Towards Development of Specular Reflection Vascular Imaging
Abstract
:1. Introduction
1.1. Light-Tissue Interactions
1.2. Specular Reflection in Photoplethysmography
1.3. Motivation for Present Work
1.4. Specular Reflection Vascular Imaging
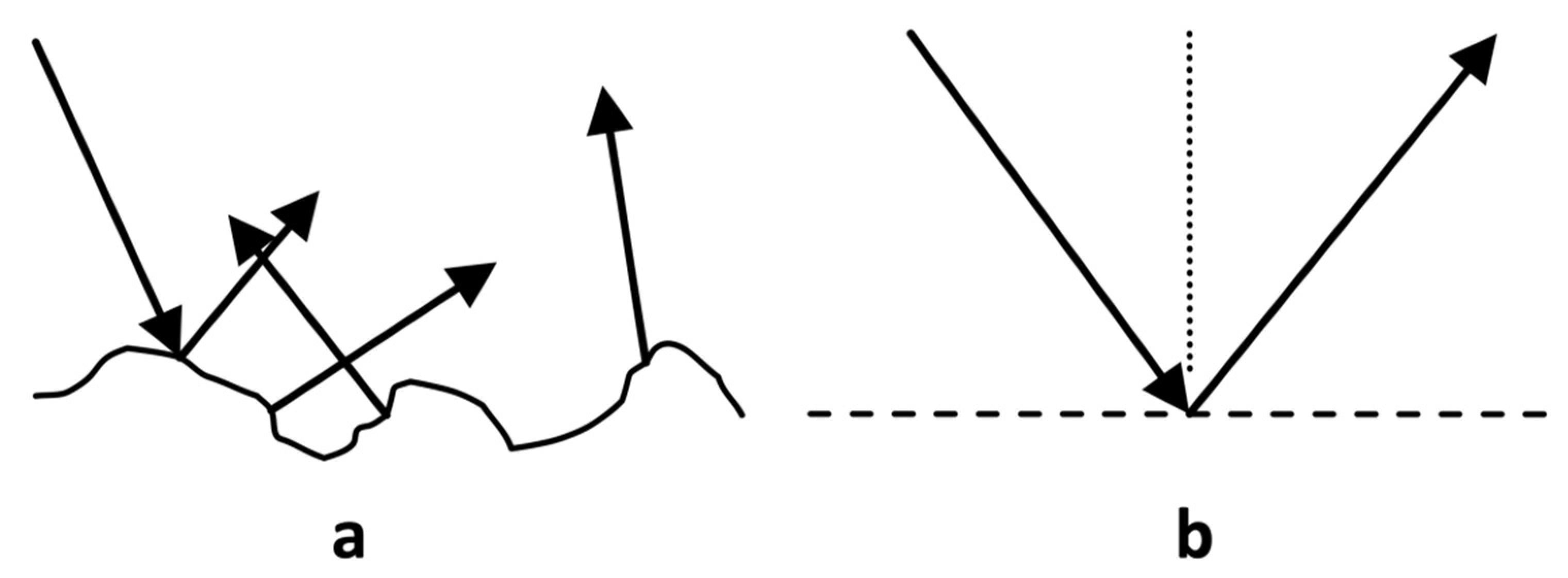
2. Theory
2.1. Specular Reflection
2.2. Model
- (1)
- The rays are always divergent, so the image is virtual and located on the opposite side of the mirror from the source.
- (2)
- If the source is located at distance P from the mirror, then the image is located at distance Q, which is given by the mirror formula (here all distances are positive)
- (3)
- The magnification of the image, m, is given by the following expression:
2.3. Translation from Theoretical to Experimental
3. Materials and Methods
3.1. Data Collection
3.2. Data Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heron, M.P. Deaths: Leading causes for 2017. Natl. Vital Stat. Rep. 2019, 68, 1–77. [Google Scholar] [PubMed]
- Azmal, G.M.; Al-Jumaily, A.; Al-Jaafreh, M. Continuous Measurement of Oxygen Saturation Level using Photoplethysmography Signal. In Proceedings of the 2006 International Conference on Biomedical and Pharmaceutical Engineering, Singapore, 11–14 December 2006; pp. 504–507. [Google Scholar]
- Katakami, N.; Osonoi, T.; Takahara, M.; Saitou, M.; Matsuoka, T.; Yamasaki, Y.; Shimomura, I. Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc. Diabetol. 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, C.; Kang, M.; Hong, J.H.; Yang, T.D.; Xing, J.; Yoo, H.; Choi, Y.; Choi, W. Removal of back-reflection noise at ultrathin imaging probes by the single-core illumination and wide-field detection. Sci. Rep. 2017, 7, 6524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Zeng, H.; Hamzavi, I.; McLean, D.I.; Lui, H. Rapid near-infrared Raman spectroscopy system for real-time in vivo skin measurements. Opt. Lett. 2001, 26, 1782–1784. [Google Scholar] [CrossRef]
- Kyriacou, P.A.; Allen, J. Photoplethysmography: Technology, Signal Analysis and Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Přibil, J.; Přibilová, A.; Frollo, I. Comparative Measurement of the PPG Signal on Different Human Body Positions by Sensors Working in Reflection and Transmission Modes. Eng. Proc. 2020, 2, 69. [Google Scholar] [CrossRef]
- Banik, P.P.; Hossain, S.; Kwon, T.-H.; Kim, H.; Kim, K.-D. Development of a Wearable Reflection-Type Pulse Oximeter System to Acquire Clean PPG Signals and Measure Pulse Rate and SpO2 with and without Finger Motion. Electronics 2020, 9, 1905. [Google Scholar] [CrossRef]
- Grubb, M.R.; Carpenter, J.; Crowe, J.A.; Teoh, J.; Marlow, N.; Ward, C.; Mann, C.; Sharkey, D.; Hayes-Gill, B.R. Forehead reflectance photoplethysmography to monitor heart rate: Preliminary results from neonatal patients. Physiol. Meas. 2014, 35, 881. [Google Scholar] [CrossRef]
- Mendelson, Y.; Ochs, B.D. Noninvasive pulse oximetry utilizing skin reflectance photoplethysmography. IEEE Trans. Biomed. Eng. 1988, 35, 798–805. [Google Scholar] [CrossRef]
- Douplik, A.; Saiko, G.; Schelkanova, I.; Tuchin, V. The response of tissue to laser light. In Lasers for Medical Applications. Diagnostics, Therapy and Surger; Jelinkova, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 47–109. [Google Scholar]
- Tan, K.; Cheng, X. Specular Reflection Effects Elimination in Terrestrial Laser Scanning Intensity Data Using Phong Model. Remote Sens. 2017, 9, 853. [Google Scholar] [CrossRef] [Green Version]
- Tan, R.T.; Nishino, K.; Ikeuchi, K. Separating reflection components based on chromaticity and noise analysis. IEEE Trans. Pattern Anal. Mach. Intell. 2004, 26, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- Leahy, M.J.; Enfield, J.G.; Clancy, N.T.; O’Doherty, J.; McNamara, P.; Nilsson, G.E. Biophotonic methods in microcirculation imaging. Med. Laser Appl. 2007, 22, 105–126. [Google Scholar] [CrossRef]
- García-López, I.; Rodriguez-Villegas, E. Extracting the jugular venous pulse from anterior neck contact photoplethysmography. Sci. Rep. 2020, 10, 3466. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; O’Gara, P. The history and physical examination: An evidence-based approach. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 10th ed.; Mann, D., Zipes, D., Libby, P., Bonow, R., Braunwald, E., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Applefeld, M. The Jugular Venous Pressure and Pulse Contour. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H., Hall, W., Hurst, J., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Moço, A.V.; Stuijk, S.; de Haan, G. New insights into the origin of remote PPG signals in visible light and infrared. Sci. Rep. 2018, 8, 8501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjoding, M.W.; Dickson, R.P.; Iwashyna, T.J.; Gay, S.E.; Valley, T.S. Racial bias in pulse oximetry measurement. N. Engl. J. Med. 2020, 383, 2477–2478. [Google Scholar] [CrossRef]
- Lê Cook, B.; McGuire, T.G.; Zuvekas, S.H. Measuring trends in racial/ethnic health care disparities. Med. Care Res. Rev. 2009, 66, 23–48. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Shan, C. Impact of makeup on remote-ppg monitoring. Biomed. Phys. Eng. Express 2020, 6, 35004. [Google Scholar] [CrossRef]
- Kamshilin, A.A.; Nippolainen, E.; Sidorov, I.S.; Vasilev, P.V.; Erofeev, N.P.; Podolian, N.P.; Romashko, R.V. A new look at the essence of the imaging photoplethysmography. Sci. Rep. 2015, 5, 10494. [Google Scholar] [CrossRef] [Green Version]
- Lu, R. Light Scattering Technology for Food Property, Quality and Safety Assessment; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1482263351. [Google Scholar]
- Baena, C.P.; Lotufo, P.A.; Fonseca, M.G.M.; Santos, I.S.; Goulart, A.C.; Bensenor, I.M.J. Neck circumference is independently associated with cardiometabolic risk factors: Cross-sectional analysis from ELSA-Brasil. Metab. Syndr. Relat. Disord. 2016, 14, 145–153. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Qamar, A.; Imram, M.; Fahim, M.F. Comparison of neck length, relative neck length and height with incidence of cervical spondylosis. Pak. J. Med. Sci. 2020, 36, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Burrows, J.W.W. Derivation of the mirror equation. Am. J. Phys. 1986, 54, 432–434. [Google Scholar] [CrossRef]
- Lim, C.L.; Keshava, S.N.; Lea, M. Anatomical variations of the internal jugular veins and their relationship to the carotid arteries: A CT evaluation. Australas. Radiol. 2006, 50, 314–318. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, H.; Mi, W.-D. Anatomic relationship of the internal jugular vein and the common carotid artery in Chinese people. Chin. Med. J. 2010, 123, 3226–3230. [Google Scholar] [PubMed]
- Buades, A.; Coll, B.; Morel, J.-M. A review of image denoising algorithms, with a new one. Multiscale Model. Simul. 2005, 4, 490–530. [Google Scholar] [CrossRef]
- Troy, T.L.; Thennadil, S.N. Optical properties of human skin in the near infrared wavelength range of 1000 to 2200 nm. J. Biomed. Opt. 2001, 6, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, A.A. Baseline drift filtering for an arterial pulse signal. Meas. Tech. 2014, 57, 91–96. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, B. Comparison of different approaches for removal of baseline wander from ECG signal. In Proceedings of the International Conference & Workshop on Emerging Trends in Technology, Mumbai, India, 25–26 February 2011; pp. 1290–1294. [Google Scholar]
- Liu, S.; Yuen, P.C.; Zhang, S.; Zhao, G. 3D mask face anti-spoofing with remote photoplethysmography. In Proceedings of the European Conference on Computer Vision, Amsterdam, The Netherlands, 11–14 October 2016; Springer: Berlin/Heidelberg, Germany, 2016; pp. 85–100. [Google Scholar]
- Van Luijtelaar, R.; Wang, W.; Stuijk, S.; de Haan, G. Automatic roi detection for camera-based pulse-rate measurement. In Proceedings of the Asian Conference on Computer Vision, Singapore, 1–5 November 2014; Springer: Berlin/Heidelberg, Germany, 2014; pp. 360–374. [Google Scholar]
- Lee, C.H.; Xiao, H.B.; Gibson, D.G. Jugular venous’a’wave in dilated cardiomyopathy: Sign of abbreviated right ventricular filling time. Heart 1991, 65, 342–345. [Google Scholar] [CrossRef]
- Briers, D.; Duncan, D.D.; Hirst, E.R.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser speckle contrast imaging: Theoretical and practical limitations. J. Biomed. Opt. 2013, 18, 66018. [Google Scholar] [CrossRef] [Green Version]
- Iredahl, F.; Löfberg, A.; Sjöberg, F.; Farnebo, S.; Tesselaar, E. Non-invasive measurement of skin microvascular response during pharmacological and physiological provocations. PLoS ONE 2015, 10, e0133760. [Google Scholar] [CrossRef]
- Saiko, G.; Burton, T.; Douplik, A. Feasibility of Specular Reflection Imaging for Extraction of Neck Vessel Pressure Waveforms. Front. Bioeng. Biotechnol. 2022, 10, 830231. [Google Scholar] [CrossRef]






| Output | Description |
|---|---|
| Local SRVIs | SRVIs measured in 5 × 5 pixels area |
| Global SRVI | SRVI measured across all pixels in the ROI |
| Correlation False Color Map | Pearson correlation of each local SRVI to the global SVRI, shown as a false color map overlaid on the first frame |
| Spatial Clustering-Derived Sub-regions | Sub-regions within the ROI that exhibit internal high correlation and low correlation across sub-regions. The SRVIs are averaged within the sub-regions to generate representative signals. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burton, T.; Saiko, G.; Douplik, A. Towards Development of Specular Reflection Vascular Imaging. Sensors 2022, 22, 2830. https://doi.org/10.3390/s22082830
Burton T, Saiko G, Douplik A. Towards Development of Specular Reflection Vascular Imaging. Sensors. 2022; 22(8):2830. https://doi.org/10.3390/s22082830
Chicago/Turabian StyleBurton, Timothy, Gennadi Saiko, and Alexandre Douplik. 2022. "Towards Development of Specular Reflection Vascular Imaging" Sensors 22, no. 8: 2830. https://doi.org/10.3390/s22082830
APA StyleBurton, T., Saiko, G., & Douplik, A. (2022). Towards Development of Specular Reflection Vascular Imaging. Sensors, 22(8), 2830. https://doi.org/10.3390/s22082830








