Performance Evaluation of a Smart Bed Technology against Polysomnography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Overnight Polysomnography
2.4. Sleep Monitoring with Smart Bed Technology
2.5. Data Curation and Cleaning and PSG-Smart Bed Synchronization
2.6. Epoch-by-Epoch Analysis
2.7. Analysis of All-Night Summary Variables
2.8. Statistical Analysis
3. Results
3.1. Dataset Characteristics
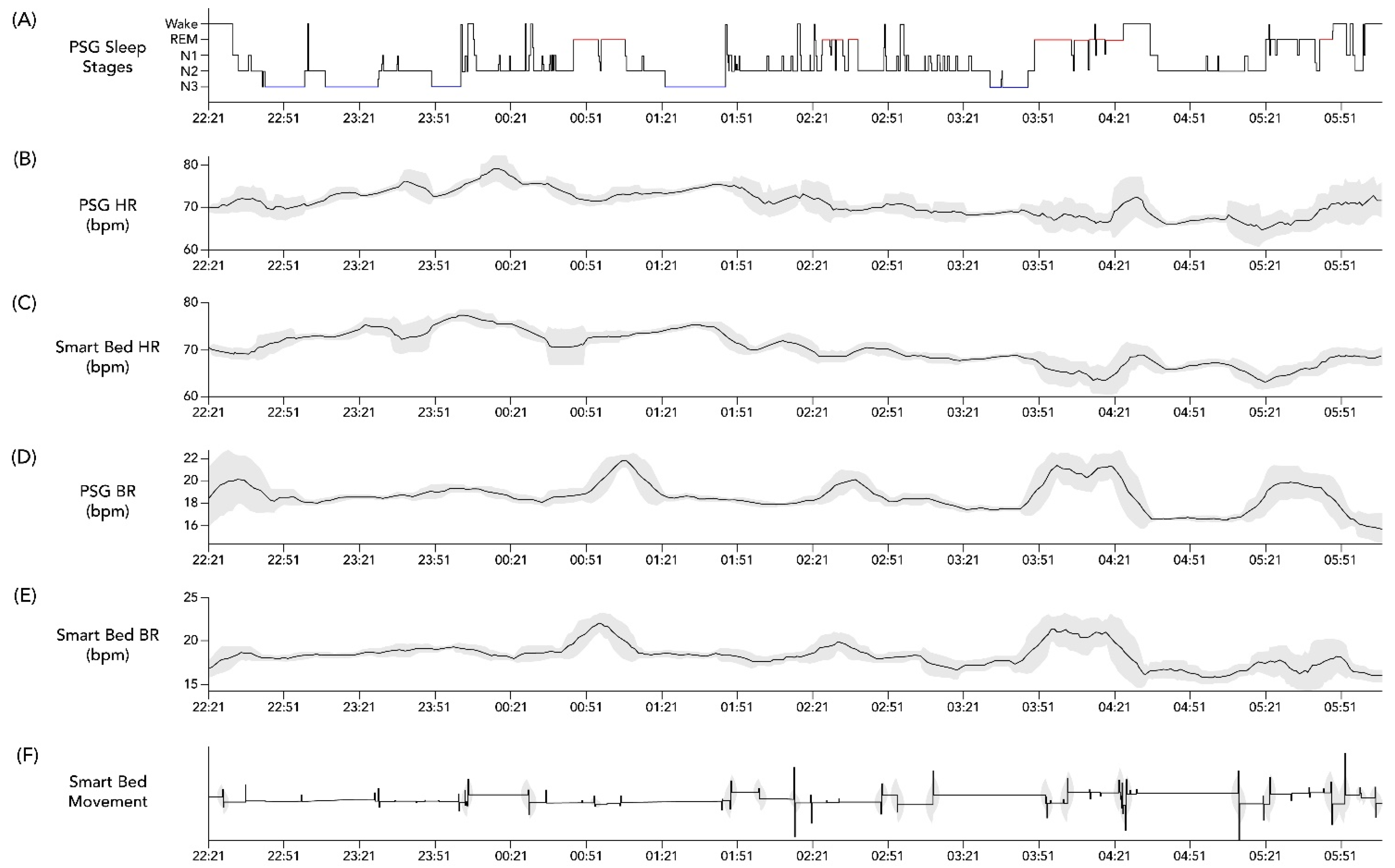
3.2. Analysis of Epoch-by-Epoch and All-Night BR and HR
3.3. Analysis of Epoch-by-Epoch and All-Night Sleep/Wake Detection
3.4. Analysis of All-Night Summary Variables
3.5. Influence of Demographic and Health Factors on PSG vs. Smart Bed Temporal Concordance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Profiles of HR and BR with Regard to Rapid Eye Movement (REM) and Non-REM (NREM) Sleep Stages
| Heart Rate, bpm | Breathing Rate, bpm | |||
|---|---|---|---|---|
| Mean (SD) | Smart Bed | PSG | Smart Bed | PSG |
| Wake | 70.58 (2.52) | 71.16 (3.78) | 14.56 (1.48) | 15.05 (1.10) |
| Light sleep | 67.91 (2.16) | 67.11 (2.87) | 14.96 (0.98) | 15.05 (0.90) |
| Deep sleep | 68.11 (1.42) | 67.24 (1.75) | 15.76 (0.66) | 15.66 (0.75) |
| REM | 67.85 (2.51) | 67.89 (2.94) | 16.00 (1.34) | 15.97 (1.12) |
| All epochs | 67.96 (2.04) | 67.72 (2.71) | 15.22 (1.04) | 15.31 (0.93) |

| Differences | Correlation | |||
|---|---|---|---|---|
| Mean [95% CI] | ΔHR (bpm) | Limits of Agreement (bpm) | r | Number of Epochs (Percent No Coverage) |
| Over entire night | −0.15 [−1.08, 0.77] | −6.21 [−7.85, −4.57]–5.90 [4.26, 7.54] | 0.94 [0.91–0.96] | - |
| Wake | 0.57 [−0.5, 1.65] | −6.28 [−8.04, −4.53]–7.43 [5.68, 9.19] | 0.79 [0.78–0.80] | 5891 (5.9%) |
| Light sleep | −0.36 [−1.52, 0.79] | −7.76 [−9.67, −5.86]–7.03 [5.13, 8.94] | 0.81 [0.81–0.82] | 19,555 (7.1%) |
| Deep sleep | −0.87 [−1.87, 0.13] | −7.18 [−8.80, −5.56]–5.45 [3.83, 7.07] | 0.83 [0.83–0.84] | 5870 (0.40%) |
| REM | 0.06 [−0.98, 1.09] | −6.48 [−8.16, −4.8]–6.59 [4.91, 8.28] | 0.83 [0.82–0.84] | 5934 (7.31%) |
| All epochs | −0.23 [−1.26, 0.78] | −6.65 [−8.33, −4.97]–6.19 [4.48, 7.85] | 0.81 [0.81–0.82] | 37,250 (6.40%) |
| Differences | Correlation | |||
|---|---|---|---|---|
| Mean [95% CI] | ΔBR (bpm) | Limits of Agreement (bpm) | r | Number of Epochs (Percent No Coverage) |
| Over entire night | 0.09 [−0.03, 0.21] | −0.69 [−0.90, −0.48]–0.87 [0.66, 1.08] | 0.96 [0.94–0.98] | - |
| Wake | 0.49 [0.11, 0.88] | −1.97 [−2.45, −1.49]–2.96 [2.47, 3.44] | 0.5 [0.48–0.52] | 6238 (1.3%) |
| Light sleep | 0.05 [−0.29, 0.4] | −2.14 [−2.5, −1.78]–2.25 [1.89, 2.61] | 0.75 [0.75–0.76] | 20,919 (0.6%) |
| Deep sleep | −0.10 [−0.47, 0.27] | −2.46 [−2.87, −2.05]–2.26 [1.85, 2.67] | 0.77 [0.75–0.78] | 6105 (0.0%) |
| REM | −0.02 [−0.41, 0.36] | −2.45 [−2.9, −2.0]–2.4 [1.96, 2.85] | 0.72 [0.71–0.73] | 6368 (0.0%) |
| All epochs | 0.08 [−0.25, 0.41] | −2.02 [−2.36, −1.68]–2.19 [1.85, 2.53] | 0.71 [0.70–0.71] | 39,630 (0.6%) |
References
- Ko, P.R.; Kientz, J.A.; Choe, E.K.; Kay, M.; Landis, C.A.; Watson, N.F. Consumer sleep technologies: A review of the landscape. J. Clin. Sleep Med. 2015, 11, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I. Sleep applications to assess sleep quality. Sleep Med. Clin. 2016, 11, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Kolla, B.; Mansukhani, S.; Mansukhani, M. Consumer sleep tracking devices: A review of mechanisms, validity and utility. Expert Rev. Med. Devices 2016, 13, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Stippig, A.; Hubers, U.; Emerich, M. Apps in sleep medicine. Sleep Breath 2015, 19, 411–417. [Google Scholar] [CrossRef]
- Toften, S.; Pallesen, S.; Hrozanova, M.; Moen, F.; Gronli, J. Validation of sleep stage classificiation using non-contact radar technology and machine learning (Somnofy®). Sleep Med. 2020, 75, 54–61. [Google Scholar] [CrossRef]
- Arnal, P.J.; Thorey, V.; Debellemaniere, E.; Ballard, M.E.; Bou Hernandez, A.; Guillot, A.; Jourde, H.; Harris, M.; Guillard, M.; van Beers, P.; et al. The Dreem Headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep 2020, 43, zsaa097. [Google Scholar] [CrossRef]
- Garcia-Molina, G.; Tsoneva, T.; Jasko, J.; Steele, B.; Aquino, A.; Baher, K.; Pastoor, S.; Pfundtner, S.; Ostrowski, L.; Miller, B.; et al. Closed-loop system to enhance slow-wave activity. J. Neural Eng. 2018, 15, 066018. [Google Scholar] [CrossRef]
- Ranta, J.; Aittokoski, T.; Tenhunen, M.; Alasaukko-Oja, M. EMFIT QS heart rate and respiration rate validation. Biomed. Phys. Eng. Express 2019, 5, 025016. [Google Scholar] [CrossRef]
- Zimlichman, E.; Szyper-Kravitz, M.; Shinar, Z.; Klap, T.; Levkovich, S.; Unterman, A.; Rozenblum, R.; Rothschild, J.M.; Amital, H.; Shoenfeld, Y. Early recognition of acutely deteriorating patients in non-intensive care units: Assessment of an innovative monitoring technology. J. Hosp. Med. 2012, 7, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Zink, M.D.; Bruser, C.; Stuben, B.O.; Napp, A.; Stohr, R.; Leonhardt, S.; Marx, N.; Mischke, K.; Schulz, J.B.; Schiefer, J. Unobtrusive nocturnal heartbeat monitoring by a ballistocardiographic sensor in patients with sleep disordered breathing. Sci. Rep. 2017, 7, 13175. [Google Scholar] [CrossRef] [Green Version]
- Migliorini, M.; Kortelainen, J.M.; Pärkkä, J.; Tenhunen, M.; Himanen, S.L.; Bianchi, A.M. Monitoring nocturnal heart rate with bed sensor. Methods Inf. Med. 2014, 53, 308–313. [Google Scholar] [PubMed] [Green Version]
- Giovangrandi, L.; Inan, O.T.; Wiard, R.M.; Etemadi, M.; Kovacs, G.T.A. Ballistocardiography-A Method Worth Revisiting. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–4 September 2011; pp. 4279–4282. [Google Scholar]
- Gubner, R.; Rodstein, M.; Ungerleider, H. Ballistocardiography: An appraisal of technic, physiologic principles, and clinical value. Circulation 1953, 7, 268–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.M.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring sleep: Accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Muzet, A.; Werner, S.; Fuchs, G.; Roth, T.; Saoud, J.B.; Viola, A.U.; Schaffhauser, J.Y.; Luthringer, R. Assessing sleep architecture and continuity measures through the analysis of heart rate and wrist movement recordings in healthy subjects: Comparison with results based on polysomnography. Sleep Med. 2016, 21, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Waldeck, M.; Lambert, M. Heart rate during sleep: Implications for monitoring training status. J. Sports Sci. Med. 2003, 2, 133–138. [Google Scholar]
- De Zambotti, M.; Goldstone, A.; Claudatos, S.; Colrain, I.; Baker, F.C. A validation study of Fitbit Charge™ compared with polysomnography in adults. Chronobiol. Int. 2018, 35, 465–476. [Google Scholar] [CrossRef]
- De Zambotti, M.; Rosas, L.; Colrain, I.M.; Baker, F.C. The sleep of the ring: Comparison of the OURA sleep tracker against polysomnography. Behav. Sleep Med. 2019, 17, 124–136. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y. A look at wearable abandonment. In Proceedings of the 2017 18th IEEE International Conference on Mobile Data Management (MDM), Daejeon, Korea, 29 May–1 June 2017; pp. 392–393. [Google Scholar]
- Depner, C.M.; Cheng, P.C.; Devine, J.K.; Khosla, S.; de Zambotti, M.; Robillard, R.; Vakulin, A.; Drummond, S.P.A. Wearable technologies for developing sleep and circadian biomarkers: A summary of workshop discussions. Sleep 2020, 43, zsz254. [Google Scholar] [CrossRef] [Green Version]
- Moinester, M.A.; Gottfried, R. Sample size estimation for correlations with pre-specified confidence interval. Quant. Methods Psychol. 2014, 10, 124–130. [Google Scholar] [CrossRef]
- Greengard, S. The Internet of Things; MIT Press: Cambridge, MA, USA, 2015; p. 253. [Google Scholar]
- Boudreau, P.; Yeh, W.H.; Dumont, G.A.; Boivin, D.B. Circadian variation of heart rate variability across sleep stages. Sleep 2013, 36, 1919–1928. [Google Scholar] [CrossRef] [Green Version]
- De Zambotti, M.; Trinder, J.; Silvani, A.; Colrain, I.M.; Baker, F.C. Dynamic coupling between the central and autonomic nervous systems during sleep: A review. Neurosci. Biobehav. Rev. 2018, 90, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Douglas, N.; White, D.; Pickett, C.; Weil, J.; Zwillich, C. Respiration during sleep in normal man. Thorax 1982, 37, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, G.; Williams, J.; Alrehaili, G.A.; McLean, A.; Pirouz, R.; Amdur, R.; Jain, V.; Ahari, J.; Bawa, A.; Kimbro, S. Respiratory rate variability in sleeping adults without obstructive sleep apnea. Physiol. Rep. 2016, 4, e12949. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Yu, Z.; Tang, Y.; Shi, W.; FKluge, T.; Ser, W. Deep learning method for sleep stage classification. In Proceedings of the International Conference on Neural Information Processing, ICONIP 2017, Guangzhou, China, 14–18 November 2017; Liu, D., Xie, S.X., Li, Y., Zhao, D., El-Alfy, E., Eds.; Springer: Guangzhou, China, 2017. [Google Scholar]
- Li, Q.; Li, Q.; Liu, C.; Shashikumar, S.P.; Nemati, S.; Clifford, G.D. Deep learning in the cross-time frequency domain for sleep staging from a single-lead electrocardiogram. Physiol. Meas. 2018, 39, 124005. [Google Scholar] [CrossRef]
- Mousavi, S.; Afghah, F.; Acharya, U.R. SleepEEGNet: Automated sleep stage scoring with sequence to sequence deep learning approach. PLoS ONE 2019, 14, e0216456. [Google Scholar] [CrossRef] [Green Version]
- Phan, H.; Andreotti, F.; Cooray, N.; Chen, O.Y.; De Vos, M. Joint classification and prediction CNN framework for automatic sleep stage classification. IEEE Trans. Biomed. Eng. 2019, 66, 1285–1296. [Google Scholar] [CrossRef]
- Roberts, D.M.; Schade, M.M.; Mathew, G.M.; Gartenberg, D.; Buxton, O.M. Detecting sleep using heart rate and motion data from multisensor consumer-grade wearables, relative to wrist actigraphy and polysomnography. Sleep 2020, 43, zsaa045. [Google Scholar] [CrossRef]
- Sun, H.; Ganglberger, W.; Panneerselvam, E.; Leone, M.J.; Quadri, S.A.; Goparaju, B.; Tesh, R.A.; Akeju, O.; Thomas, R.J.; Westover, M.B. Sleep staging from electrocardiography and respiration with deep learning. Sleep 2020, 43, zsz306. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, O.; Baloglu, U.B.; Acharya, U.R. A deep learning model for automated sleep stages classification using PSG signals. Int. J. Env. Res. Public Health 2019, 16, 599. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, Z.; Lan, K.; Liu, X.; Zhang, Z.; Li, P.; Cao, D.; Zheng, J.; Pan, J. Sleep Stage Classification Using Bidirectional LSTM in Wearable Multi-sensor Systems. In Proceedings of the IEEE INFOCOM 2019 Workshops, IEEE Conference on Computer Communications Workshops (INFOCOM WKSHPS), Paris, France, 29 April–2 May 2019; pp. 443–448. [Google Scholar]
- Martinez, J.P.; Almeida, R.; Olmos, S.; Rocha, A.P.; Laguna, P. A wavelet-based ECG delineator: Evaluation on standard databases. IEEE Trans. Biomed. Eng. 2004, 51, 570–581. [Google Scholar] [CrossRef]
- Menghini, L.; Cellini, N.; Goldstone, A.; Baker, F.C.; de Zambotti, M. A standardized framework for testing the performance of sleep-tracking technology: Step-by-step guidelines and open-source code. Sleep 2021, 44, zsaa170. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Haghayegh, S.; Kang, H.-A.; Khoshnevis, S.; Smolensky, M.; Diller, K. A comprehensive guideline for Bland-Altman and intra class correlation calculations to properly compare two methods of measurement and interpret findings. Physiol. Meas. 2020, 41, 055012. [Google Scholar] [CrossRef]
- Byrt, T.; Bishop, J.; Carlin, J. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef]
- Kortelainen, J.M.; Mendez, M.O.; Bianchi, A.M.; Matteucci, M.; Cerutti, S. Sleep staging based on signals acquired through bed sensor. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 776–785. [Google Scholar] [CrossRef]
- Mendez, M.O.; Matteucci, M.; Cerutti, S.; Bianchi, A.M.; Kortelainen, J.M. Automatic detection of sleep macrostructure based on bed sensors. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 5555–5558. [Google Scholar]
- Migliorini, M.; Bianchi, A.M.; Nistico, D.; Kortelainen, J.; Arce-Santana, E.; Cerutti, S.; Mendez, M.O. Automatic sleep staging based on ballistocardiographic signals recorded through bed sensors. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2010, 2010, 3273–3276. [Google Scholar]
- Hori, T.; Hayashi, M.; Morikawa, T. Topographical EEG changes and the hypnagogic experience. In Sleep Onset: Normal and Abnormal Processes; American Psychological Association: Washington, DC, USA, 1994; pp. 237–253. [Google Scholar]
- Kelly, J.M.; Strecker, R.E.; Bianchi, M.T. Recent developments in home sleep-monitoring devices. ISRN Neurol. 2012, 2012, 768794. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, L.J.; Hiruma, L.S.; Avis, K.; Montgomery-Downs, H.; Valentin, J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 2015, 38, 1323–1330. [Google Scholar] [CrossRef] [Green Version]




| Variable | All Participants (N = 45) |
|---|---|
| Male sex, n (%) | 20 (44.4) |
| Mean age, years (SD) | 41.2 (10.5) |
| Mean BMI, kg/m2 (SD) | 25.9 (4.4) |
| Mean lights out, hh:mm (SD) | 22:16 (00:29) |
| Mean lights on, hh:mm (SD) | 05:55 (00:32) |
| Mean TST, min (SD) | 388.7 (46.3) |
| Mean WASO, min (SD) | 54.2 (33.9) |
| Mean SE, % (SD) | 87 (7) |
| Mean SOL, min (SD) | 16.4 (13.6) |
| Mean BR, bpm * (SD) | 14.9 (2.2) |
| Mean HR, bpm * (SD) | 66.4 (9.9) |
| Mean AHI, events/h (SD) | 6.53 (15.1) |
| Performance Measure | Smart Bed Machine Learning Algorithm |
|---|---|
| AUC | 0.86 |
| Accuracy (mean ± SD) | 0.86 ± 0.11 |
| Balanced accuracy (mean ± SD) | 0.75 ± 0.12 |
| d′ (mean ± SD) | 1.47 ± 0.38 |
| Kappa | 0.45 ± 0.17 |
| Adjusted kappa (mean ± SD) | 0.74 ± 0.11 |
| Precision (mean ± SD) | 0.90 ± 0.06 |
| Sensitivity (mean ± SD) | 0.94 ± 0.05 |
| Specificity (mean ± SD) | 0.48 ± 0.18 |
| SOL (min) | WASO (min) | TST (min) | SE (%) | |
|---|---|---|---|---|
| Smart bed (SD) | 13.4 (11.4) | 76.8 (43.8) | 369.1 (52.7) | 0.8 (0.1) |
| PSG (SD) | 16.4 (13.7) | 54.2 (33.9) | 388.7 (46.4) | 0.9 (0.1) |
| Bias | 3.0 (16.8) | 15.3 − 0.5 × ref | 150.6 − 0.4 × ref | 0.5 − 0.5 × ref |
| Bias CI | [−1.9, 7.7] | b0 = [1.1, 27.6], b1 = [−0.7, −0.3] | b0 = [63.0, 213.9], b1 = [−0.5, −0.1] | b0 = [0.3, 0.6], b1 = [−0.6, −0.3] |
| Lower LOA | bias − 2.5 (−3.4 + 0.9 × ref) | bias − 2.5 (16.3 + 0.1 × ref) | bias − 61.8 | bias − 0.1 |
| Upper LOA | bias + 2.5 (−3.4 + 0.9 × ref) | bias + 2.5 (16.3 + 0.1 × ref) | bias + 61.8 | bias + 0.1 |
| LOA CI | c0 = [−22.9, −10.8], c1 = [1.4, 2.2] | c0 = [4.2, 19.4], c1 = [0.0, 0.2] | bias ± [52.8, 74.1] | bias ± [0.1, 0.1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siyahjani, F.; Garcia Molina, G.; Barr, S.; Mushtaq, F. Performance Evaluation of a Smart Bed Technology against Polysomnography. Sensors 2022, 22, 2605. https://doi.org/10.3390/s22072605
Siyahjani F, Garcia Molina G, Barr S, Mushtaq F. Performance Evaluation of a Smart Bed Technology against Polysomnography. Sensors. 2022; 22(7):2605. https://doi.org/10.3390/s22072605
Chicago/Turabian StyleSiyahjani, Farzad, Gary Garcia Molina, Shawn Barr, and Faisal Mushtaq. 2022. "Performance Evaluation of a Smart Bed Technology against Polysomnography" Sensors 22, no. 7: 2605. https://doi.org/10.3390/s22072605
APA StyleSiyahjani, F., Garcia Molina, G., Barr, S., & Mushtaq, F. (2022). Performance Evaluation of a Smart Bed Technology against Polysomnography. Sensors, 22(7), 2605. https://doi.org/10.3390/s22072605






