Head Pitch Angular Velocity Discriminates (Sub-)Acute Neck Pain Patients and Controls Assessed with the DidRen Laser Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Participants
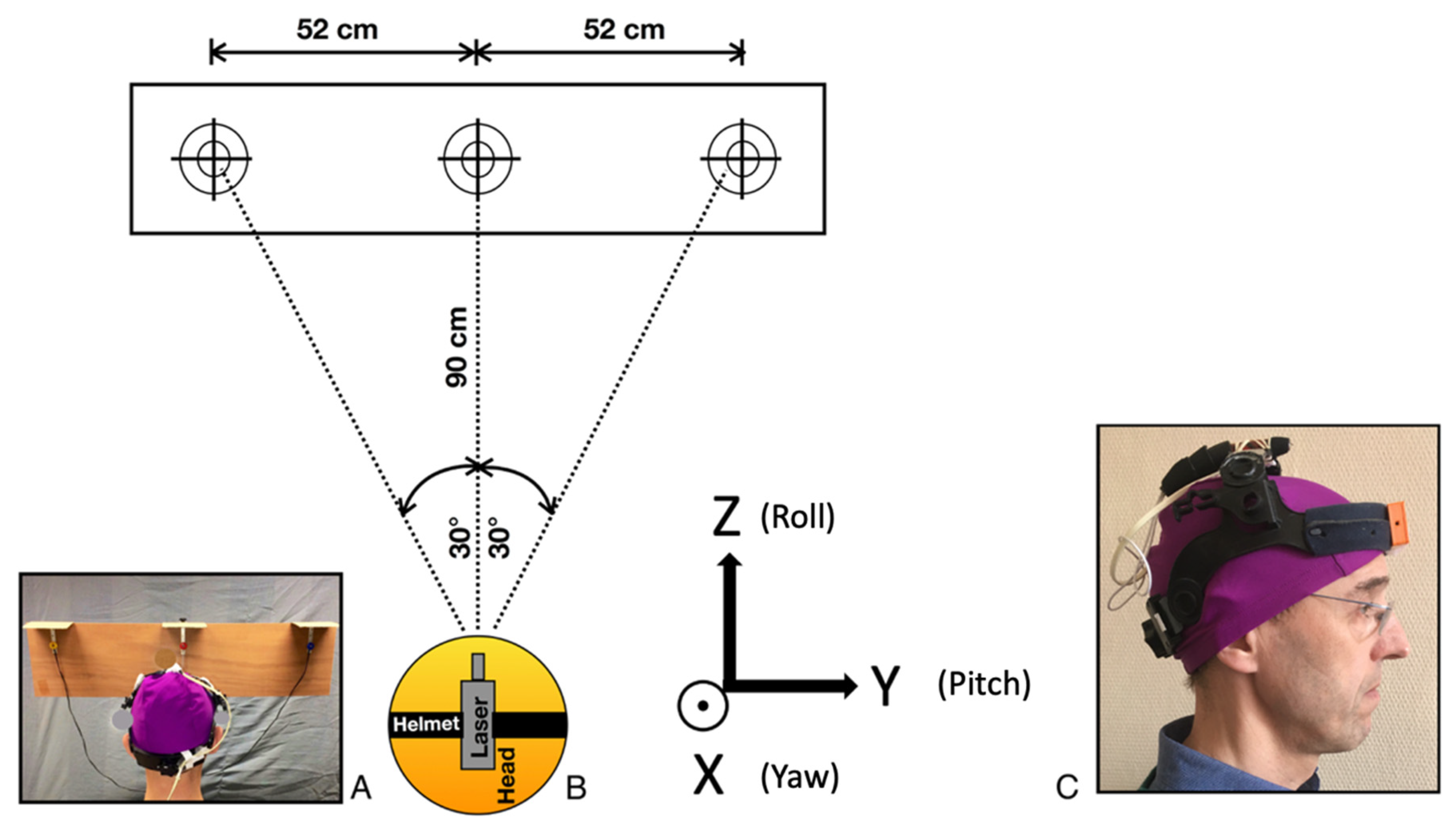
2.2. Protocol
2.3. Data Analysis
2.3.1. Dataset and Pre-Processing
2.3.2. ML Algorithms and Determination of the Best Performer
2.3.3. Determination of Most Informative Kinematic Features and Logistic Regressions
3. Results
3.1. Optimal Hyperparameters and Performance Metrics of ML Algorithms
3.2. Most Discriminative Features and Logistic Regressions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [Green Version]
- Hoy, D.G.; Protani, M.; De, R.; Buchbinder, R. The epidemiology of neck pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Kazeminasab, S.; Nejadghaderi, S.A.; Amiri, P.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Neck pain: Global epidemiology, trends and risk factors. BMC Musculoskelet. Disord. 2022, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Blanpied, P.R.; Gross, A.R.; Elliott, J.M.; Devaney, L.L.; Clewley, D.; Walton, D.M.; Sparks, C.; Robertson, E.K. Neck Pain: Revision 2017. J. Orthop. Sports Phys. Ther. 2017, 47, A1–A83. [Google Scholar] [CrossRef] [Green Version]
- Childs, J.D.; Cleland, J.A.; Elliott, J.M.; Teyhen, D.S.; Wainner, R.S.; Whitman, J.M.; Sopky, B.J.; Godges, J.J.; Flynn, T.W.; American Physical Therapy Association. Neck pain: Clinical practice guidelines linked to the International Classification of Functioning, Disability, and Health from the Orthopedic Section of the American Physical Therapy Association. J. Orthop. Sports Phys. Ther. 2008, 38, A1–A34. [Google Scholar] [CrossRef] [Green Version]
- Coulter, I.D.; Crawford, C.; Vernon, H.; Hurwitz, E.L.; Khorsan, R.; Booth, M.S.; Herman, P.M. Manipulation and Mobilization for Treating Chronic Nonspecific Neck Pain: A Systematic Review and Meta-Analysis for an Appropriateness Panel. Pain Physician 2019, 22, e55–e70. [Google Scholar] [CrossRef]
- Pool, J.J.; Ostelo, R.W.; Knol, D.; Bouter, L.M.; de Vet, H.C. Are psychological factors prognostic indicators of outcome in patients with sub-acute neck pain? Man Ther. 2010, 15, 111–116. [Google Scholar] [CrossRef] [Green Version]
- de Zoete, R.M.J.; Osmotherly, P.G.; Rivett, D.A.; Farrell, S.F.; Snodgrass, S.J. Sensorimotor Control in Individuals With Idiopathic Neck Pain and Healthy Individuals: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 1257–12571. [Google Scholar] [CrossRef] [Green Version]
- Sjolander, P.; Michaelson, P.; Jaric, S.; Djupsjobacka, M. Sensorimotor disturbances in chronic neck pain—Range of motion, peak velocity, smoothness of movement, and repositioning acuity. Man Ther. 2008, 13, 122–131. [Google Scholar] [CrossRef]
- Sarig Bahat, H.; Chen, X.; Reznik, D.; Kodesh, E.; Treleaven, J. Interactive cervical motion kinematics: Sensitivity, specificity and clinically significant values for identifying kinematic impairments in patients with chronic neck pain. Man Ther. 2015, 20, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Hage, R.; Detrembleur, C.; Dierick, F.; Brismée, J.M.; Roussel, N.; Pitance, L. Sensorimotor performance in acute-subacute non-specific neck pain: A non-randomized prospective clinical trial with intervention. BMC Musculoskelet. Disord. 2021, 22, 1017. [Google Scholar] [CrossRef] [PubMed]
- Roijezon, U.; Djupsjobacka, M.; Bjorklund, M.; Hager-Ross, C.; Grip, H.; Liebermann, D.G. Kinematics of fast cervical rotations in persons with chronic neck pain: A cross-sectional and reliability study. BMC Musculoskelet. Disord. 2010, 11, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarig Bahat, H.; Weiss, P.L.; Laufer, Y. The effect of neck pain on cervical kinematics, as assessed in a virtual environment. Arch. Phys. Med. Rehabil. 2010, 91, 1884–1890. [Google Scholar] [CrossRef]
- Bahat, H.S.; Igbariya, M.; Quek, J.; Treleaven, J. Cervical Kinematics of Fast Neck Motion across Age. J. Nov. Physiother. 2016, 6, 306. [Google Scholar] [CrossRef]
- Hage, R.; Dierick, F.; Roussel, N.; Pitance, L.; Detrembleur, C. Age-related kinematic performance should be considered during fast head-neck rotation target task in individuals aged from 8 to 85 years old. PeerJ 2019, 7, e7095. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Buisseret, F.; Pitance, L.; Brismee, J.M.; Detrembleur, C.; Dierick, F. Head-neck rotational movements using DidRen laser test indicate children and seniors’ lower performance. PLoS ONE 2019, 14, e0219515. [Google Scholar] [CrossRef]
- Hage, R.; Ancenay, E. Identification of a relationship between cervical spine function and rotational movement control. Ann. Phys. Rehabil. Med. 2009, 52, 653–667. [Google Scholar] [CrossRef] [Green Version]
- Falla, D.; Farina, D. Neural and muscular factors associated with motor impairment in neck pain. Curr. Rheumatol. Rep. 2007, 9, 497–502. [Google Scholar] [CrossRef]
- Falla, D.; Farina, D. Neuromuscular adaptation in experimental and clinical neck pain. J. Electromyogr. Kinesiol. 2008, 18, 255–261. [Google Scholar] [CrossRef]
- Treleaven, J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man Ther. 2008, 13, 2–11. [Google Scholar] [CrossRef]
- Kristjansson, E.; Treleaven, J. Sensorimotor function and dizziness in neck pain: Implications for assessment and management. J. Orthop. Sports Phys. Ther. 2009, 39, 364–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, A.N.; Kambhampati, C.; Monson, J.R.; Drew, P.J. Artificial intelligence in medicine. Ann. R. Coll. Surg. Engl. 2004, 86, 334–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, M.; Khanna, R. Machine Learning. Efficient Learning Machines: Theories, Concepts, and Applications for Engineers and System Designers; Apress: Berkeley, CA, USA, 2015; pp. 1–18. [Google Scholar]
- Uddin, S.; Khan, A.; Hossain, M.E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inform. Decis. Mak. 2019, 19, 281. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Kuppili, V.; Saba, L.; Edla, D.R.; Suri, H.S.; Cuadrado-Godia, E.; Laird, J.R.; Marinhoe, R.T.; Sanches, J.M.; Nicolaides, A.; et al. State-of-the-art review on deep learning in medical imaging. Front. Biosci. 2019, 24, 392–426. [Google Scholar]
- Kohli, M.; Prevedello, L.M.; Filice, R.W.; Geis, J.R. Implementing Machine Learning in Radiology Practice and Research. AJR Am. J. Roentgenol. 2017, 208, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Luk, K.D.; Cheung, J.P.Y.; Hu, Y. Prognosis of cervical myelopathy based on diffusion tensor imaging with artificial intelligence methods. NMR Biomed. 2019, 32, e4114. [Google Scholar] [CrossRef]
- Tack, C. Artificial intelligence and machine learning|applications in musculoskeletal physiotherapy. Musculoskelet. Sci. Pract. 2019, 39, 164–169. [Google Scholar] [CrossRef]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef]
- Cleland, J.A.; Childs, J.D.; Whitman, J.M. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef]
- Meisingset, I.; Stensdotter, A.K.; Woodhouse, A.; Vasseljen, O. Neck motion, motor control, pain and disability: A longitudinal study of associations in neck pain patients in physiotherapy treatment. Man Ther. 2016, 22, 94–100. [Google Scholar] [CrossRef]
- Meisingset, I.; Woodhouse, A.; Stensdotter, A.K.; Stavdahl, Ø.; Lorås, H.; Gismervik, S.; Andresen, H.; Austreim, K.; Vasseljen, O. Evidence for a general stiffening motor control pattern in neck pain: A cross sectional study. BMC Musculoskelet. Disord. 2015, 16, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaffi, F.; Stancati, A.; Silvestri, C.A.; Ciapetti, A.; Grassi, W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur. J. Pain 2004, 8, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Stewart, R.E.; Köke, A.J.; Oosterwijk, R.F.; Swaan, J.L.; Schreurs, K.M.; Schiphorst Preuper, H.R. Cut-Off Points for Mild, Moderate, and Severe Pain on the Numeric Rating Scale for Pain in Patients with Chronic Musculoskeletal Pain: Variability and Influence of Sex and Catastrophizing. Front. Psychol. 2016, 7, 1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, G.M.; Jull, G.; Thomas, K.; Smith, A.; Emery, C.; Faris, P.; Cook, C.; Frizzell, B.; Salo, P. Derivation of a clinical decision guide in the diagnosis of cervical facet joint pain. Arch. Phys. Med. Rehabil. 2014, 95, 1695–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hage, R.; Detrembleur, C.; Dierick, F.; Pitance, L.; Jojczyk, L.; Estievenart, W.; Buisseret, F. DYSKIMOT: An Ultra-Low-Cost Inertial Sensor to Assess Head’s Rotational Kinematics in Adults during the Didren-Laser Test. Sensors 2020, 20, 833. [Google Scholar] [CrossRef] [Green Version]
- Kononenko, I. Machine learning for medical diagnosis: History, state of the art and perspective. Artif. Intell. Med. 2001, 23, 89–109. [Google Scholar] [CrossRef] [Green Version]
- Berrar, D. Cross-Validation. Encycl. Bioinform. Comput. Biol. 2018, 1, 542–545. [Google Scholar] [CrossRef]
- Ndiaye, E.; Le, T.; Fercoq, O.; Salmon, J.; Takeuchi, I. Safe grid search with optimal complexity. In Proceedings of the 36th International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019. [Google Scholar]
- Kumar, V.M. Sonajharia. Feature Selection: A literature Review. Smart Comput. Rev. 2014, 4, 211–229. [Google Scholar] [CrossRef]
- Franov, E.; Straub, M.; Bauer, C.M.; Ernst, M.J. Head kinematics in patients with neck pain compared to asymptomatic controls: A systematic review. BMC Musculoskelet. Disord. 2022, 23, 156. [Google Scholar] [CrossRef]
- Fukuchi, R.K.; Eskofier, B.M.; Duarte, M.; Ferber, R. Support vector machines for detecting age-related changes in running kinematics. J. Biomech. 2011, 44, 540–542. [Google Scholar] [CrossRef]
- Lai, D.T.; Levinger, P.; Begg, R.K.; Gilleard, W.L.; Palaniswami, M. Automatic recognition of gait patterns exhibiting patellofemoral pain syndrome using a support vector machine approach. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Suda, E.Y.; Watari, R.; Matias, A.B.; Sacco, I.C.N. Recognition of Foot-Ankle Movement Patterns in Long-Distance Runners With Different Experience Levels Using Support Vector Machines. Front. Bioeng. Biotechnol. 2020, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sadeqi, A.; Miller, E.L.; Sonkusale, S. Head motion classification using thread-based sensor and machine learning algorithm. Sci. Rep. 2021, 11, 2646. [Google Scholar] [CrossRef] [PubMed]
- Jairam, V.; Park, H.S. Strengths and limitations of large databases in lung cancer radiation oncology research. Transl. Lung Cancer Res. 2019, 8 (Suppl. 2), S172–S183. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.E.; Shah, Y.; Ichesco, E.; Gerstner, G.E.; Peltier, S.J. Multivariate classification of pain-evoked brain activity in temporomandibular disorder. Pain Rep. 2016, 1, e572. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhou, C.; Wang, C.; Tsai, T.-Y.; Yu, Y.; Wang, W.; Li, G.; Cha, T. In vivo primary and coupled segmental motions of the healthy female head-neck complex during dynamic head axial rotation. J. Biomech. 2021, 123, 110513. [Google Scholar] [CrossRef] [PubMed]
- Moghaddas, D.; de Zoete, R.M.J.; Edwards, S.; Snodgrass, S.J. Differences in the kinematics of the cervical and thoracic spine during functional movement in individuals with or without chronic neck pain: A systematic review. Physiotherapy 2019, 105, 421–433. [Google Scholar] [CrossRef]
- Betsch, M.W.; Blizzard, S.R.; Shinseki, M.S.; Yoo, J.U. Prevalence of degenerative changes of the atlanto-axial joints. Spine J. 2015, 15, 275–280. [Google Scholar] [CrossRef]
- Pan, F.; Arshad, R.; Zander, T.; Reitmaier, S.; Schroll, A.; Schmidt, H. The effect of age and sex on the cervical range of motion—A systematic review and meta-analysis. J. Biomech. 2018, 75, 13–27. [Google Scholar] [CrossRef]
- Liew, B.X.W.; Rugamer, D.; De Nunzio, A.M.; Falla, D. Interpretable machine learning models for classifying low back pain status using functional physiological variables. Eur. Spine J. 2020, 29, 1845–1859. [Google Scholar] [CrossRef] [Green Version]
- Falla, D.; Devecchi, V.; Jiménez-Grande, D.; Rügamer, D.; Liew, B.X.W. Machine learning approaches applied in spinal pain research. J. Electromyogr. Kinesiol. 2021, 61, 102599. [Google Scholar] [CrossRef] [PubMed]
- NOMADe. DidRen VR. Available online: https://www.youtube.com/watch?v=Pqrty4Bj_5A&t=16s (accessed on 12 March 2022).

| ANSP Patients (n = 38) | HCP (n = 42) | p-Values | |
|---|---|---|---|
| Age (years), mean ± SD | 46.2 ± 16.3 | 24.3 ± 6.8 | <0.001 |
| Gender n (men/women), (%) | 21 (55%)/17 (45%) | 27 (64%)/15 (36%) | 0.55 |
| BMI (kg m−2), mean ± SD | 23.5 ± 3.2 | 21.5 ± 4.2 | 0.014 |
| NDI (100), median [Q1–Q3] | 22 [16–31.5] | 0 [0–0] | <0.001 |
| NPRS, median [Q1–Q3] | 6 [4–7] | 0 [0–0] | <0.001 |
| ML Algorithm | Hyperparameters |
|---|---|
| BF KNN | n_neighbors = 5, weights = “distance” |
| Linear SVM | kernel = “linear”, C = 10 |
| SVM RBF | gamma = 0.001, C = 100 |
| DT | max_depth = 1, criterion = “entropy”, splitter = “best” |
| RF | max_depth = 10, n_estimators = 100, max_features = 10 |
| ML Algorithm | Accuracy | AUC Score |
|---|---|---|
| BF KNN | 0.66 ± 0.03 | 0.51 ± 0.07 |
| Linear SVM | 0.82 ± 0.03 | 0.84 ± 0.04 |
| SVM RBF | 0.65 ± 0.05 | 0.57 ± 0.09 |
| DT | 0.74 ± 0.03 | 0.70 ± 0.04 |
| RF | 0.76 ± 0.03 | 0.76 ± 0.04 |
| AdaBoost | 0.75 ± 0.04 | 0.76 ± 0.05 |
| GaussianNB | 0.77 ± 0.03 | 0.82 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hage, R.; Buisseret, F.; Houry, M.; Dierick, F. Head Pitch Angular Velocity Discriminates (Sub-)Acute Neck Pain Patients and Controls Assessed with the DidRen Laser Test. Sensors 2022, 22, 2805. https://doi.org/10.3390/s22072805
Hage R, Buisseret F, Houry M, Dierick F. Head Pitch Angular Velocity Discriminates (Sub-)Acute Neck Pain Patients and Controls Assessed with the DidRen Laser Test. Sensors. 2022; 22(7):2805. https://doi.org/10.3390/s22072805
Chicago/Turabian StyleHage, Renaud, Fabien Buisseret, Martin Houry, and Frédéric Dierick. 2022. "Head Pitch Angular Velocity Discriminates (Sub-)Acute Neck Pain Patients and Controls Assessed with the DidRen Laser Test" Sensors 22, no. 7: 2805. https://doi.org/10.3390/s22072805
APA StyleHage, R., Buisseret, F., Houry, M., & Dierick, F. (2022). Head Pitch Angular Velocity Discriminates (Sub-)Acute Neck Pain Patients and Controls Assessed with the DidRen Laser Test. Sensors, 22(7), 2805. https://doi.org/10.3390/s22072805








