Magnetic Nanoparticles as Effective Heavy Ion Adsorbers in Natural Samples
Abstract
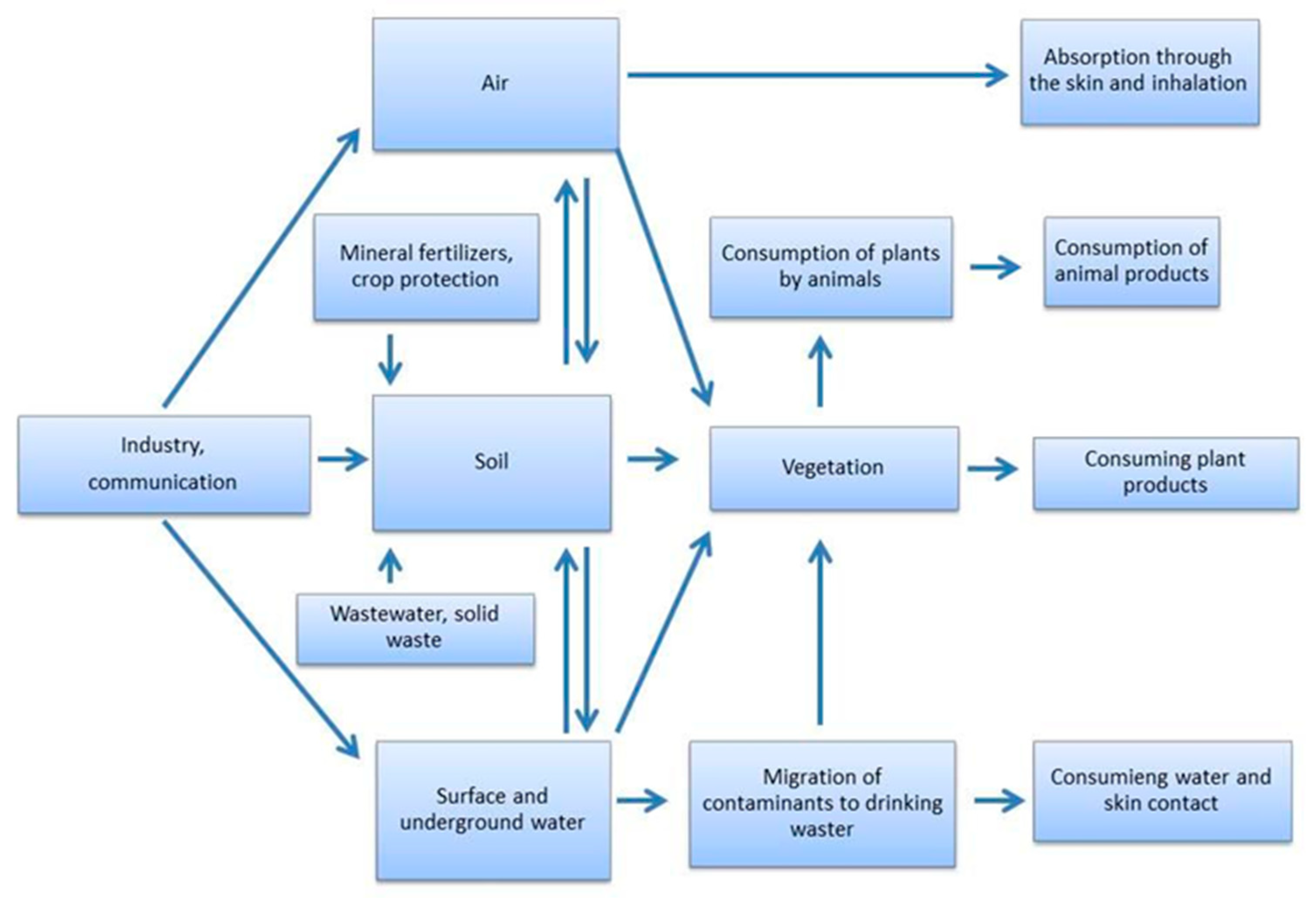
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
- (i)
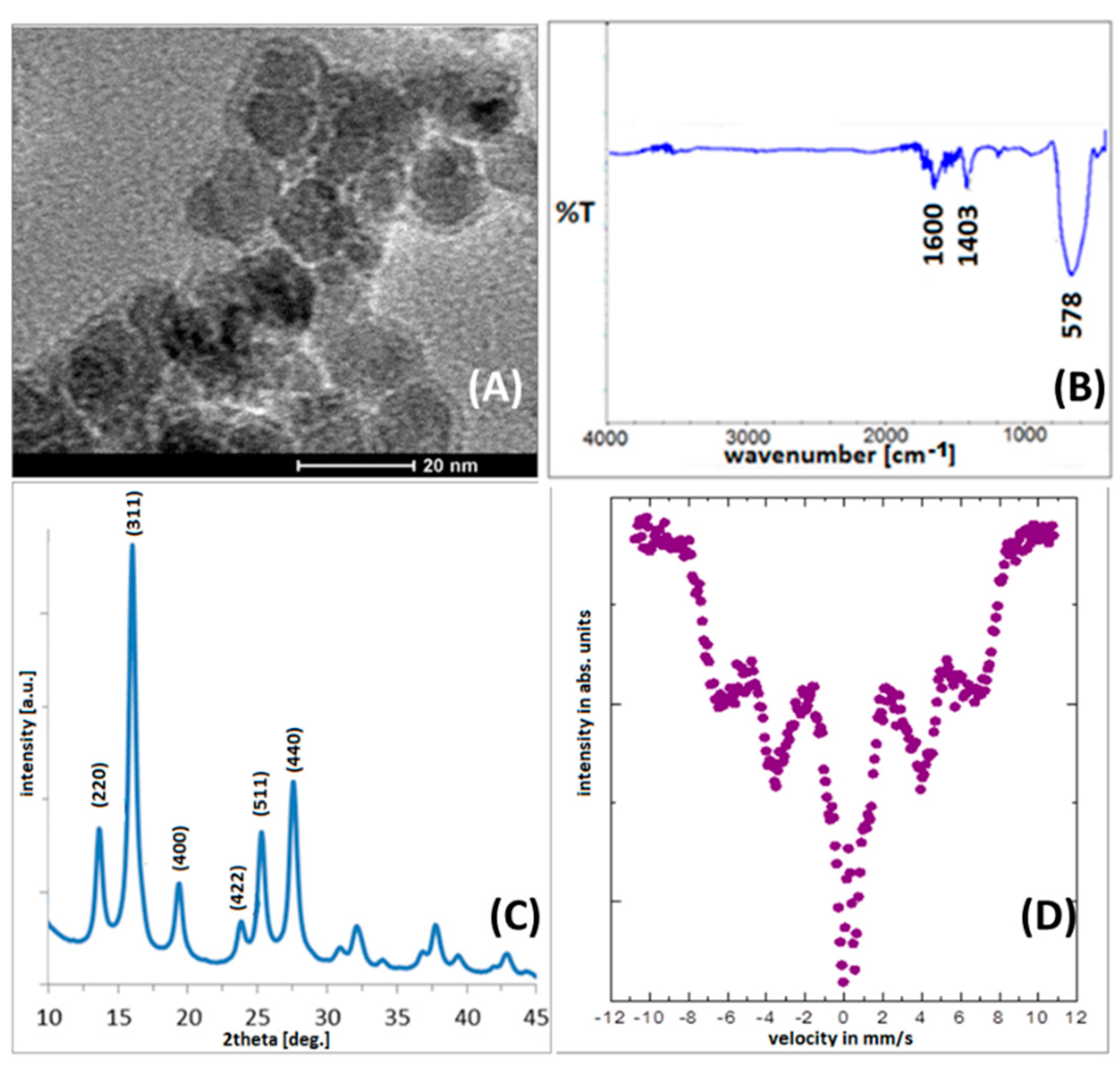
- X-ray diffractometry (XRD) (Agilent Technologies SuperNova diffractometer with a Mo micro-focused source (Kα2 = 0.713067 Å))—placing a small amount of powder on a nylon loop using a high viscosity oil—to determine the crystal structure;
- (ii)
- Transmission electron microscopy (TEM) (FEI Tecnai G2 X-TWIN 200 kV microscope—prefixing a drop of nanoparticle solution, on a carbon-covered 400 mesh Cu grid—to control particle morphology, shape, and size;
- (iii)
- Infrared spectroscopy (IR) in the spectral range between 500 and 4000 cm−1 (using a Nicolet 6700 spectrometer working in transmission mode)—positioning a small amount of particle powder on a diamond window and squeezing via a stamp—to confirm surface functionalization;
- (iv)
- Scanning electron microscope (INSPEC 60)—placing a small amount of particle powder on the microscopic table via conducting carbon tape—to examine the morphology of the obtained particle film;
- (v)
- Mössbauer spectroscopy with a spectrometer working in constant acceleration mode with a 57Co in Rh matrix radioactive source—mixing the particle powder with BN and forming a disc—to establish the magnetic state of particles. The spectra were calibrated using α-Fe as a reference foil at room temperature (RT).
2.3. Synthesis of Mn2+ Doped Ferrite Nanoparticles
2.4. Modification of Nanoparticles PA, SA, AA, 3-PPA, and 16-PHDA
2.5. Preparation of Food Samples Solution for FAAS
3. Results
3.1. Physicochemical Characterization of Pristine and Modified Ferrite Nanoparticles
3.2. Adsorption Tests
3.3. Scanning Electron Microscopy
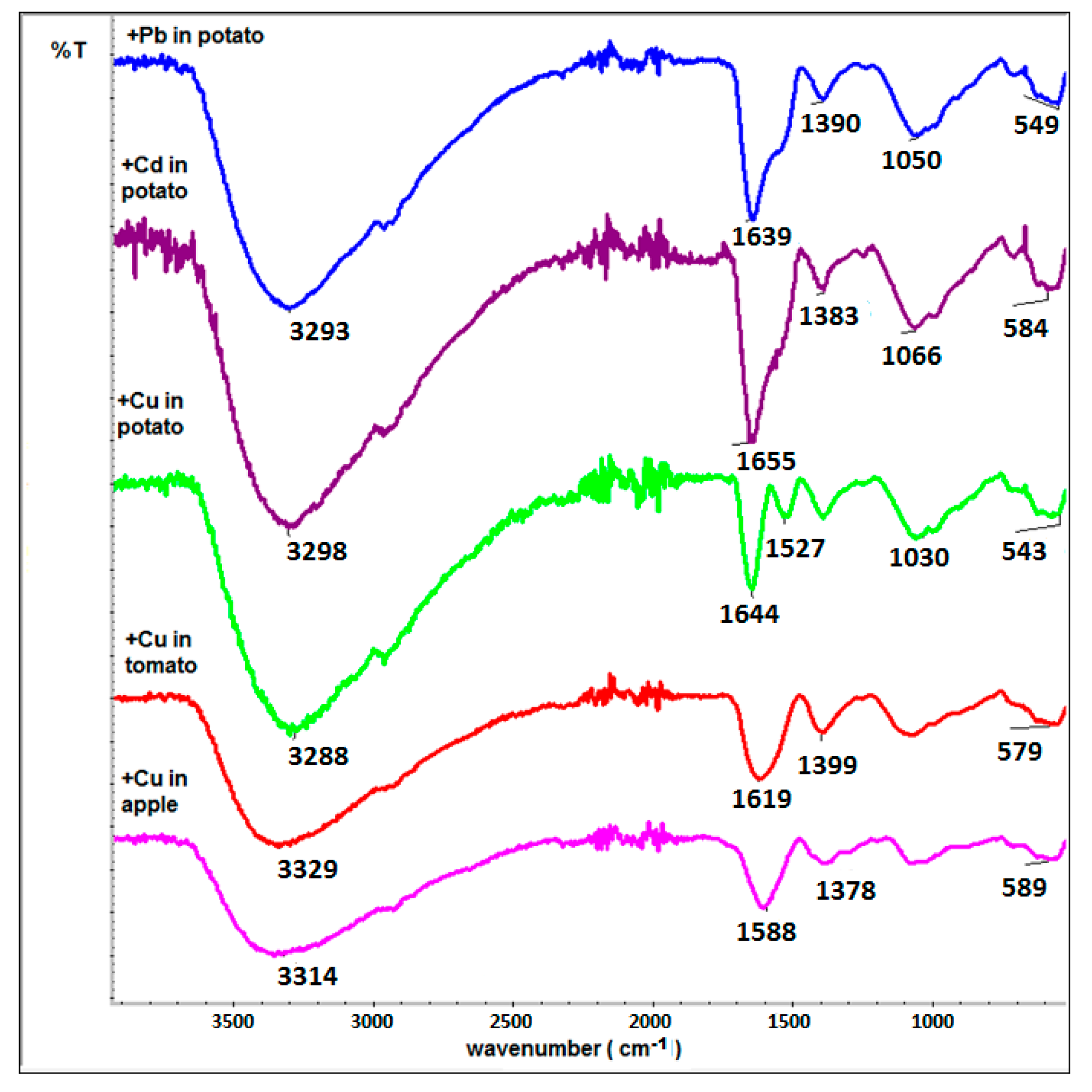
3.4. Infrared Spectroscopy
3.5. Flame Atomic Absorption Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Simal-Gandara, J. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788. [Google Scholar] [CrossRef] [PubMed]
- Dee, G.; Gun’ko, Y.K. Magnetic nanoparticles and nanoobjects used for medical applications. In Magnetic Materials and Technologies for Medical Applications; School of Chemistry, Trinity College Dublin: Dublin, Ireland, 2022; pp. 59–105. [Google Scholar]
- Youssef, A.M.; Malhat, F.M. Selective Removal of Heavy Metals from Drinking Water Using Titanium Dioxide Nanowire. Macromol. Symp. 2014, 337, 96–101. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar]
- Zhong, W.-S.; Ren, T.; Zhao, L.-J. Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J. Food Drug Anal. 2016, 24, 46–55. [Google Scholar] [CrossRef] [Green Version]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy OA, A.; Mishrif, M.R. Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- CAS, No. International Agency for Research on Cancer. Agents Classified by the IARC Monographs. 2012, pp. 1–106. Available online: http://monographs.iarc.fr/ENG/Classification/index.php (accessed on 20 January 2022).
- Hashem, E.Y.; Seleim, M.M.; El-Zohry, A.M. Environmental method for spectrophotometric determination of copper(II). Green Chem. Lett. Rev. 2011, 4, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Gouder de Beauregard, A.C.; Mahy, G. Phytoremediation of heavy metals: The role of macrophytes in a stormwater basin. Int. J. Ecohydrol. Hydrobiol. 2002, 2, 1–4. [Google Scholar]
- Vásquez-Murrieta, M.; Migueles-Garduño, I.; Franco-Hernández, O.; Govaerts, B.; Dendooven, L. C and N mineralization and microbial biomass in heavy-metal contaminated soil. Eur. J. Soil Biol. 2006, 42, 89–98. [Google Scholar] [CrossRef]
- Fato, F.P.; Li, S.W.; Zhao, L.J.; Qiu, K.; Long, Y.T. Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles. ACS Omega 2019, 4, 7543–7549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water 2020, 12, 446. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kumar, N.; Mehra, R.; Kumar, H.; Singh, V.P. Progress and challenges in the detection of residual pesticides using nanotechnology based colorimetric techniques. Trends Environ. Anal. Chem. 2020, 26, e00086. [Google Scholar] [CrossRef]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. 2021, 28, 58994–59002. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Carlos, L.; Garcia Einschlag, F.S.G.; Gonzalez, M.C.; Martire, D.O. Applications of Magnetite Nanoparticles for Heavy Metal Removal from Wastewater. Waste Water Treat. Technol. Recent Anal. Dev. 2013, 3, 64–73. [Google Scholar]
- Tatarchuk, T.; Myslin, M.; Lapchuk, I.; Shyichuk, A.; Murthy, A.P.; Gargula, R.; Pędziwiatr, A.T. Magnesium-zinc ferrites as magnetic adsorbents for Cr(VI) and Ni(II) ions removal: Cation distribution and antistructure modeling. Chemosphere 2021, 270, 129414. [Google Scholar] [CrossRef]
- Kaur, N.; Mehta, A.; Mishra, A.; Chaudhary, S.; Rawat, M.; Basu, S. Amphiphilic carbon dots derived by cationic surfactant for selective and sensitive detection of metal ions. Mater. Sci. Eng. C 2019, 95, 72–77. [Google Scholar] [CrossRef]
- Wasiewska, L.A.; Seymour, I.; Patella, B.; Inguanta, R.; Burgess, C.M.; Duffy, G.; O’Riordan, A. Reagent free electrochemical-based detection of silver ions at interdigitated microelectrodes using in-situ pH control. Sens. Actuators B Chem. 2021, 333, 129531. [Google Scholar] [CrossRef]
- Klekotka, U.; Piotrowska, B.; Satuła, D.; Giersig, M.; Kalska-Szostko, B. Ferrite Core-Shell Nanoparticles Synthesized by Seed-Based Method Characterization and Potential Application. Phys. Status Solidi A 2018, 215, 1700901. [Google Scholar] [CrossRef]
- Pirouz, M.J.; Beyki, M.H.; Shemirani, F. Anhydride functionalised calcium ferrite nanoparticles: A new selective magnetic material for enrichment of lead ions from water and food samples. Food Chem. 2015, 170, 131–137. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.; You, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169. [Google Scholar]
- Bhadani, A.; Okano, T.; Ogura, T.; Misono, T.; Sakai, K.; Abe, M.; Sakai, H. Structural features and surfactant properties of core–shell type micellar aggregates formed by gemini piperidinium surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 147–155. [Google Scholar] [CrossRef]
- Klekotka, U.; Wińska, E.; Zambrzycka-Szelewa, E.; Satuła, D.; Kalska-Szostko, B. Heavy-metal detectors based on modified ferrite nanoparticles. Beilstein J. Nanotechnol. 2018, 9, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Kalska-Szostko, B.; Kropiewnicka, K. The influence of the transition metal substitution on chemically prepared ferrite nanoparticles—Mossbauer studies. Curr. Appl. Phys. 2012, 12, 896–902. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Zubowska, M.; Satuła, D. Studies of the magnetite nanoparticles by means of Mössbauer spectroscopy. Acta Phys. Pol. A 2006, 109, 365–369. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Rogowska, M.; Satuła, D. Organophosphorous functionalization of magnetite nanoparticles. Colloids Surf. B Biointerfaces 2013, 111, 656–662. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra. A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Panda, R.N.; Gajbhiye, N.S.; Balaji, G. Magnetic properties of interacting single domain Fe3O4 particles. J. Alloys Compd. 2001, 326, 50–53. [Google Scholar] [CrossRef]
- Topsoe, H.; Dumesic, J.; Boudart, M. Mossbauer spectra of stoichiometric and nonstoichiometric Fe3O4 microcrystals. J. Phys. Colloq. 1974, 35, 411–413. [Google Scholar] [CrossRef]
- Thermo Elemental. AAS, GFAAS, ICP or ICP-MS? Which Technique Should I Use? Thermo Elemental: Waltham, WA, USA, 2001; pp. 1–11. [Google Scholar]

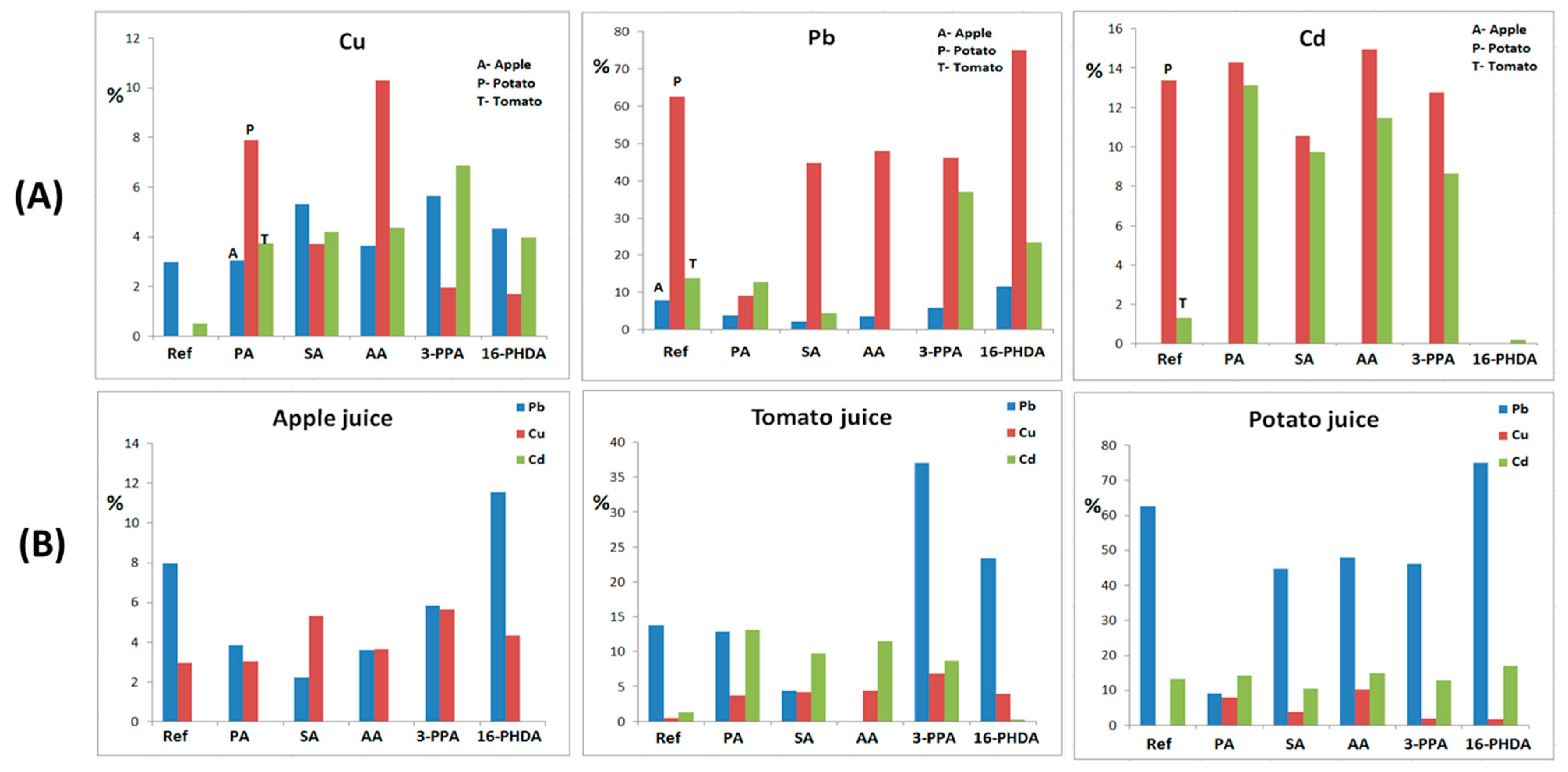
| Sample Type | % Adsorbed ± 0.05 | ||
|---|---|---|---|
| Apple | Potato | Tomato | |
| I | II | III | |
| Pb | |||
| Mn0.2Fe2.8O4 NP’s | 7.98 | 62.53 | 13.80 |
| Mn0.2Fe2.8O4 + PA | 3.84 | 9.19 | 12.82 |
| Mn0.2Fe2.8O4 + SA | 2.22 | 44.80 | 4.38 |
| Mn0.2Fe2.8O4 + AA | 3.62 | 48.00 | <LOD |
| Mn0.2Fe2.8O4 + 3-PPA | 5.83 | 46.22 | 37.01 |
| Mn0.2Fe2.8O4 + 16-PHDA | 11.52 | 75.02 | 23.38 |
| Cu | |||
| Mn0.2Fe2.8O4 NP’s | 2.98 | <LOD | 0.50 |
| Mn0.2Fe2.8O4 + PA | 3.04 | 7.89 | 3.76 |
| Mn0.2Fe2.8O4 + SA | 5.32 | 3.72 | 4.21 |
| Mn0.2Fe2.8O4 + AA | 3.64 | 10.31 | 4.38 |
| Mn0.2Fe2.8O4 + 3-PPA | 5.66 | 1.97 | 6.89 |
| Mn0.2Fe2.8O4 + 16-PHDA | 4.33 | 1.69 | 3.98 |
| Cd | |||
| Mn0.2Fe2.8O4 NP’s | 0.01 | 13.38 | 1.33 |
| Mn0.2Fe2.8O4 + PA | 0.02 | 14.3 | 13.13 |
| Mn0.2Fe2.8O4 + SA | 0.02 | 10.56 | 9.75 |
| Mn0.2Fe2.8O4 + AA | 0.03 | 14.93 | 11.47 |
| Mn0.2Fe2.8O4 + 3-PPA | 0.01 | 12.75 | 8.64 |
| Mn0.2Fe2.8O4 + 16-PHDA | <LOD | 16.89 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klekotka, U.; Wińska, E.; Zambrzycka-Szelewa, E.; Satuła, D.; Kalska-Szostko, B. Magnetic Nanoparticles as Effective Heavy Ion Adsorbers in Natural Samples. Sensors 2022, 22, 3297. https://doi.org/10.3390/s22093297
Klekotka U, Wińska E, Zambrzycka-Szelewa E, Satuła D, Kalska-Szostko B. Magnetic Nanoparticles as Effective Heavy Ion Adsorbers in Natural Samples. Sensors. 2022; 22(9):3297. https://doi.org/10.3390/s22093297
Chicago/Turabian StyleKlekotka, Urszula, Ewelina Wińska, Elżbieta Zambrzycka-Szelewa, Dariusz Satuła, and Beata Kalska-Szostko. 2022. "Magnetic Nanoparticles as Effective Heavy Ion Adsorbers in Natural Samples" Sensors 22, no. 9: 3297. https://doi.org/10.3390/s22093297






