The Size Dependence of Microwave Permeability of Hollow Iron Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ultrasonic Spray Pyrolysis and Reduction with Hydrogen
2.2. Characterization
3. Results and Discussion
3.1. X-ray Difraction Analysis
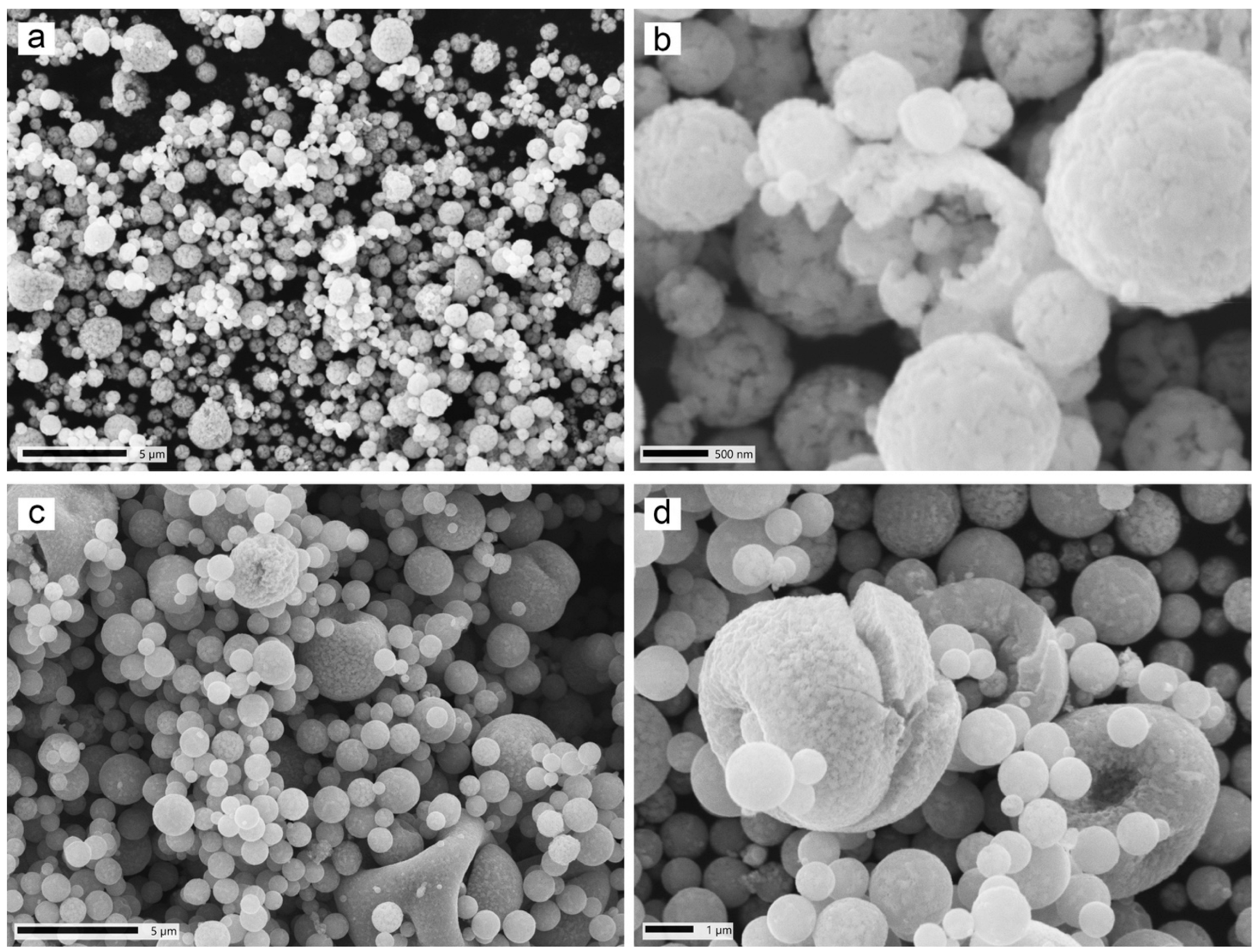
3.2. SEM and EDX Analyses
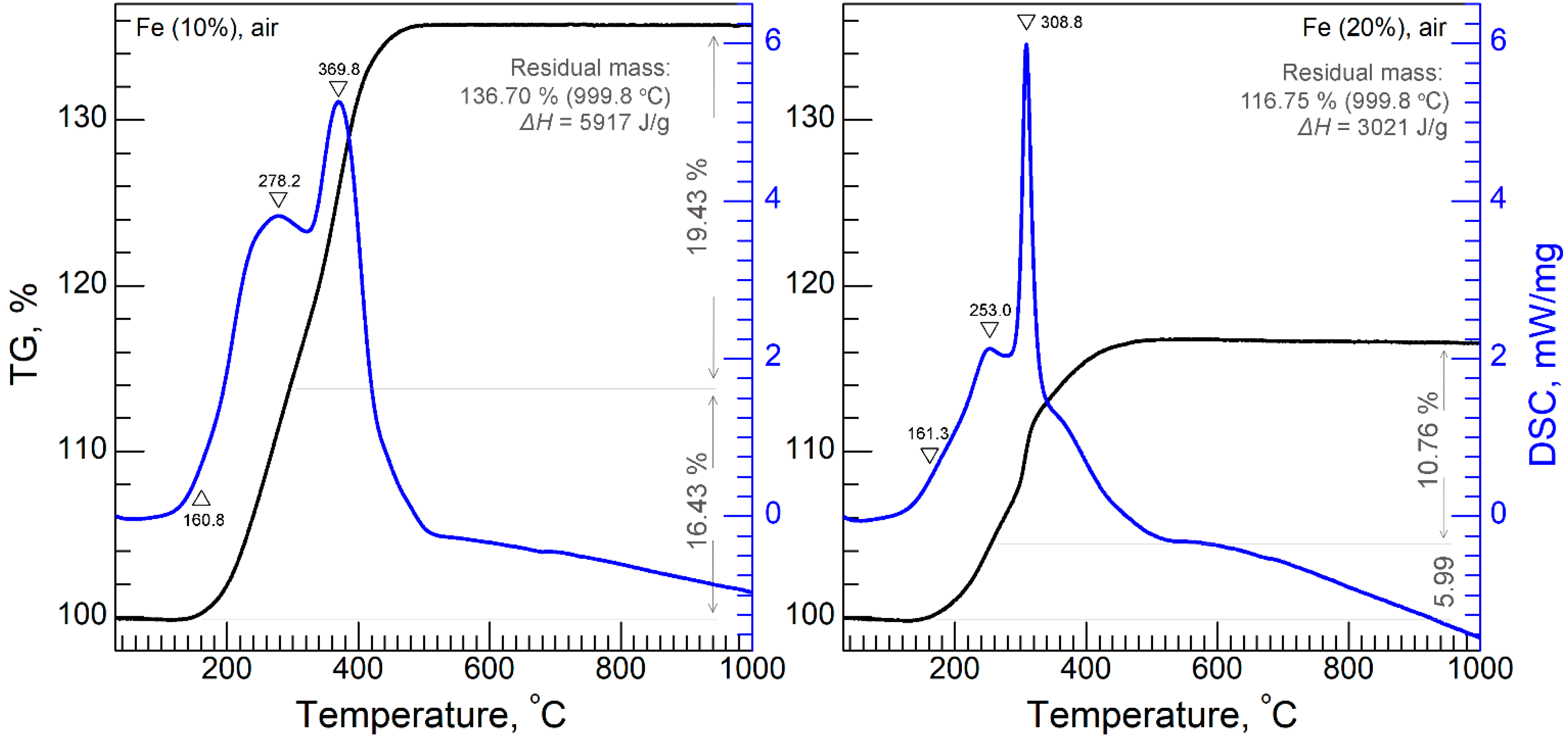
3.3. STA Analysis
3.4. Magnetic Properties
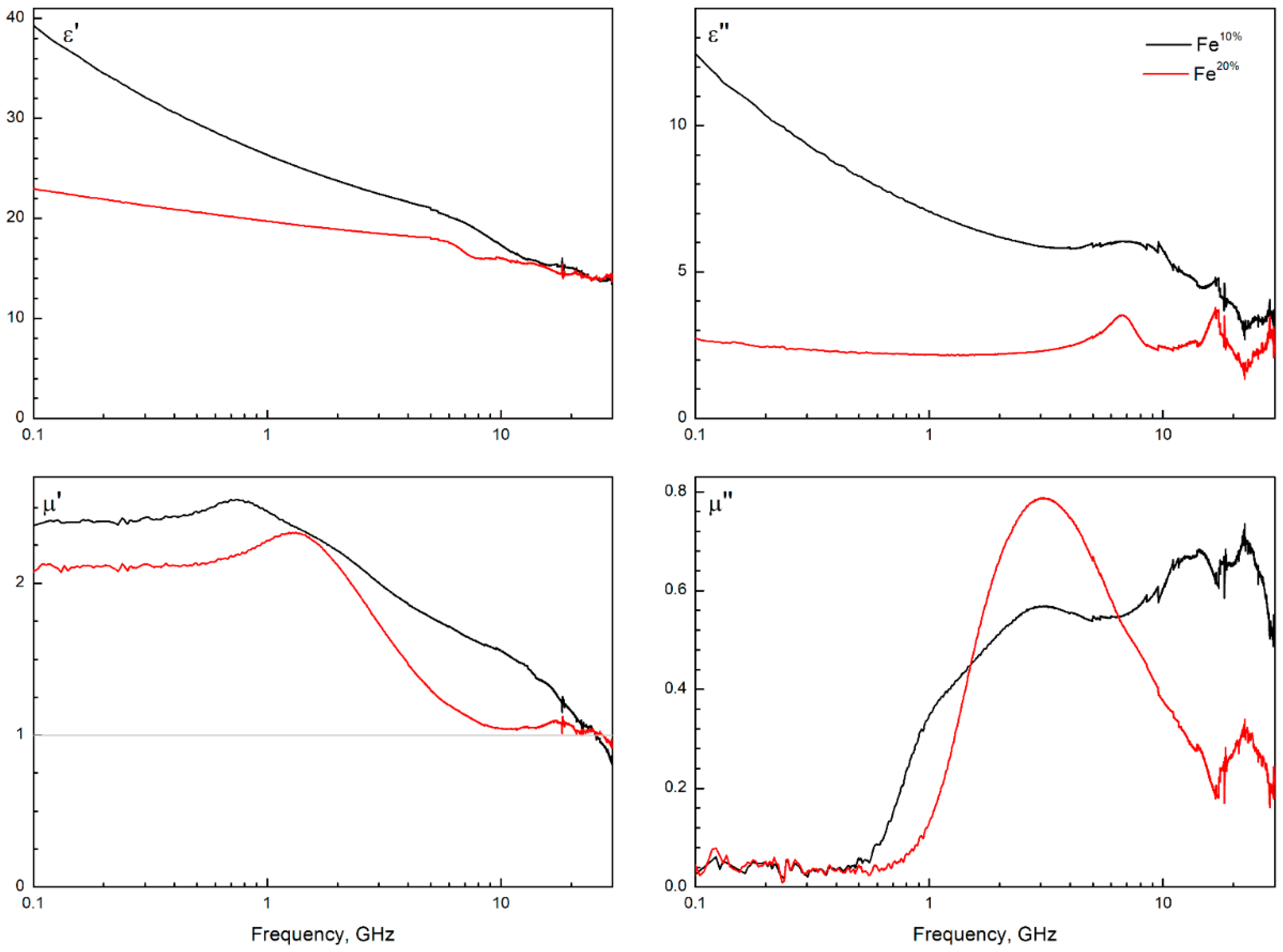
3.5. Microwave Measurement of Composites
3.6. Hollow or Not
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Maklakov, S.S.; Lagarkov, A.N.; Maklakov, S.A.; Adamovich, Y.A.; Petrov, D.A.; Rozanov, K.N.; Ryzhikov, I.A.; Zarubina, A.Y.; Pokholok, K.V.; Filimonov, D.S. Corrosion-resistive magnetic powder Fe@SiO2 for microwave applications. J. Alloys Compd. 2017, 706, 267–273. [Google Scholar] [CrossRef]
- Dolmatov, A.V.; Maklakov, S.S.; Zezyulina, P.A.; Osipov, A.V.; Petrov, D.A.; Naboko, A.S.; Polozov, V.I.; Maklakov, S.A.; Starostenko, S.N.; Lagarkov, A.N. Deposition of a SiO2 Shell of Variable Thickness and Chemical Composition to Carbonyl Iron: Synthesis and Microwave Measurements. Sensors 2021, 21, 4624. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, E.; Donten, M.; Kowalczyk, A.; Bystrzejewski, M.; Grudzinski, I.P.; Nowicka, A.M. A novel type of electrochemical sensor based on ferromagnetic carbon-encapsulated iron nanoparticles for direct determination of hemoglobin in blood samples. Biosens. Bioelectron. 2015, 64, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Mistlberger, G.; Borisov, S.M.; Klimant, I. Ferromagnetic and permanent magnetic spheres as platform for single- and dual-analyte optical sensors. Procedia Eng. 2010, 5, 997–1000. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Gatel, C.; Li, Z.-A.; Richter, G. Synthesis of magnetic Fe and Co nano-whiskers and platelets via physical vapor deposition. Mater. Des. 2021, 208, 109914. [Google Scholar] [CrossRef]
- Söderberg, O.; Ge, Y.; Haimi, E.; Heczko, O.; Oja, M.; Laine, J.; Suhonen, T.; Aaltonen, A.; Kalliokari, K.; Borak, B.; et al. Morphology of ferromagnetic sol—gel submicron silica powders doped with iron and nickel particles. Mater. Lett. 2007, 61, 3171–3173. [Google Scholar] [CrossRef]
- Ulbrich, K.F.; Campos, C.E.M. Nanocrystalline Ni3S2 prepared by mechanochemistry and its behavior at high temperatures and high pressure. J. Magn. Magn. Mater. 2020, 493, 165706. [Google Scholar] [CrossRef]
- Gurmen, S.; Ebin, B.; Stopić, S.; Friedrich, B. Nanocrystalline spherical iron–nickel (Fe–Ni) alloy particles prepared by ultrasonic spray pyrolysis and hydrogen reduction (USP-HR). J. Alloys Compd. 2009, 480, 529–533. [Google Scholar] [CrossRef]
- Gurmen, S.; Guven, A.; Ebin, B.; Stopić, S.; Friedrich, B. Synthesis of nano-crystalline spherical cobalt–iron (Co–Fe) alloy particles by ultrasonic spray pyrolysis and hydrogen reduction. J. Alloys Compd. 2009, 481, 600–604. [Google Scholar] [CrossRef]
- Kastrinaki, G.; Lorentzou, S.; Karagiannakis, G.; Rattenbury, M.; Woodhead, J.; Konstandopoulos, A.G. Parametric synthesis study of iron based nanoparticles via aerosol spray pyrolysis route. J. Aerosol Sci. 2018, 115, 96–107. [Google Scholar] [CrossRef]
- Krasnikova, I.V.; Mishakov, I.V.; Bauman, Y.I.; Karnaukhov, T.M.; Vedyagin, A.A. Preparation of NiO-CuO-MgO fine powders by ultrasonic spray pyrolysis for carbon nanofibers synthesis. Chem. Phys. Lett. 2017, 684, 36–38. [Google Scholar] [CrossRef]
- Yudin, A.; Shatrova, N.; Khaydarov, B.; Kuznetsov, D.; Dzidziguri, E.; Issi, J.-P. Synthesis of hollow nanostructured nickel oxide microspheres by ultrasonic spray atomization. J. Aerosol Sci. 2016, 98, 30–40. [Google Scholar] [CrossRef]
- Tischendorf, R.; Simmler, M.; Weinberger, C.; Bieber, M.; Reddemann, M.; Fröde, F.; Lindner, J.; Pitsch, H.; Kneer, R.; Tiemann, M.; et al. Examination of the evolution of iron oxide nanoparticles in flame spray pyrolysis by tailored in situ particle sampling techniques. J. Aerosol Sci. 2021, 154, 105722. [Google Scholar] [CrossRef]
- Oh, S.W.; Bang, H.J.; Bae, Y.C.; Sun, Y.-K. Effect of calcination temperature on morphology, crystallinity and electrochemical properties of nano-crystalline metal oxides (Co3O4, CuO, and NiO) prepared via ultrasonic spray pyrolysis. J. Power Sources 2007, 173, 502–509. [Google Scholar] [CrossRef]
- Shatrova, N.; Yudin, A.; Levina, V.; Kuznetsov, D.; Novakova, A.; Dzidziguri, E.; Perov, N.; Issi, J.-P. Characteristics of Co3O4 and cobalt nanostructured microspheres: Morphology, structure, reduction process, and magnetic properties. Mater. Res. Bull. 2018, 99, 189–195. [Google Scholar] [CrossRef]
- Septiani, E.L.; Kikkawa, J.; Cao, K.L.A.; Hirano, T.; Okuda, N.; Matsumoto, H.; Enokido, Y.; Ogi, T. Direct synthesis of submicron FeNi particles via spray pyrolysis using various reduction agents. Adv. Powder Technol. 2021, 32, 4263–4272. [Google Scholar] [CrossRef]
- Kosevich, A.; Petrusevich, E.; Maklakov, S.; Naboko, A.; Kolesnikov, E.; Petrov, D.; Zezyulina, P.; Pokholok, K.; Filimonov, D.; Han, M. Low Weight Hollow Microspheres of Iron with Thin Dielectric Coating: Synthesis and Microwave Permeability. Coatings 2020, 10, 995. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Akl, A.A. Microstructure and electrical properties of iron oxide thin films deposited by spray pyrolysis. Appl. Surf. Sci. 2004, 221, 319–329. [Google Scholar] [CrossRef]
- Dghoughi, L.; Elidrissi, B.; Bernède, C.; Addou, M.; Lamrani, M.A.; Regragui, M.; Erguig, H. Physico-chemical, optical and electrochemical properties of iron oxide thin films prepared by spray pyrolysis. Appl. Surf. Sci. 2006, 253, 1823–1829. [Google Scholar] [CrossRef]
- Yu, F.; Wang, J.; Sheng, Z.; Su, L. Synthesis of carbon-encapsulated magnetic nanoparticles by spray pyrolysis of iron carbonyl and ethanol. Carbon 2005, 43, 3018–3021. [Google Scholar] [CrossRef]
- Han, S.; Yoo, K.S.; Kim, D.; Kim, J.; Othman, M.R. Metal-silica spherical particles development by spray pyrolysis: Effect of metal species on surface area and toluene adsorption. J. Anal. Appl. Pyrolysis 2021, 156, 105049. [Google Scholar] [CrossRef]
- McKeever, C.; Ogrin, F.Y.; Aziz, M.M. Microwave magnetization dynamics in ferromagnetic spherical nanoshells. Phys. Rev. B 2019, 100, 054425. [Google Scholar] [CrossRef]
- Li, Z.W.; Yang, Z.H. Microwave absorption properties and mechanism for hollow Fe3O4 nanosphere composites. J. Magn. Magn. Mater. 2015, 387, 131–138. [Google Scholar] [CrossRef]
- Chen, G.; Xu, D.; Chen, P.; Guo, X.; Yu, Q.; Qiu, H. Constructing and optimizing hollow bird-nest-patterned C@Fe3O4 composites as high-performance microwave absorbers. J. Magn. Magn. Mater. 2021, 532, 167990. [Google Scholar] [CrossRef]
- Nicolson, A.M.; Ross, G.F. Measurement of the Intrinsic Properties of Materials by Time-Domain Techniques. IEEE Trans. Instrum. Meas. 1970, 19, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Weir, W.B. Automatic measurement of complex dielectric constant and permeability at microwave frequencies. Proc. IEEE 1974, 62, 33–36. [Google Scholar] [CrossRef]
- Petrov, D.A.; Rozanov, K.N.; Koledintseva, M.Y. Influence of Higher-order Modes in Coaxial Waveguide on Measurements of Material Parameters. In Proceedings of the 2018 IEEE Symposium on Electromagnetic Compatibility, Signal Integrity and Power Integrity (EMC, SI & PI), Long Beach, CA, USA, 30 July–3 August 2018; pp. 66–70. [Google Scholar]
- Lindsley, D.H. Chapter 1. The CRYSTAL CHEMISTRY and STRUCTURE of OXIDE MINERALS as EXEMPLIFIED by the Fe-Ti OXIDES. In Oxide Minerals; Rumble, D., Ed.; De Gruyter: Berlin, Germany, 1976; 60p. [Google Scholar]
- Jeon, S.-H.; Son, Y.-H.; Choi, W.-I.; Song, G.; Hur, D. Simulating Porous Magnetite Layer Deposited on Alloy 690TT Steam Generator Tubes. Materials 2018, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Snyder, R.L. RIR-Measurement and Use in Quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Shatrova, N.; Yudin, A.; Levina, V.; Dzidziguri, E.; Kuznetsov, D.; Perov, N.; Issi, J.-P. Elaboration, characterization and magnetic properties of cobalt nanoparticles synthesized by ultrasonic spray pyrolysis followed by hydrogen reduction. Mater. Res. Bull. 2017, 86, 80–87. [Google Scholar] [CrossRef]
- Wen, D.; Song, P.; Zhang, K.; Qian, J. Thermal oxidation of iron nanoparticles and its implication for chemical-looping combustion. J. Chem. Technol. Biotechnol. 2011, 86, 375–380. [Google Scholar] [CrossRef]
- Huang, D.H.; Tran, T.N.; Yang, B. Investigation on the reaction of iron powder mixture as a portable heat source for thermoelectric power generators. J. Therm. Anal. Calorim. 2014, 116, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Mrlík, M.; Ilčíková, M.; Cvek, M.; Pavlínek, V.; Zahoranová, A.; Kroneková, Z.; Kasak, P. Carbonyl iron coated with a sulfobetaine moiety as a biocompatible system and the magnetorheological performance of its silicone oil suspensions. RSC Adv. 2016, 6, 32823–32830. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of Magnetite (Fe3O4) Oxidation to Hematite (Fe2O3) in Air for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2014, 53, 13320–13328. [Google Scholar] [CrossRef]
- Chung, K.H.; Wu, C.S.; Malawer, E.G. Thermomagnetometry and thermogravimetric analysis of carbonyl iron powder. Thermochim. Acta 1989, 154, 195–204. [Google Scholar] [CrossRef]
- Somunkiran, I.; Buytoz, S.; Dagdelen, F. Determination of curie temperatures and thermal oxidation behavior of Fe-Cr matrix composites produced by hot pressing. J. Alloys Compd. 2019, 777, 302–308. [Google Scholar] [CrossRef]
- Velasco, S.; Román, F.L. Determining the Curie Temperature of Iron and Nickel. Phys. Teach. 2007, 45, 387–389. [Google Scholar] [CrossRef]
- Manohar, A.; Krishnamoorthi, C. Low Curie-transition temperature and superparamagnetic nature of Fe3O4 nanoparticles prepared by colloidal nanocrystal synthesis. Mater. Chem. Phys. 2017, 192, 235–243. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kwon, S.H.; Liu, Y.D.; Lee, J.-S.; You, C.-Y.; Choi, H.J. Core–shell-structured cross-linked poly(glycidyl methacrylate)-coated carbonyl iron microspheres and their magnetorheology. J. Mater. Sci. 2014, 49, 1345–1352. [Google Scholar] [CrossRef]
- Bombard, A.J.F.; Joekes, I.; Alcântara, M.R.; Knobel, M. Magnetic Susceptibility and Saturation Magnetization of some Carbonyl Iron Powders used in Magnetorheological Fluids. Mater. Sci. Forum 2003, 416–418, 753. [Google Scholar] [CrossRef]
- Tuo, Y.; Liu, G.; Dong, B.; Zhou, J.; Wang, A.; Wang, J.; Jin, R.; Lv, H.; Dou, Z.; Huang, W. Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds. Sci. Rep. 2015, 5, 13515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenenko, V.N.; Chistyaev, V.A.; Politiko, A.A.; Kibets, S.G.; Kisel, V.N.; Gallagher, C.P.; McKeever, C.; Hibbins, A.P.; Ogrin, F.Y.; Sambles, J.R. Complex Permittivity and Permeability of Composite Materials Based on Carbonyl Iron Powder Over an Ultrawide Frequency Band. Phys. Rev. Appl. 2021, 16, 014062. [Google Scholar] [CrossRef]
- Iakubov, I.T.; Lagarkov, A.N.; Maklakov, S.A.; Osipov, A.V.; Rozanov, K.N.; Ryzhikov, I.A.; Starostenko, S.N. Microwave permeability of composites filled with thin Fe films. J. Magn. Magn. Mater. 2006, 300, e74–e77. [Google Scholar] [CrossRef]
- Rozanov, K.N.; Koledintseva, M.Y. Application of generalized Snoek’s law over a finite frequency range: A case study. J. Appl. Phys. 2016, 119, 073901. [Google Scholar] [CrossRef]
- McKeever, C.; Ogrin, F.Y.; Aziz, M.M. Influence of surface anisotropy on exchange resonance modes in spherical shells. J. Phys. D Appl. Phys. 2018, 51, 305003. [Google Scholar] [CrossRef] [Green Version]

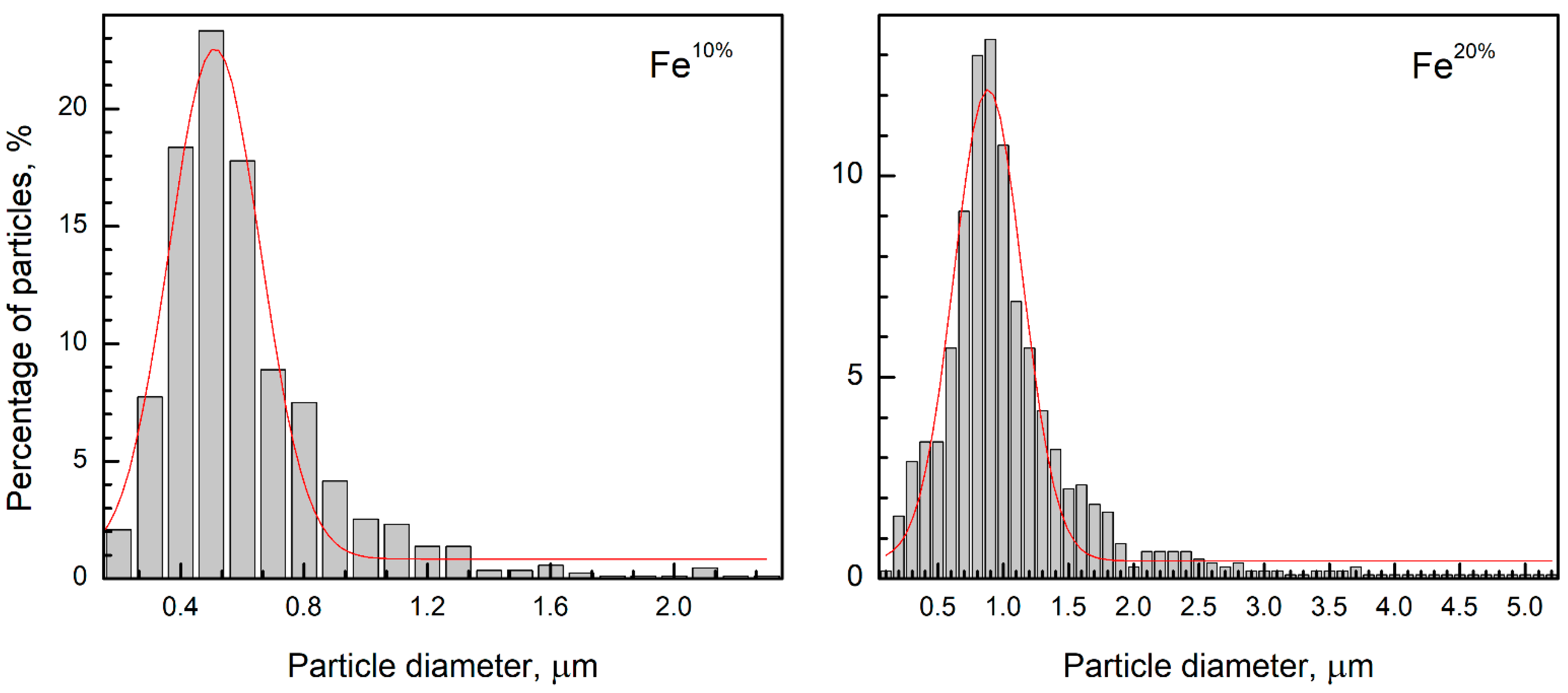
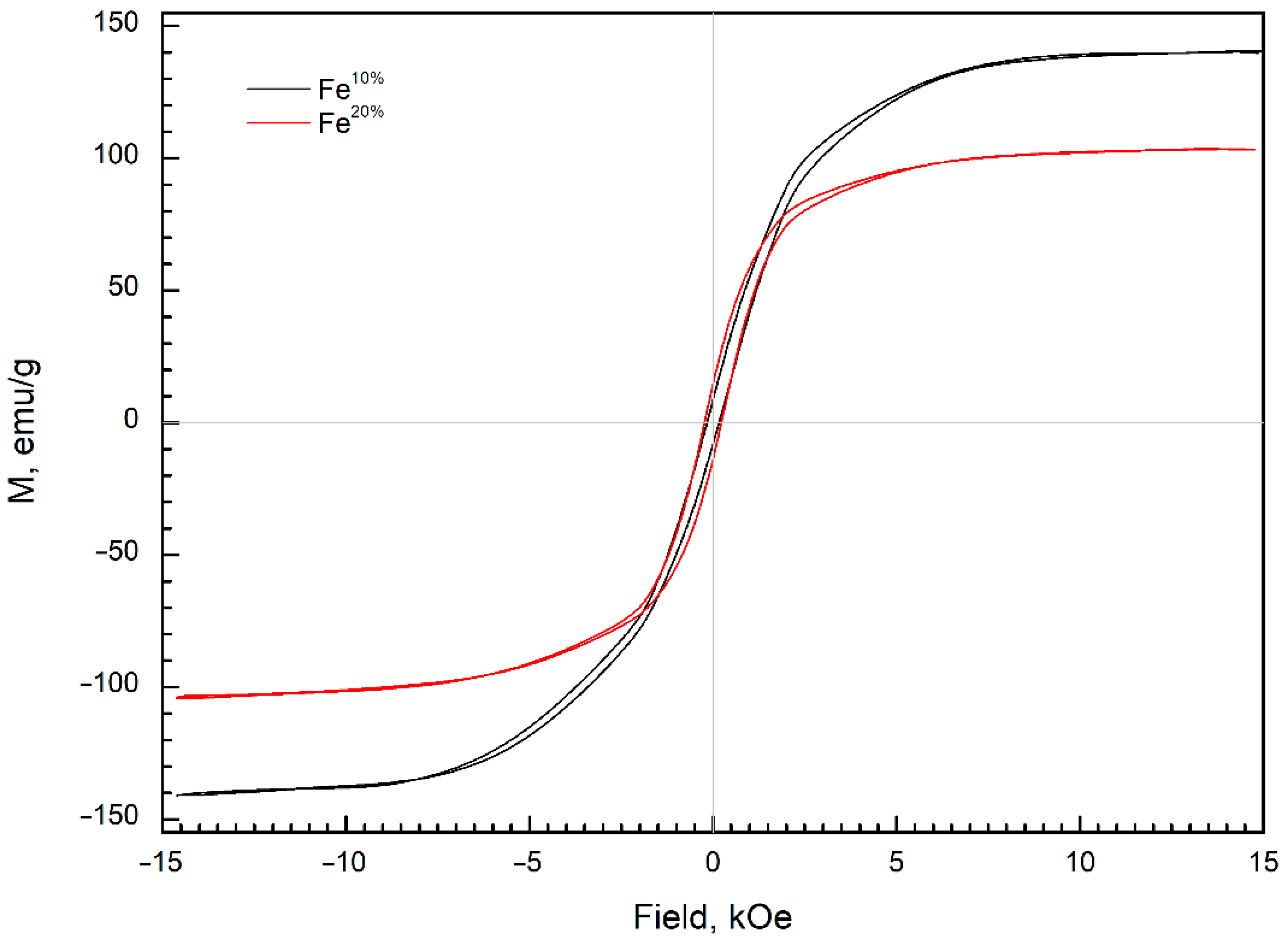
| Sample | Phases | a, Å | CS, nm | ωFe, wt.% | ωoxide, wt.% | Ms, emu/g | Hc, Oe |
|---|---|---|---|---|---|---|---|
| Fe10% | α-Fe (#60696) | 2866 | 37 | 95 | 5 | 140 | 160 |
| Fe3O4 (#11111) | 8371 | 23 | |||||
| Fe20% | α-Fe (#60696) | 2866 | 29 | 30 | 70 | 103 | 225 |
| Fe3O4 (#11111) | 8414 | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artemova, A.V.; Maklakov, S.S.; Osipov, A.V.; Petrov, D.A.; Shiryaev, A.O.; Rozanov, K.N.; Lagarkov, A.N. The Size Dependence of Microwave Permeability of Hollow Iron Particles. Sensors 2022, 22, 3086. https://doi.org/10.3390/s22083086
Artemova AV, Maklakov SS, Osipov AV, Petrov DA, Shiryaev AO, Rozanov KN, Lagarkov AN. The Size Dependence of Microwave Permeability of Hollow Iron Particles. Sensors. 2022; 22(8):3086. https://doi.org/10.3390/s22083086
Chicago/Turabian StyleArtemova, Anastasia V., Sergey S. Maklakov, Alexey V. Osipov, Dmitriy A. Petrov, Artem O. Shiryaev, Konstantin N. Rozanov, and Andrey N. Lagarkov. 2022. "The Size Dependence of Microwave Permeability of Hollow Iron Particles" Sensors 22, no. 8: 3086. https://doi.org/10.3390/s22083086
APA StyleArtemova, A. V., Maklakov, S. S., Osipov, A. V., Petrov, D. A., Shiryaev, A. O., Rozanov, K. N., & Lagarkov, A. N. (2022). The Size Dependence of Microwave Permeability of Hollow Iron Particles. Sensors, 22(8), 3086. https://doi.org/10.3390/s22083086







