Paper-Strip-Based Sensors for H2S Detection: A Proof-of-Principle Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. General
3. Results and Discussion
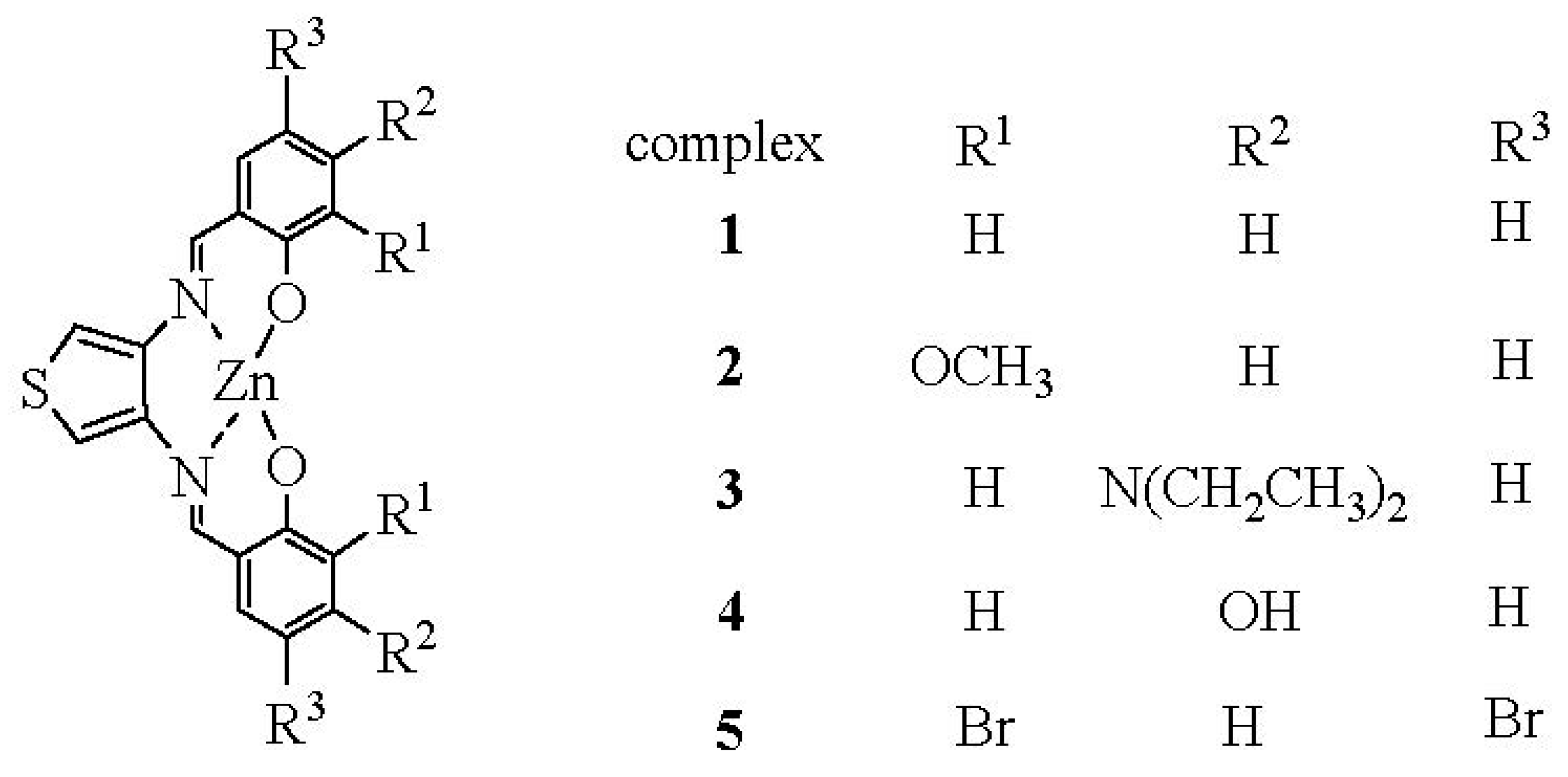
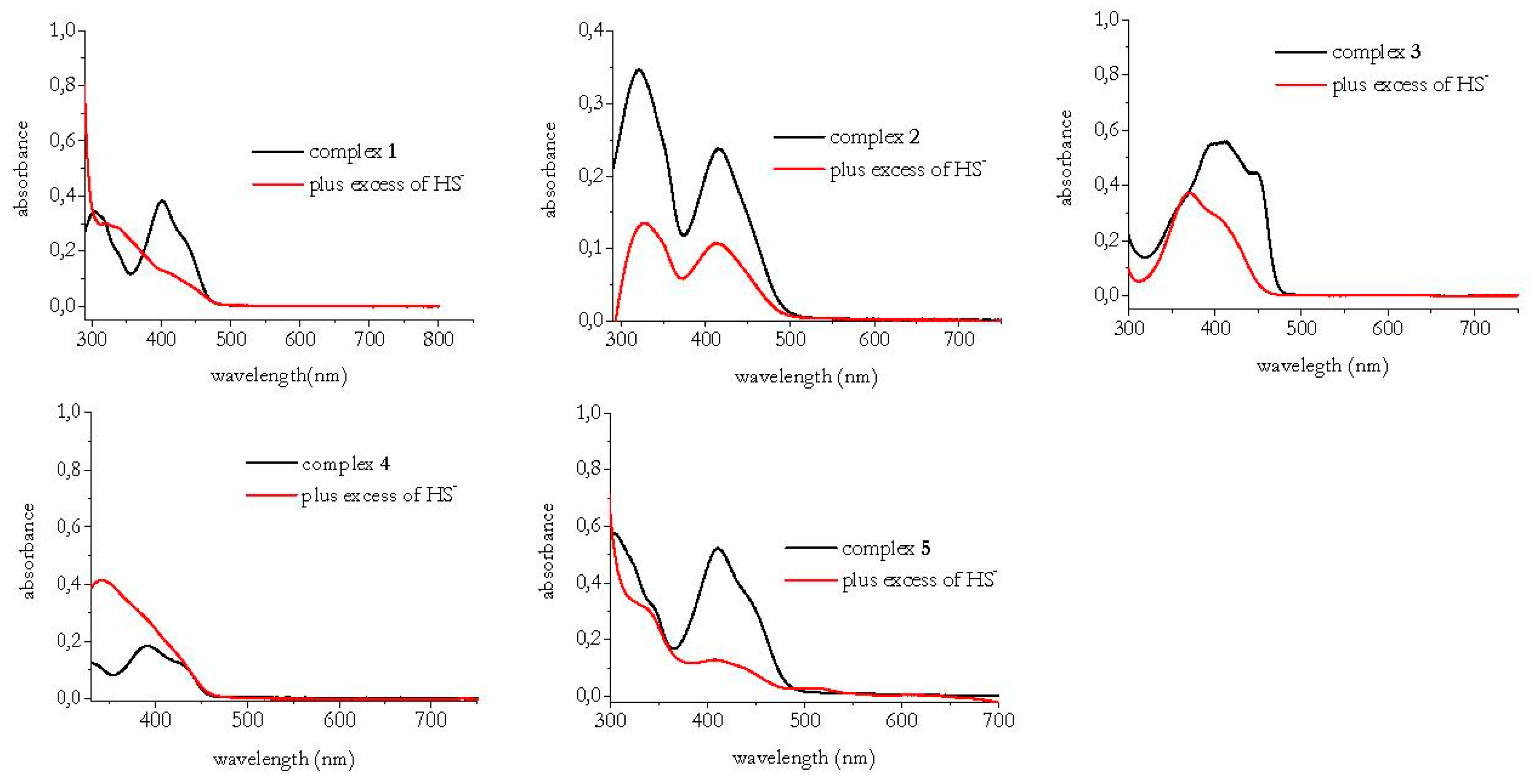
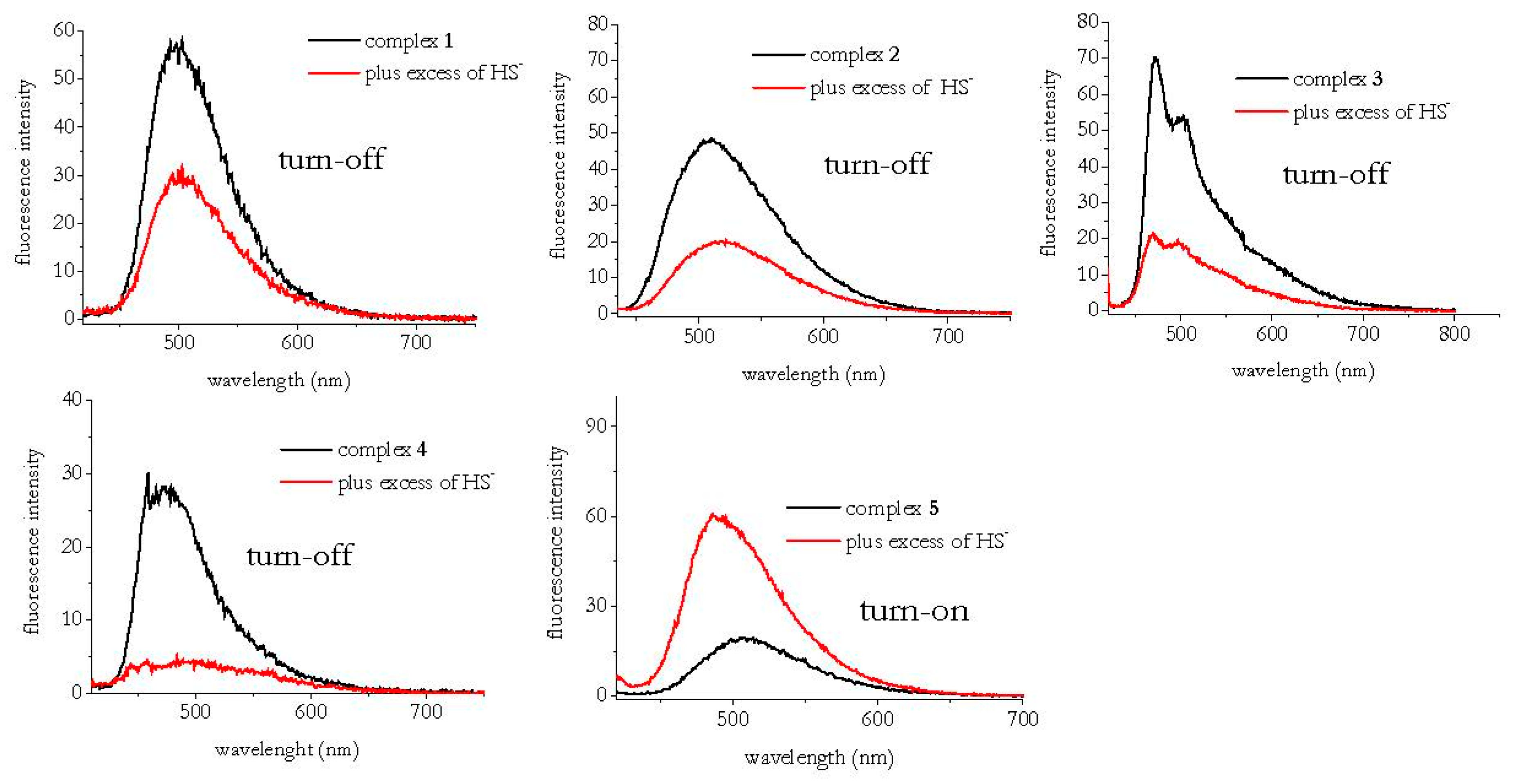
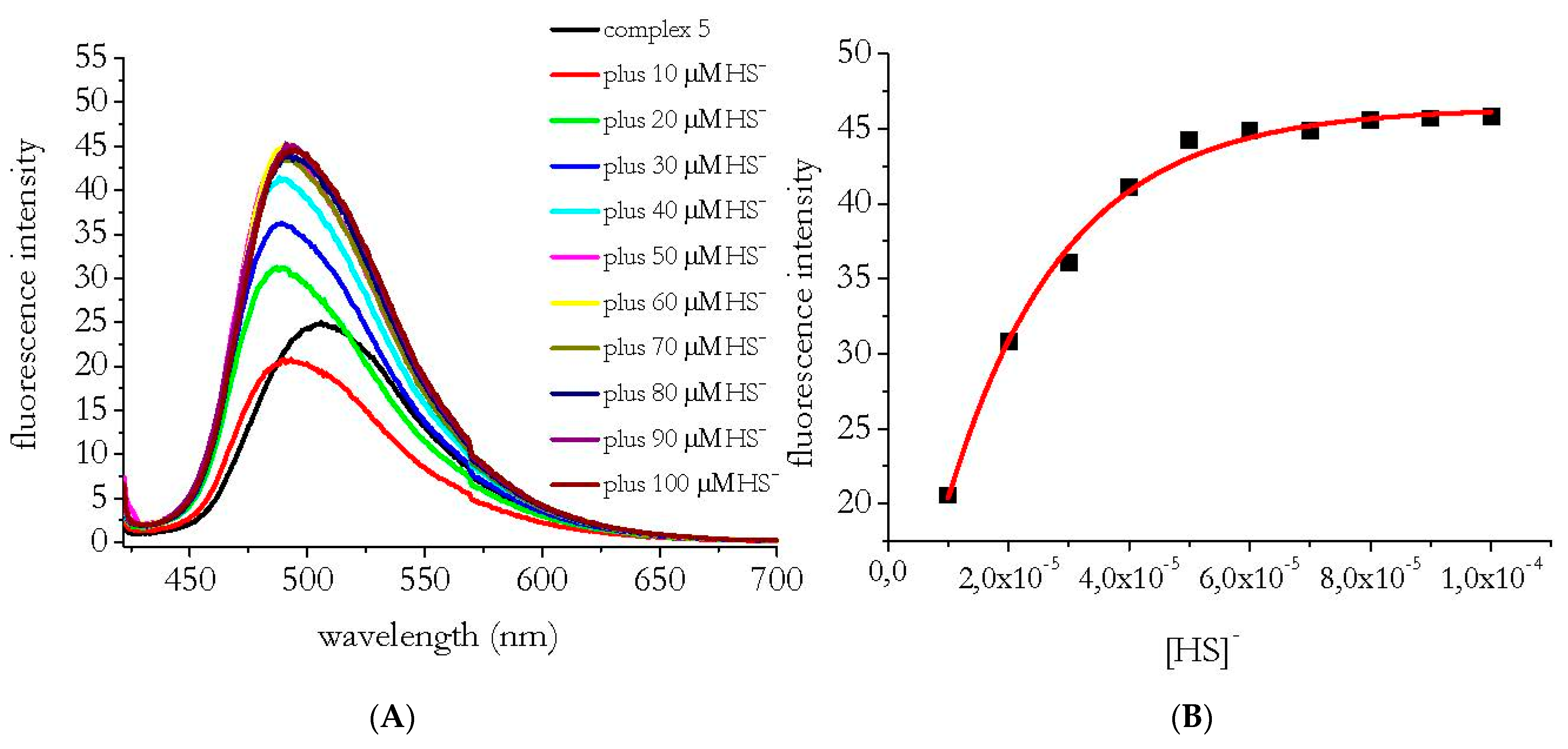
3.1. HS− Interaction with Complexes 1–5
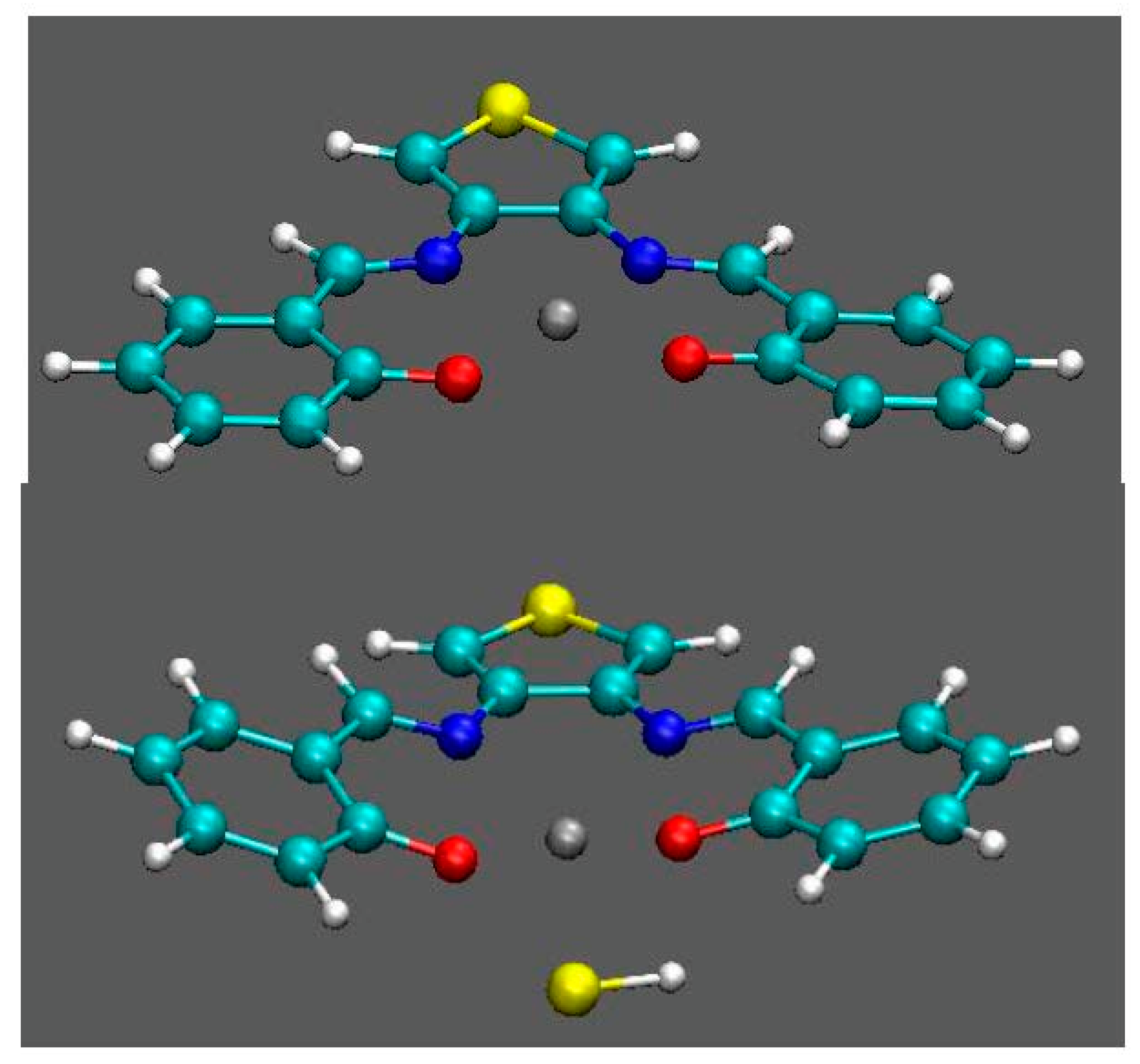
3.2. Computational Study
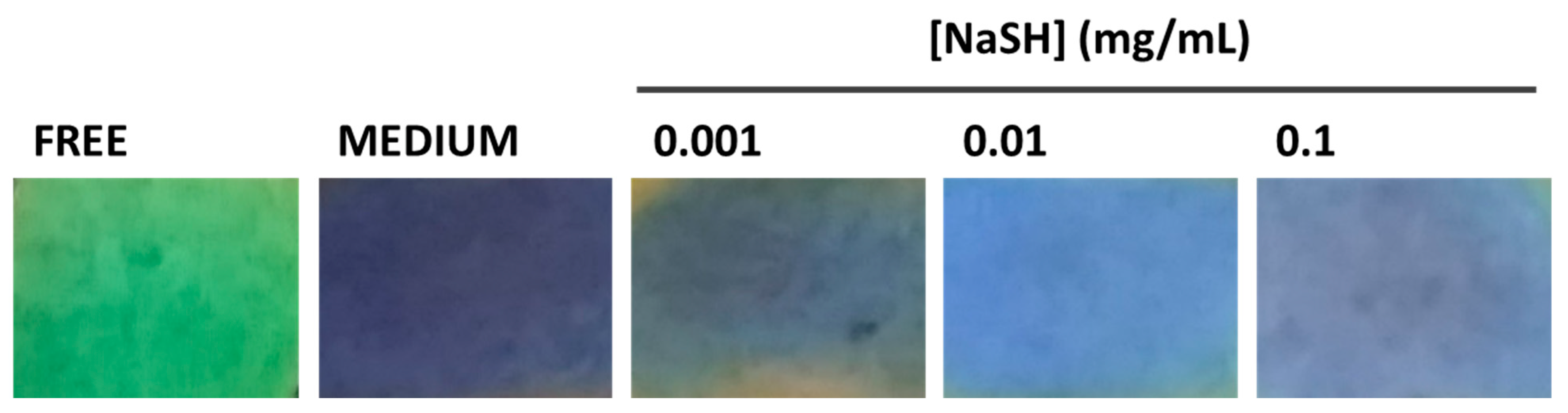
3.3. Sensing Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, G.D.; Wu, L.Y.; Jiang, B.; Yang, W.; Qi, J.S.; Cao, K.; Meng, Q.H.; Mustafa, A.K.; Mu, W.T.; Zhang, S.M.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartle, M.D.; Tillotson, M.R.; Prell, J.S.; Pluth, M.D. Spectroscopic investigation of the reaction of metallo-protoporphyrins with hydrogen sulfide. J. Inorg. Biochem. 2017, 173, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Hartle, M.D.; Sommer, S.K.; Dietrich, S.R.; Pluth, M.D. Chemically Reversible Reactions of Hydrogen Sulfide with Metal Phthalocyanines. Inorg. Chem. 2014, 53, 7800–7802. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Milione, S.; Bertolasi, V.; Pellecchia, C.; Grassi, A. Heteroscorpionate-Based Co2+, Zn2+, and Cu2+ Complexes: Coordination Behavior, Aerobic Oxidation, and Hydrogen Sulfide Detection. Inorg. Chem. 2011, 50, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Mirra, S.; Bertolasi, V.; Milione, S.; Pellecchia, C. Organometallic sulfur complexes: Reactivity of the hydrogen sulfide anion with cobaloximes. New J. Chem. 2015, 39, 4093–4099. [Google Scholar] [CrossRef]
- Strianese, M.; Mirra, S.; Lamberti, M.; Pellecchia, C. Zinc (II) porphyrins as viable scaffolds to stabilize hydrogen sulfide binding at the metal center. Inorg. Chim. Acta 2017, 466, 426–431. [Google Scholar] [CrossRef]
- Strianese, M.; Varriale, A.; Staiano, M.; Pellecchia, C.; D’Auria, S. Absorption into fluorescence. A method to sense biologically relevant gas molecules. Nanoscale 2011, 3, 298–302. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Pellecchia, C. Interaction of monohydrogensulfide with a family of fluorescent pyridoxal-based Zn(ii) receptors. Dalton Trans. 2018, 47, 17392–17400. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Persico, A.; Pellecchia, C. Reactivity of monohydrogensulfide with a suite of pyridoxal-based complexes: A combined NMR, ESI-MS, UVGÇôvisible and fluorescence study. Inorg. Chim. Acta 2019, 501, 119235. [Google Scholar] [CrossRef]
- Strianese, M.; Pappalardo, D.; Mazzeo, M.; Lamberti, M.; Pellecchia, C. Salen-type aluminum and zinc complexes as two-faced Janus compounds: Contribution to molecular sensing and polymerization catalysis. Dalton Trans. 2020, 49, 16533–16550. [Google Scholar] [CrossRef]
- Strianese, M.; Guarnieri, D.; Lamberti, M.; Landi, A.; Peluso, A.; Pellecchia, C. Fluorescent salen-type Zn(II) Complexes As Probes for Detecting Hydrogen Sulfide and Its Anion: Bioimaging Applications. Inorg. Chem. 2020, 59, 15977–15986. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, J.; Li, Y.; Sun, P.; Zhou, H.; Yang, J.; Zhang, S.; Jin, B.; Tian, Y. A Sulfur-Terminal Zn(II) Complex and Its Two-Photon Microscopy Biological Imaging Application. J. Am. Chem. Soc. 2009, 131, 5208–5213. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hong, Z.; Xie, Z.; Tong, S.; Wong, O.; Lee, C.S.; Wong, N.; Hung, L.; Lee, S. A bis-salicylaldiminato Schiff base and its zinc complex as new highly fluorescent red dopants for high performance organic electroluminescence devices. Chem. Commun. 2003, 1664–1665. [Google Scholar] [CrossRef]
- Xie, D.; Jing, J.; Cai, Y.B.; Tang, J.; Chen, J.J.; Zhang, J.L. Construction of an orthogonal ZnSalen/Salophen library as a colour palette for one- and two-photon live cell imaging. Chem. Sci. 2014, 5, 2318–2327. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.J.R.A.; et al. Gaussian 16, Revision C.01, Gaussian: Wallingford, CT, USA, 2016.
- Cramer, C.J.; Truhlar, D.G. Density functional theory for transition metals and transition metal chemistry. Phys. Chem. Chem. Phys. 2009, 11, 10757–10816. [Google Scholar] [CrossRef]
- Bjornsson, R.; Bühl, M. Electric field gradients of transition metal complexes from density functional theory: Assessment of functionals, geometries and basis sets. Dalton Trans. 2010, 39, 5319–5324. [Google Scholar] [CrossRef] [Green Version]
- Landi, A.; Troisi, A.; Peluso, A. Explaining different experimental hole mobilities: Influence of polymorphism on dynamic disorder in pentacene. J. Mater. Chem. C 2019, 7, 9665–9670. [Google Scholar] [CrossRef]
- Landi, A.; Peluso, A.; Troisi, A. Quantitative Prediction of the Electro-Mechanical Response in Organic Crystals. Adv. Mater. 2021, 33, 2008049. [Google Scholar] [CrossRef]
- Gao, X.; Bai, S.; Fazzi, D.; Niehaus, T.; Barbatti, M.; Thiel, W. Evaluation of Spin-Orbit Couplings with Linear-Response Time-Dependent Density Functional Methods. J. Chem. Theory Comput. 2017, 13, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Amilan Jose, D. Recent progress in hydrogen sulphide (H2S) sensors by metal displacement approach. Coord. Chem. Rev. 2017, 347, 141–157. [Google Scholar] [CrossRef]
- Strianese, M.; Lamberti, M.; Pellecchia, C. Chemically reversible binding of H2S to a zinc porphyrin complex: Towards implementation of a reversible sensor via a “coordinative-based approach”. Dalton Trans. 2017, 46, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Brenna, S.; Ardizzoia, G.A.; Guarnieri, D.; Lamberti, M.; D’Auria, I.; Pellecchia, C. Imidazo-pyridine-based zinc(ii) complexes as fluorescent hydrogen sulfide probes. Dalton Trans. 2021, 50, 17075–17085. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Borrelli, R.; Landi, A.; Velardo, A.; Peluso, A. Absorption Band Shapes of a PushGÇôPull Dye Approaching the Cyanine Limit: A Challenging Case for First Principle Calculations. J. Phys. Chem. A 2016, 120, 5581–5589. [Google Scholar] [CrossRef]
- Kimura, H. Signaling Molecules: Hydrogen Sulfide and Polysulfide. Antioxid. Redox Signal. 2014, 22, 362–376. [Google Scholar] [CrossRef] [Green Version]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol.-Regul. Integr. Comp. Phys. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef] [Green Version]
- Sanokawa-Akakura, R.; Ostrakhovitch, E.A.; Akakura, S.; Goodwin, S.; Tabibzadeh, S. A H2S-Nampt Dependent Energetic Circuit Is Critical to Survival and Cytoprotection from Damage in Cancer Cells. PLoS ONE 2014, 9, e108537. [Google Scholar] [CrossRef] [Green Version]
- Quan, F.S.; Lee, G.J. Analytical Methods for Detection of Gasotransmitter Hydrogen Sulfide Released from Live Cells. BioMed Res. Internat. 2021, 2021, 5473965. [Google Scholar] [CrossRef]
- Strianese, M.; Pellecchia, C. Metal complexes as fluorescent probes for sensing biologically relevant gas molecules. Coord. Chem. Rev. 2016, 318, 16–28. [Google Scholar] [CrossRef]



| Vertical | Adiabatic | Oscillator Strength | |
|---|---|---|---|
| 1 | 3.41 | 2.75 | 0.57 |
| 1 + HS− | 3.37 | 2.78 | 0.60 |
| 5 | 3.35 | 2.80 | 0.61 |
| 5 + HS− | 3.32 | 2.73 | 0.59 |
| SOC T3-S1 | SOC T4-S1 | |
|---|---|---|
| 1 | 15.3 | 15.2 |
| 1 + HS− | 25.6 | 204 |
| 5 | 12.3 | 6.25 |
| 5 + HS− | 4.78 | 142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strianese, M.; Vykhovanets, V.; Blal, N.; Guarnieri, D.; Landi, A.; Lamberti, M.; Peluso, A.; Pellecchia, C. Paper-Strip-Based Sensors for H2S Detection: A Proof-of-Principle Study. Sensors 2022, 22, 3173. https://doi.org/10.3390/s22093173
Strianese M, Vykhovanets V, Blal N, Guarnieri D, Landi A, Lamberti M, Peluso A, Pellecchia C. Paper-Strip-Based Sensors for H2S Detection: A Proof-of-Principle Study. Sensors. 2022; 22(9):3173. https://doi.org/10.3390/s22093173
Chicago/Turabian StyleStrianese, Maria, Viktoriia Vykhovanets, Naym Blal, Daniela Guarnieri, Alessandro Landi, Marina Lamberti, Andrea Peluso, and Claudio Pellecchia. 2022. "Paper-Strip-Based Sensors for H2S Detection: A Proof-of-Principle Study" Sensors 22, no. 9: 3173. https://doi.org/10.3390/s22093173






