Electrochemical Detection of Ethanol in Air Using Graphene Oxide Nanosheets Combined with Au-WO3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of GO Membranes
2.2. Synthesis of Au-WO3 and rGO for the Sensing Electrode
2.3. Materials Characterization
2.4. Sensor Fabrication and Sensing Tests
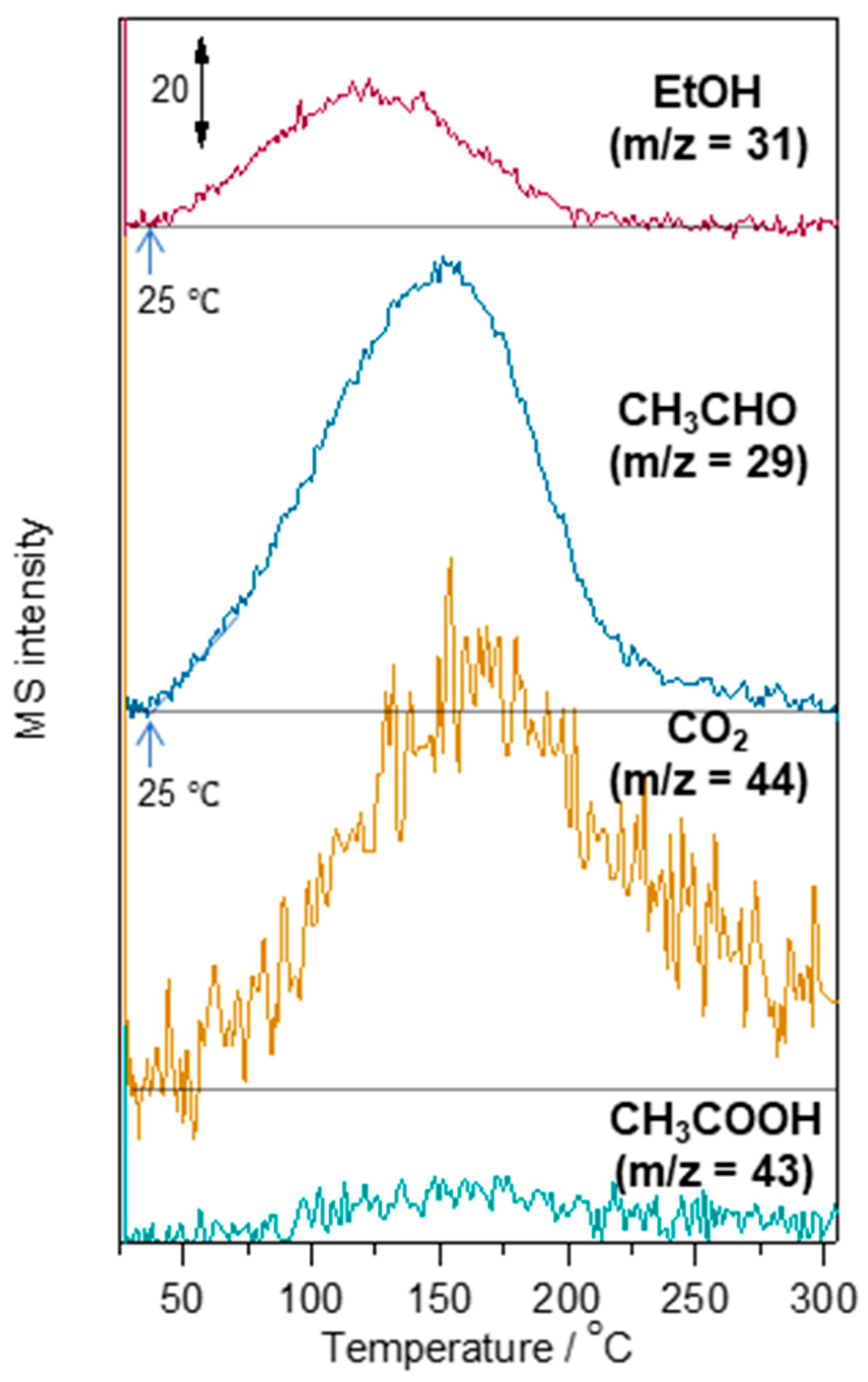
2.5. Study on Adsorption, Desorption, and Reaction of Ethanol on Au-WO3
3. Results and Discussion
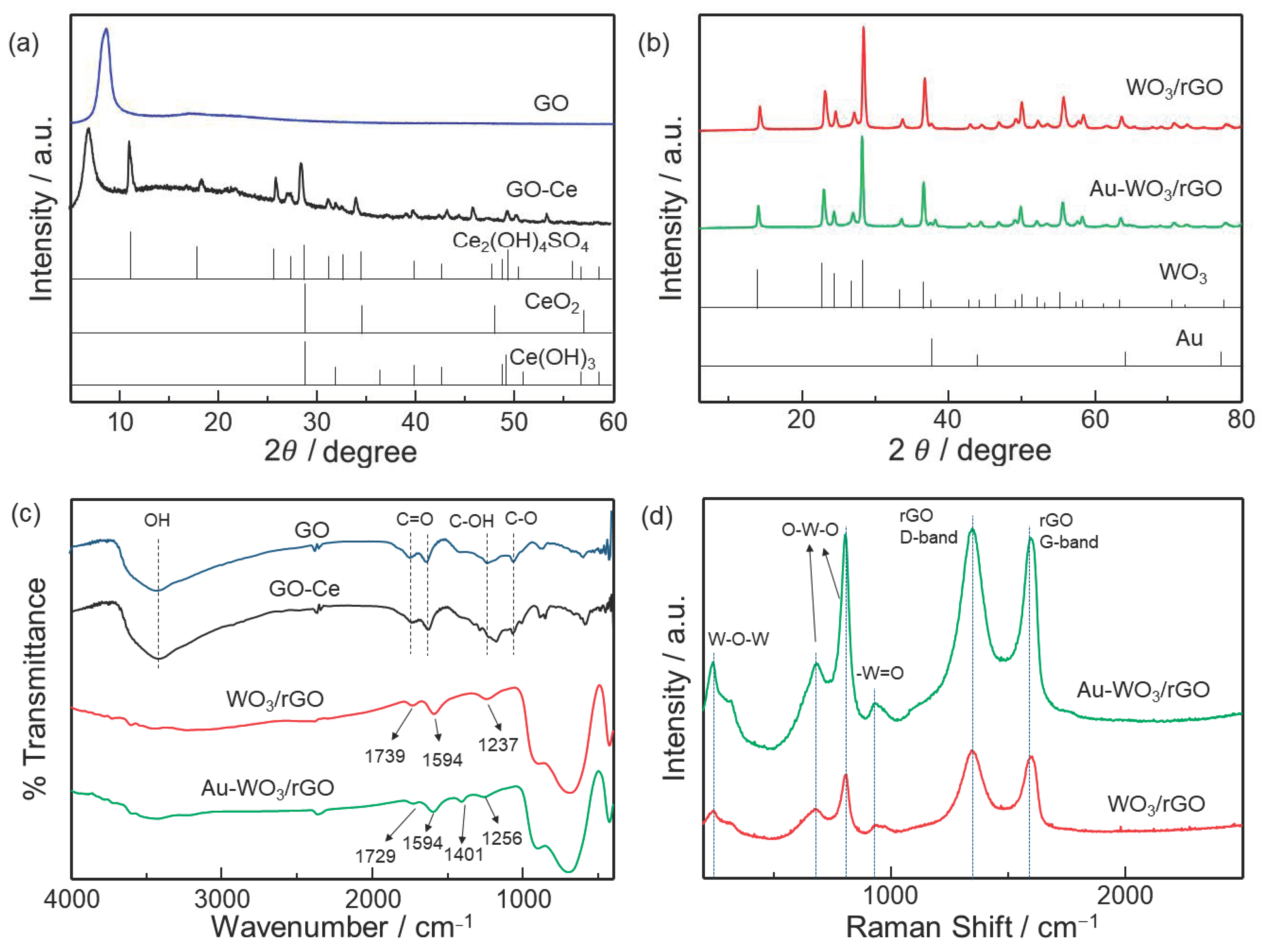
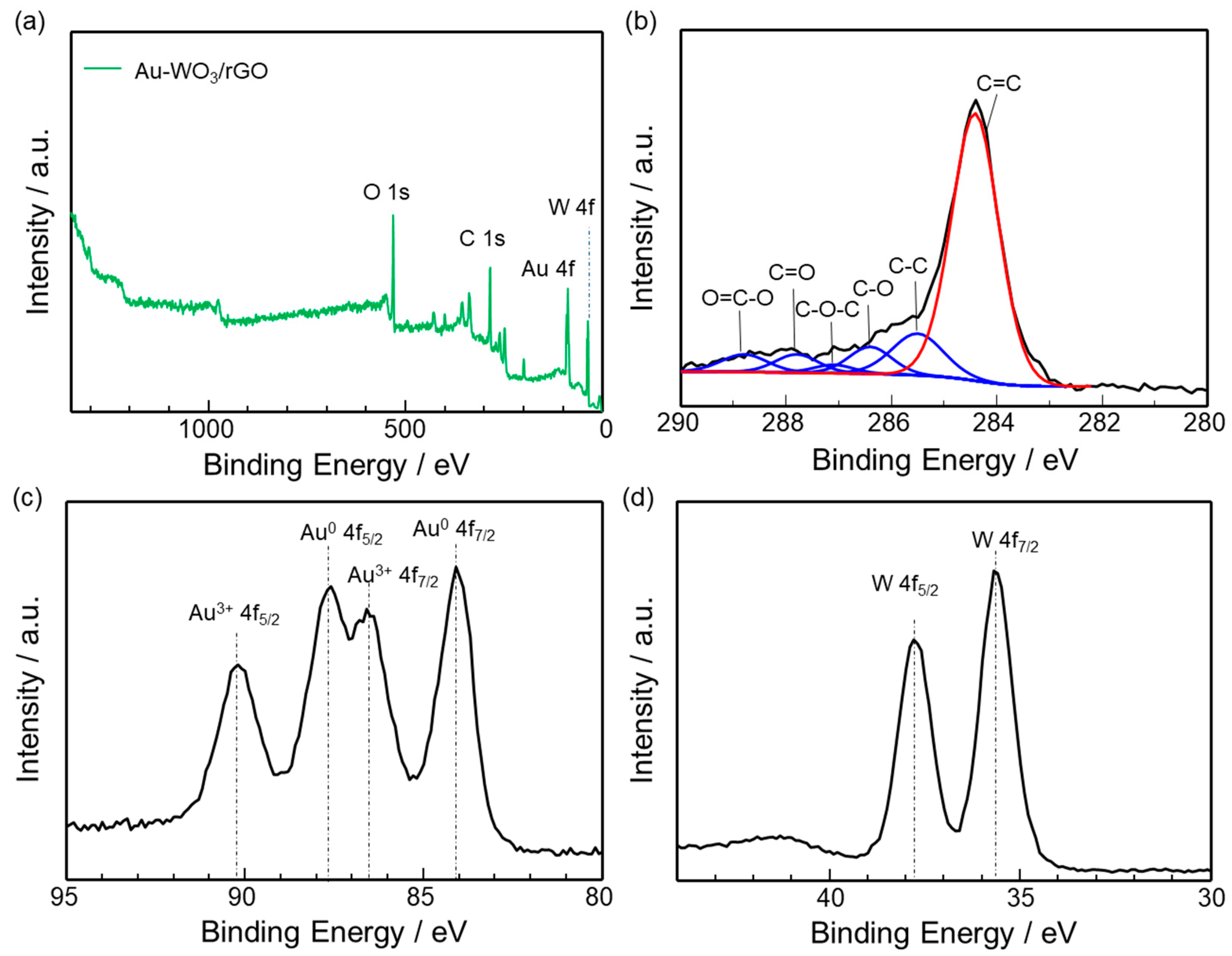
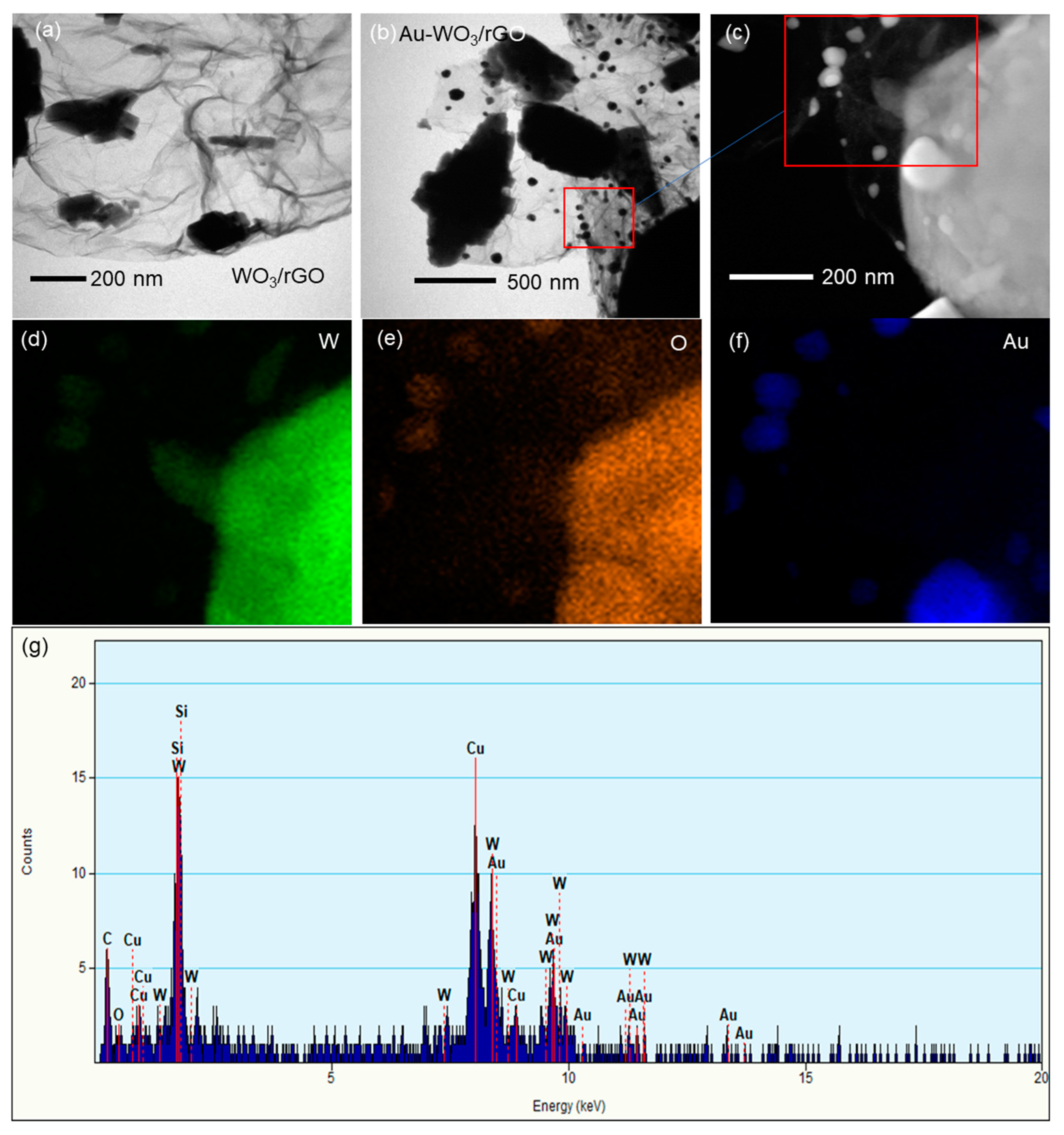
3.1. Materials Characterization
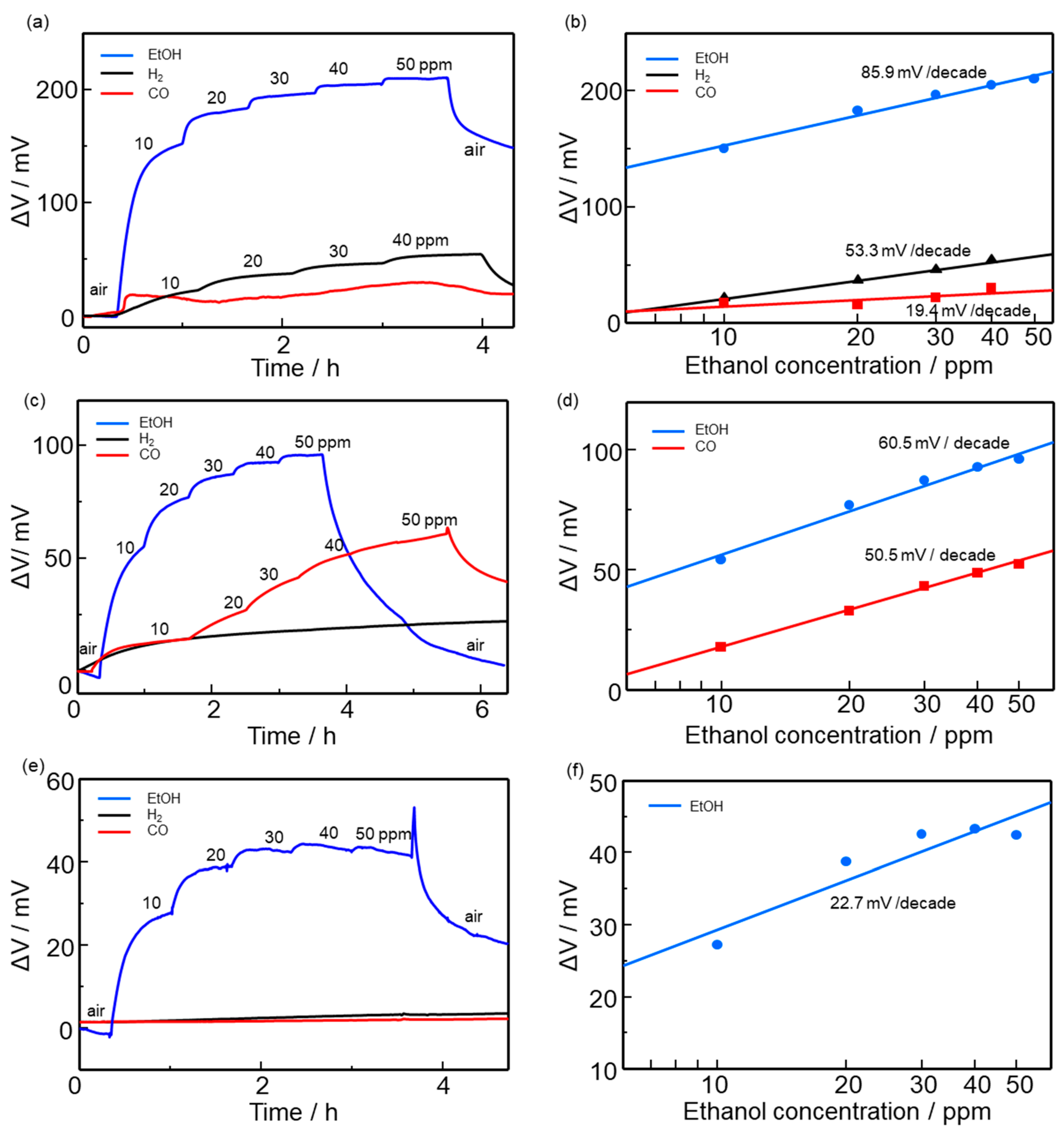
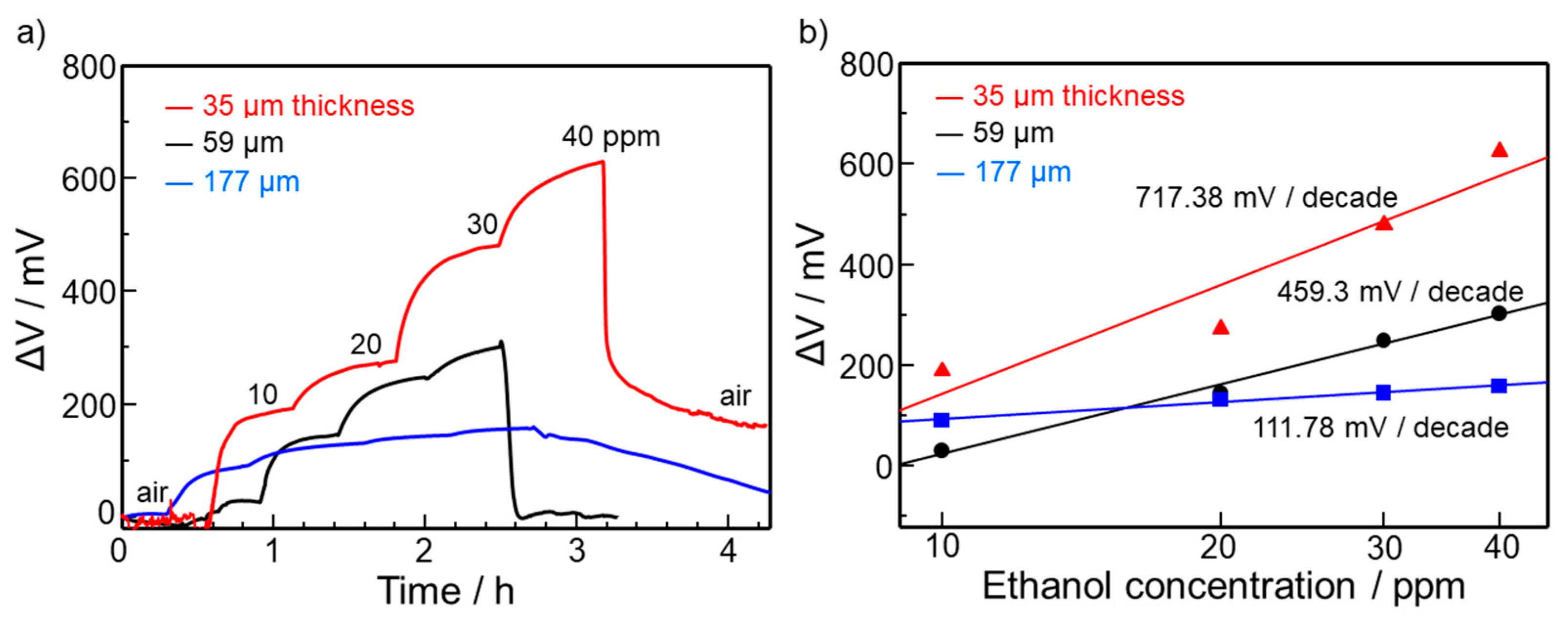
3.2. Ethanol Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kida, T.; Suematsu, K.; Hara, K.; Kanie, K.; Muramatsu, A. Ultrasensitive Detection of Volatile Organic Compounds by a Pore Tuning Approach Using Anisotropically Shaped SnO2 Nanocrystals. ACS Appl. Mater. Interfaces 2016, 8, 35485–35495. [Google Scholar] [CrossRef] [PubMed]
- Kida, T.; Doi, T.; Shimanoe, K. Synthesis of Monodispersed SnO2 Nanocrystals and Their Remarkably High Sensitivity to Volatile Organic Compounds. Chem. Mater. 2010, 22, 2662–2667. [Google Scholar] [CrossRef]
- Okamoto, H.; Obayashi, H.; Kudo, T. Carbon monoxide gas sensor made of stabilized zirconia. Solid State Ion. 1980, 1, 319–326. [Google Scholar] [CrossRef]
- Mukundan, R.L. Brosha, E.; Garzon, F. Mixed Potential Hydrocarbon Sensors Based on a YSZ Electrolyte and Oxide Electrodes. J. Electrochem. Soc. 2003, 150, H279. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef] [Green Version]
- Kida, T.; Morinaga, N.; Kishi, S.; An, K.-M.; Sim, K.-W.; Chae, B.-Y.; Kim, J.-K.; Ryu, B.-K.; Shimanoe, K. Electrochemical detection of volatile organic compounds using a Na3Zr2Si2PO12/Bi2Cu0.1V0.9O5.35 heterojunction device. Electrochim. Acta 2011, 56, 7484–7490. [Google Scholar] [CrossRef]
- Kida, T.; Harano, H.; Minami, T.; Kishi, S.; Morinaga, N.; Yamazoe, N.; Shimanoe, K. Control of electrode reactions in a mixed-potential-type gas sensor based on a BiCuVOx solid electrolyte. J. Phys. Chem. C 2010, 114, 15141–15148. [Google Scholar] [CrossRef]
- Yang, X.; Hao, X.; Liu, T.; Liu, F.; Wang, B.; Ma, C.; Liang, X.; Yang, C.; Zhu, H.; Zheng, J.; et al. CeO2-Based Mixed Potential Type Acetone Sensor Using La1-xSrxCoO3 Sensing Electrode. Sens. Actuators B Chem. 2018, 269, 118–126. [Google Scholar] [CrossRef]
- Fujio, Y.; Plashnitsa, V.V.; Elumalai, P.; Miura, N. Stabilization of sensing performance for mixed-potential-type zirconia-based hydrocarbon sensor. Talanta 2011, 85, 575–581. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Matsui, T.; Takehira, H.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T.; Nakamura, T.; Noro, S.-I.; et al. Graphene oxide nanosheet with high proton conductivity. J. Am. Chem. Soc. 2013, 135, 8097–8100. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Karim, M.R.; Ogata, C.; Tateishi, H.; Funatsu, A.; Taniguchi, T.; Koinuma, M.; Hayami, S.; Matsumoto, Y. Proton conductivities of graphene oxide nanosheets: Single, multilayer, and modified nanosheets. Angew. Chem. Int. Ed. 2014, 53, 6997–7000. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Kuwaki, Y.; Sano, T.; Hatakeyama, K.; Quitain, A.; Sasaki, M.; Kida, T. Solid Electrolyte Gas Sensor Based on a Proton-Conducting Graphene Oxide Membrane. ACS Omega 2017, 2, 2994–3001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauzi, A.S.A.; Hamidah, N.L.; Sato, S.; Shintani, M.; Putri, G.K.; Kitamura, S.; Hatakeyama, K.; Quitain, A.T.; Kida, T. Carbon-based potentiometric hydrogen sensor using a proton conducting graphene oxide membrane coupled with a WO3 sensing electrode. Sens. Actuators B Chem. 2020, 323, 128678. [Google Scholar] [CrossRef]
- Hamidah, N.L.; Shintani, M.; Ahmad Fauzi, A.S.; Mission, E.G.; Hatakeyama, K.; Quitain, A.T.; Kida, T. Improving the Proton Conductivity of Graphene Oxide Membranes by Intercalating Cations. SN Appl. Sci. 2019, 1, 630. [Google Scholar] [CrossRef] [Green Version]
- Hamidah, N.L.; Shintani, M.; Ahmad, F.A.S.; Putri, G.K.; Kitamura, S.; Hatakeyama, K.; Sasaki, M.; Quitain, A.T.; Kida, T. Graphene Oxide Membranes with Cerium-Enhanced Proton Conductivity for Water Vapor Electrolysis. ACS Appl. Nano Mater. 2020, 3, 4292–4304. [Google Scholar] [CrossRef]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Mounasamy, V.; Madanagurusamy, S. Porous Reduced Graphene Oxide (RGO)/WO3 Nanocomposites for the Enhanced Detection of NH3 at Room Temperature. Nanoscale Adv. 2019, 1, 1799–1811. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Chen, W.; Jin, L.; Cui, F.; Song, Z.; Zhu, C. High Performance Acetylene Sensor with Heterostructure Based on WO3 Nanolamellae/Reduced Graphene Oxide (RGO) Nanosheets Operating at Low Temperature. Nanomaterials 2018, 8, 909. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Zhong, X.; Shang, S.; Wang, S.; Tang, M. Effects of Sintering Temperature on Sensing Properties of WO3 and Ag-WO3 Electrode for NO2 Sensor. R. Soc. Open Sci. 2018, 5, 171691. [Google Scholar] [CrossRef] [Green Version]
- Minh Vuong, N.; Kim, D.; Kim, H. Porous Au-Embedded WO3 Nanowire Structure for Efficient Detection of CH4 and H2S. Sci. Rep. 2015, 5, 11040. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Fugetsu, B. Mass Production of Graphene Oxide from Expanded Graphite. Mater. Lett. 2013, 109, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Zanella, R.; Giorgio, S.; Henry, C.R.; Louis, C. Alternative Methods for the Preparation of Gold Nanoparticles Supported on TiO2. J. Phys. Chem. B 2002, 106, 7634–7642. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Inagaki, M. Preparation of Monodispersed Cerium(IV) Oxide Particles by Thermal Hydrolysis: Influence of the Presence of Urea and Gd Doping on Their Morphology and Growth. J. Mater. Chem. 2000, 10, 473–477. [Google Scholar] [CrossRef]
- Wang, L.; Luan, Q.; Yang, D.; Yao, X.; Zhou, K. Strong Electron-Conjugation Interaction Facilitates Electron Transfer of Hemoglobin by Ce(OH)3 Nanorods. RSC Adv. 2013, 3, 6339–6342. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Yao, Z.; Chen, Z.; Hong, M.; Zhu, R.; Liang, Y.; Zhao, J. Transition-Metal Doped Ceria Microspheres with Nanoporous Structures for CO Oxidation. Sci. Rep. 2016, 6, 23900. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Zhao, H.; Liu, Y.; Li, Z.; Song, J.; Chu, W.; Sun, Z. Efficient Conversion of Fructose into 5-Hydroxymethylfurfural over WO3/Reduced Graphene Oxide Catalysts. RSC Adv. 2017, 7, 3790–3795. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Liu, T.; Liu, J.; Liu, Q.; Jing, X.; Zhang, H.; Huang, G.; Wang, J. Controllable Synthesis and Enhanced Gas Sensing Properties of a Single-Crystalline WO3–RGO Porous Nanocomposite. RSC Adv. 2017, 7, 14192–14199. [Google Scholar] [CrossRef] [Green Version]
- Malefane, M.E.; Ntsendwana, B.; Mafa, P.J.; Mabuba, N.; Feleni, U.; Kuvarega, A.T. In-Situ Synthesis of Tetraphenylporphyrin/Tungsten (VI) Oxide/Reduced Graphene Oxide (TPP/WO3 /RGO) Nanocomposite for Visible Light Photocatalytic Degradation of Acid Blue 25. Chem. Select 2019, 4, 8379–8389. [Google Scholar] [CrossRef]
- Kumar, K.V.A.; Chandana, L.; Ghosal, P.; Subrahmanyam, C. Simultaneous Photocatalytic Degradation of P-Cresol and Cr (VI) by Metal Oxides Supported Reduced Graphene Oxide. Mol. Catal. 2018, 451, 87–95. [Google Scholar] [CrossRef]
- Assmus, T.; Balasubramanian, K.; Burghard, M.; Kern, K.; Scolari, M.; Fu, N.; Myalitsin, A.; Mews, A. Raman Properties of Gold Nanoparticle-Decorated Individual Carbon Nanotubes. Appl. Phys. Lett. 2007, 90, 173109. [Google Scholar] [CrossRef] [Green Version]
- Gürdal, E.; Dickreuter, S.; Noureddine, F.; Bieschke, P.; Kern, D.P.; Fleischer, M. Self-Assembled Quasi-Hexagonal Arrays of Gold Nanoparticles with Small Gaps for Surface-Enhanced Raman Spectroscopy. Beilstein J. Nanotechnol. 2018, 9, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS Study of Supported Gold Catalysts: The Role of Au0 and Au + δ Species as Active Sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Song, Z.; Li, W.; Niu, F.; Xu, Y.; Niu, L.; Yang, W.; Wang, Y.; Liu, J. A Novel Method to Decorate Au Clusters onto Graphene via a Mild Co-Reduction Process for Ultrahigh Catalytic Activity. J. Mater. Chem. A 2016, 5, 230–239. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported Gold Nanoparticles as Catalysts for Organic Reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Zhang, J.; Zhang, Z. Exposed Facet and Crystal Phase Tuning of Hierarchical Tungsten Oxide Nanostructures and Their Enhanced Visible-Light-Driven Photocatalytic Performance. CrystEngComm 2015, 17, 9102–9110. [Google Scholar] [CrossRef]
- Sumner, J.J.; Creager, S.E.; Ma, J.J.; Desmarteau, D.D. Proton Conductivity in Nafion 117 and in a Novel Bis[(Perfluoroalkyl)Sulfonyl]Imide Ionomer Membrane. J. Electrochem. Soc. 1998, 145, 107. [Google Scholar] [CrossRef]
- Velayutham, G.; Ramesh, C.; Murugesan, N.; Manivannan, V.; Dhathathreyan, K.S.; Periaswami, G. Nafion Based Amperometric Hydrogen Sensor. Ionics 2004, 10, 63–67. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. A comprehensive and critical review of the recent progress in electrocatalysts for the ethanol oxidation reaction. RSC Adv. 2021, 11, 16768–16804. [Google Scholar] [CrossRef]
- Liu, F.; Yang, X.; Yu, Z.; Wang, B.; Guan, Y.; Liang, X.; Sun, P.; Liu, F.; Gao, Y.; Lu, G. Highly Sensitive Mixed-Potential Type Ethanol Sensors Based on Stabilized Zirconia and ZnNb2O6 Sensing Electrode. RSC Adv. 2016, 6, 27197–27204. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauzi, A.S.A.; Hamidah, N.L.; Kitamura, S.; Kodama, T.; Sonda, K.; Putri, G.K.; Shinkai, T.; Ahmad, M.S.; Inomata, Y.; Quitain, A.T.; et al. Electrochemical Detection of Ethanol in Air Using Graphene Oxide Nanosheets Combined with Au-WO3. Sensors 2022, 22, 3194. https://doi.org/10.3390/s22093194
Fauzi ASA, Hamidah NL, Kitamura S, Kodama T, Sonda K, Putri GK, Shinkai T, Ahmad MS, Inomata Y, Quitain AT, et al. Electrochemical Detection of Ethanol in Air Using Graphene Oxide Nanosheets Combined with Au-WO3. Sensors. 2022; 22(9):3194. https://doi.org/10.3390/s22093194
Chicago/Turabian StyleFauzi, Aynul Sakinah Ahmad, Nur Laila Hamidah, Shota Kitamura, Taiga Kodama, Kosuke Sonda, Ghina Kifayah Putri, Takeshi Shinkai, Muhammad Sohail Ahmad, Yusuke Inomata, Armando T. Quitain, and et al. 2022. "Electrochemical Detection of Ethanol in Air Using Graphene Oxide Nanosheets Combined with Au-WO3" Sensors 22, no. 9: 3194. https://doi.org/10.3390/s22093194





