||||
| Pain (before) | ≈240 | _ | |||||
| Pain (1 m after) | ≈225 | _ | |||||
| Pain (6 m after) | ≈220 | _ | |||||
| IE | X for Manual manipulation (under anaesthesia) | Pain (before vs. after) | p > 0.05, for comparison in each assessment moments | ||||
| Unilateral neck or shoulder pain and active myofascial trigger points in the trapezius muscle (19.8 ± 2.4 years old) [25] | BGc | - | - | ||||
| IE | ✓ for Ischemic compression | Pain | UT (distally of muscle belly’s center) | 232.00 | 29.70 | p = 0.03 * | |
| UT2 (proximally of muscle belly’s center) | 269.00 | 42.10 | |||||
| MT | 405.30 | 192.10 | p = 0.40 | ||||
| LT (mid-point) | 347.50 | 110.40 | p = 0.29 | ||||
| LT (lateral border mid-point of muscle fibers) | 331.70 | 89.30 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, A.S.C.; Cruz, E.B.; Vilas-Boas, J.P.; Sousa, A.S.P. Scapular Dynamic Muscular Stiffness Assessed through Myotonometry: A Narrative Review. Sensors 2022, 22, 2565. https://doi.org/10.3390/s22072565
Melo ASC, Cruz EB, Vilas-Boas JP, Sousa ASP. Scapular Dynamic Muscular Stiffness Assessed through Myotonometry: A Narrative Review. Sensors. 2022; 22(7):2565. https://doi.org/10.3390/s22072565
Chicago/Turabian StyleMelo, Ana S. C., Eduardo B. Cruz, João Paulo Vilas-Boas, and Andreia S. P. Sousa. 2022. "Scapular Dynamic Muscular Stiffness Assessed through Myotonometry: A Narrative Review" Sensors 22, no. 7: 2565. https://doi.org/10.3390/s22072565
APA StyleMelo, A. S. C., Cruz, E. B., Vilas-Boas, J. P., & Sousa, A. S. P. (2022). Scapular Dynamic Muscular Stiffness Assessed through Myotonometry: A Narrative Review. Sensors, 22(7), 2565. https://doi.org/10.3390/s22072565








