S-Scheme BiOCl/MoSe2 Heterostructure with Enhanced Photocatalytic Activity for Dyes and Antibiotics Degradation under Sunlight Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of BiOCl/MoSe2
2.3. Characterization
2.4. Photocatalytic Activity Measurement
3. Results and Discussion
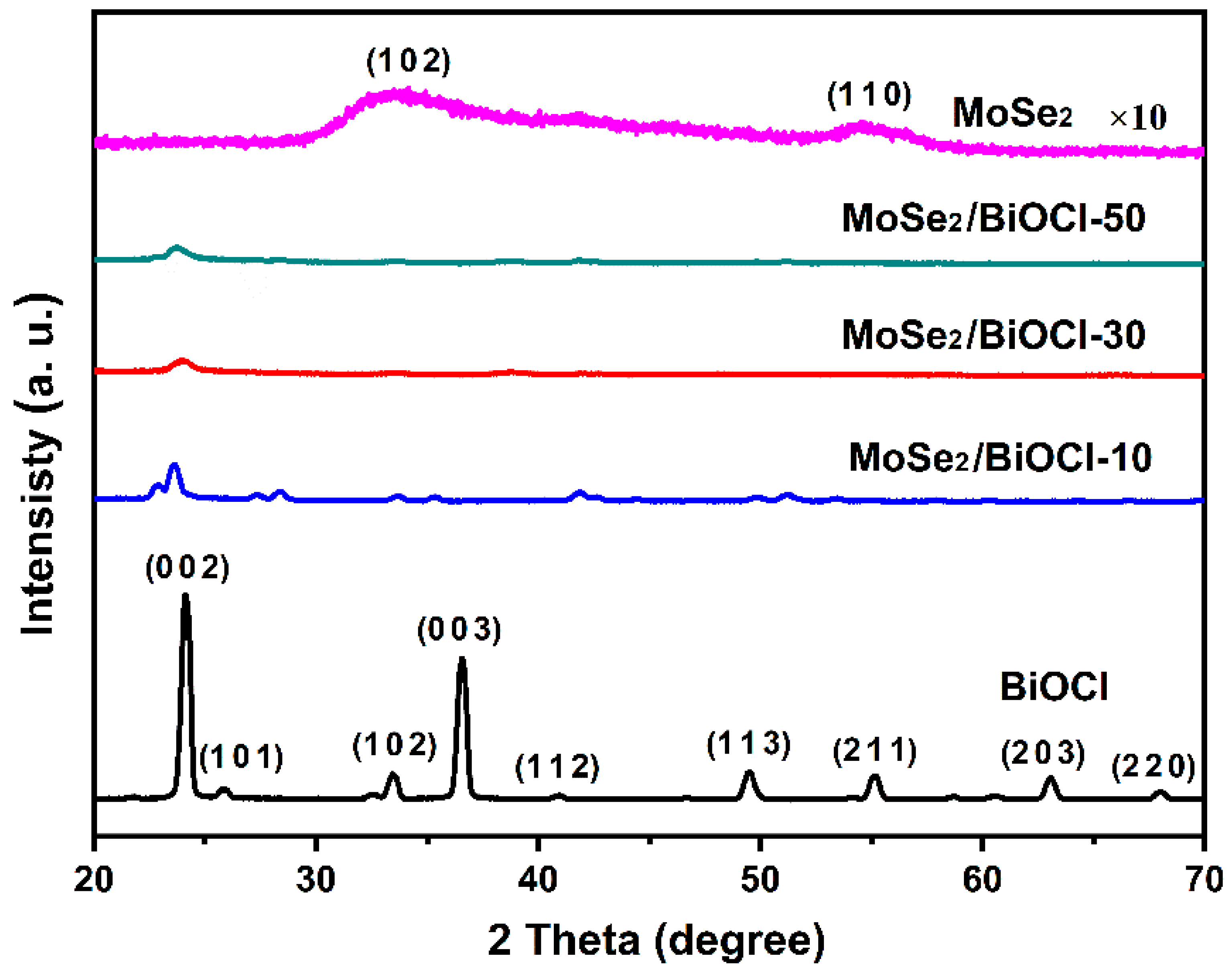
3.1. Morphological and Crystalline Structures
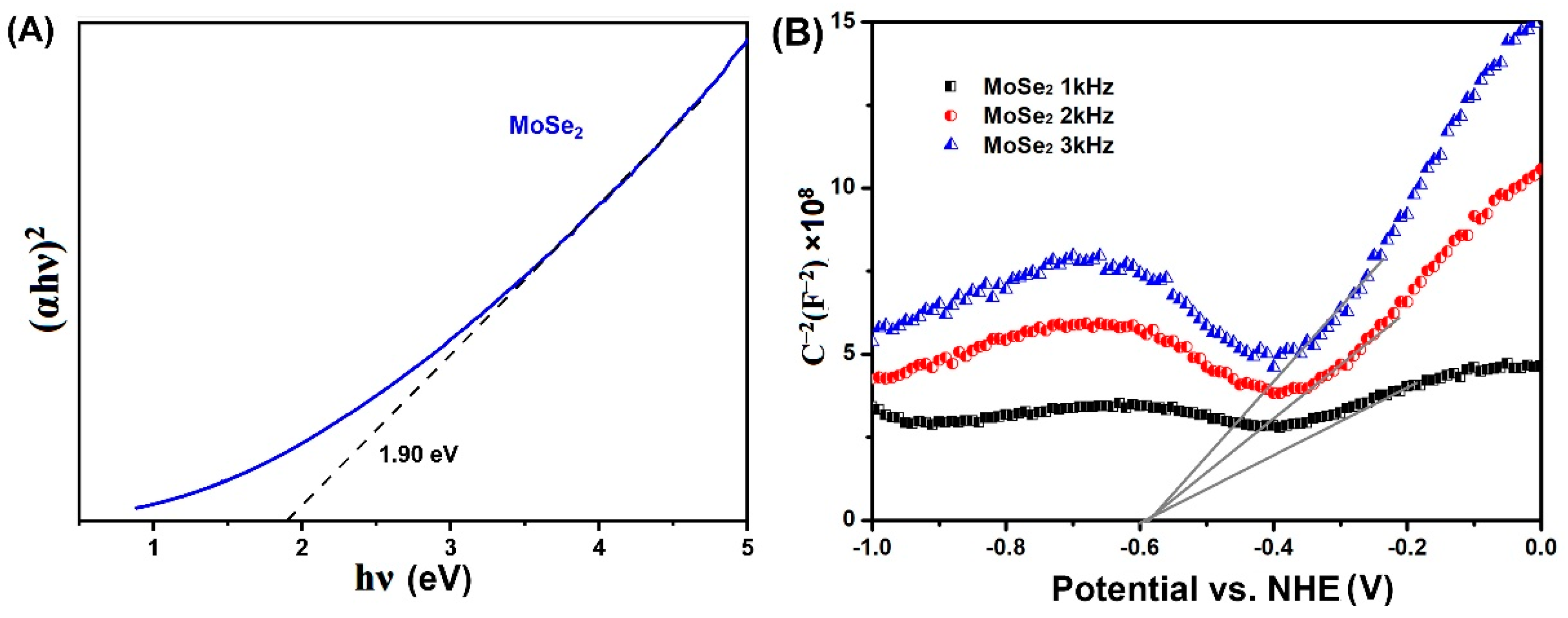
3.2. Light Absorption and PL Properties
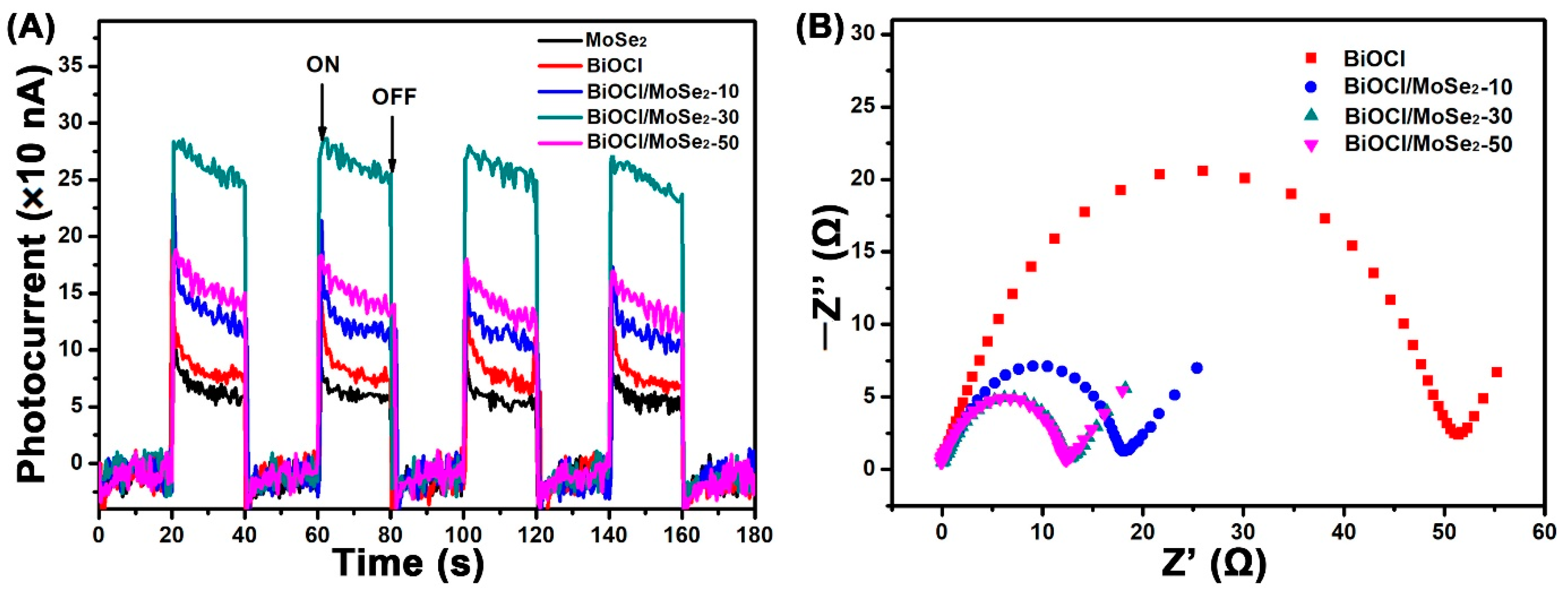
3.3. Photoelectric Characteristics
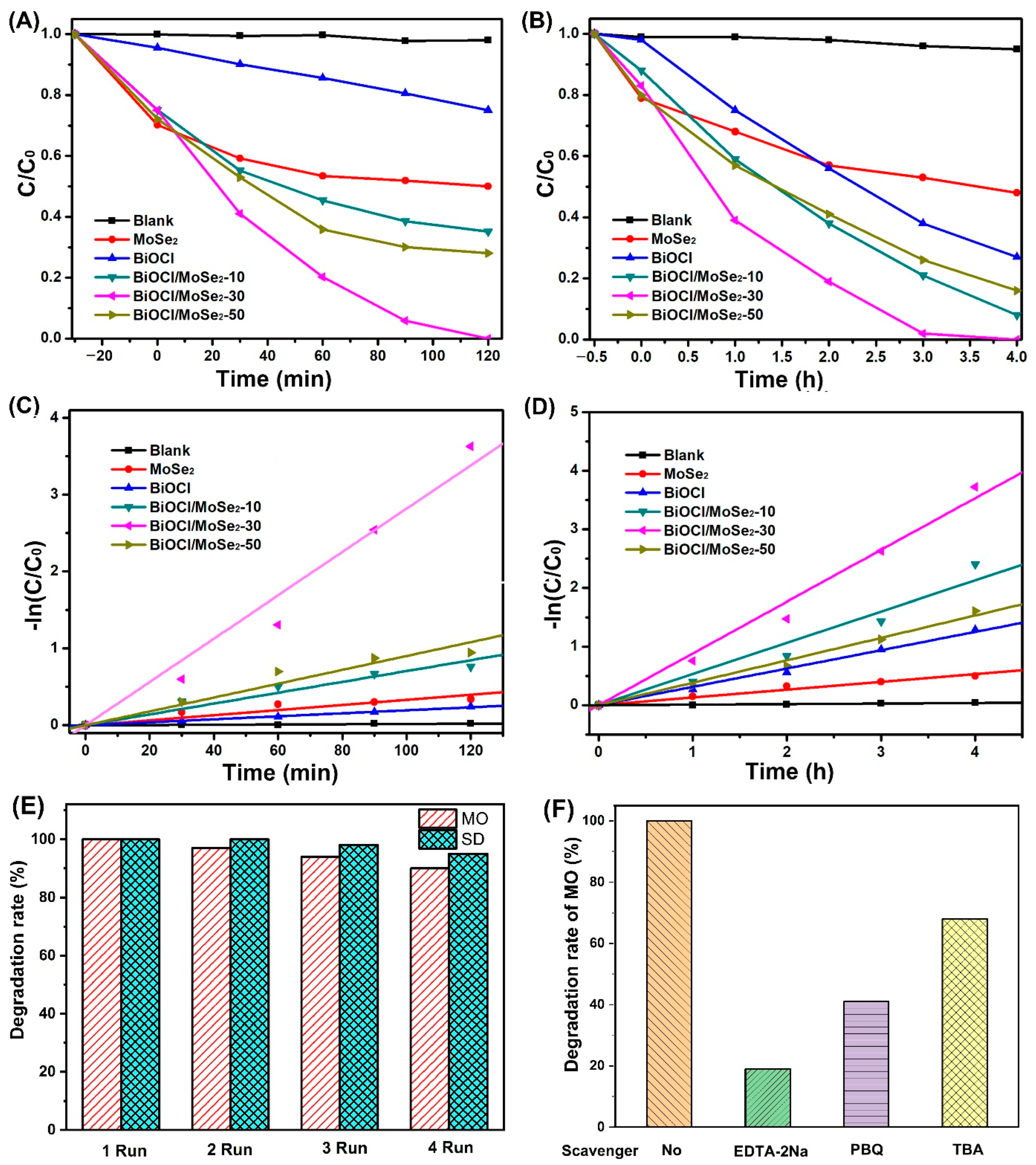
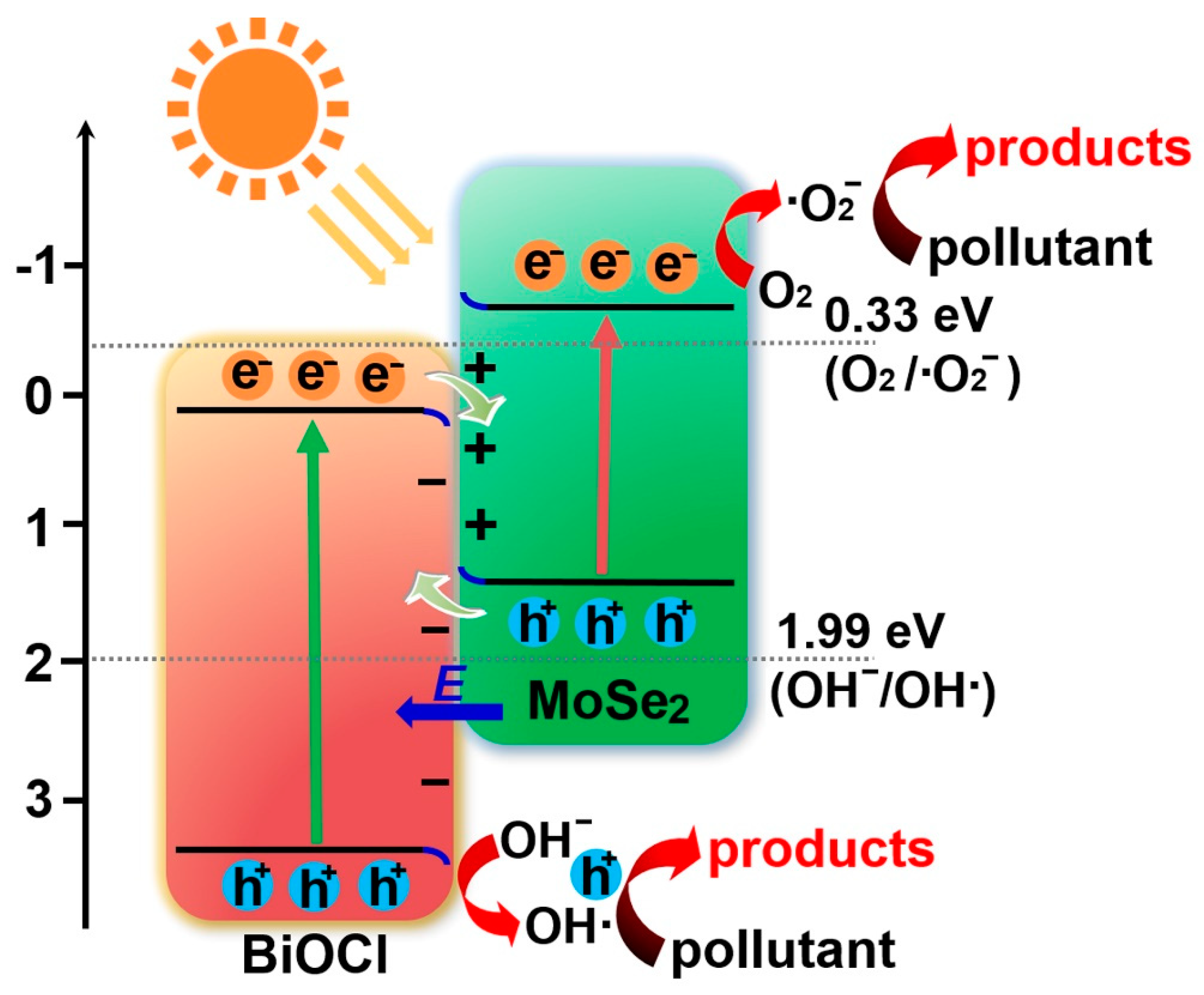
3.4. Photocatalytic Activity and Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Som, I.; Roy, M.; Saha, R. Advances in nanomaterial-based water treatment approaches for photocatalytic degradation of water pollutants. ChemCatChem 2020, 12, 3409–3433. [Google Scholar] [CrossRef]
- Ren, G.M.; Han, H.T.; Wang, Y.X.; Liu, S.T.; Zhao, J.Y.; Meng, X.C.; Li, Z.Z. Recent advances of photocatalytic application in watertreatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Pirillo, F.; Loddo, V.; Palmisano, L. Heterogeneous photocatalytic degradation of pharmaceuticals in water by using polycrystalline TiO2 and a nanofiltration membrane reactor. Catal. Today 2006, 118, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Arriaga, F.; Esplugas, S.; Giménez, J. Photocatalytic degradation of non−steroidal anti−inflammatory drugs with TiO2 and simulated solar irradiation. Water Res. 2008, 42, 585–594. [Google Scholar] [CrossRef]
- Hu, J.L.; Yang, Q.H.; Lin, H.; Ye, Y.P.; He, Q.; Zhang, J.N.; Qian, H.S. Mesoporous silica nanospheres decorated with CdS nanocrystals for enhanced photocatalytic and excellent antibacterial activities. Nanoscale 2013, 5, 6327–6332. [Google Scholar] [CrossRef]
- Dong, R.F.; Tian, B.Z.; Zhang, J.L.; Wang, T.T.; Tao, Q.S.; Bao, S.Y.; Yang, F.; Zeng, C.Y. AgBr@Ag/TiO2 core−shell composite with excellent visible light photocatalytic activity and hydrothermal stability. Catal. Commun. 2013, 38, 16–20. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Sarayu, K.; Sandhya, S. Current technologies for biological treatment of textile wastewater—A review. Appl. Biochem. Biotechnol. 2012, 167, 645–661. [Google Scholar] [CrossRef]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Peñuela, G.; Perez-Moya, M.; Mansilla, H.D. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal. Today 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Wang, P.H.; Yap, P.S.; Lim, T.T. C−N−S tridoped TiO2 for photocatalytic degradation of tetracycline under visible−light irradiation. Appl. Catal. A 2011, 399, 252–261. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Bao, S.Y.; Wu, Q.F.; Chang, S.Z.; Tian, B.Z.; Zhang, J.L. Z-scheme CdS−Au−BiVO4 with enhanced photocatalytic activity for organic contaminant decomposition. Catal. Sci. Technol. 2017, 7, 124–132. [Google Scholar] [CrossRef]
- Li, Q.Y.; Guan, Z.P.; Wu, D.; Zhao, X.G.; Bao, S.Y.; Tian, B.Z.; Zhang, J.L. Z-scheme BiOCl-Au-CdS heterostructure with enhanced sunlight-driven photocatalytic activity in degrading water dyes and antibiotics. ACS Sustain. Chem. Eng. 2017, 5, 6958–6968. [Google Scholar] [CrossRef]
- Kong, W.H.; Wang, S.L.; Wu, D.; Chen, C.R.; Luo, Y.S.; Pei, Y.T.; Tian, B.Z.; Zhang, J.L. Fabrication of 3D sponge@AgBr-AgCl/Ag and tubular photoreactor for continuous wastewater purification under sunlight irradiation. ACS Sustain. Chem. Eng. 2019, 7, 14051–14063. [Google Scholar] [CrossRef]
- Cheng, T.T.; Gao, H.J.; Liu, G.R.; Pu, Z.S.; Wang, S.F.; Yi, Z.; Wu, X.W.; Yang, H. Preparation of core-shell heterojunction photocatalysts by coating CdS nanoparticles onto Bi4Ti3O12 hierarchical microspheres and their photocatalytic removal of organic pollutants and Cr(VI) ions. Colloid Surf. A 2022, 633, 127918. [Google Scholar] [CrossRef]
- Li, L.X.; Gao, H.J.; Yi, Z.; Wang, S.F.; Wu, X.W.; Li, R.S.; Yang, H. Comparative investigation on synthesis, morphological tailoring and photocatalytic activities of Bi2O2CO3 nanostructures. Colloid Surf. A 2022, 644, 128758. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.H.; Yang, H.; Wen, L.H.; Yi, Z.; Zhou, Z.G.; Dai, B.; Zhang, J.G.; Wu, X.W.; Wu, P.H. Multi-mode surface plasmon resonance absorber based on dart-type single-layer graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef]
- Li, L.X.; Sun, X.F.; Xian, T.; Gao, H.J.; Wang, S.F.; Yi, Z.; Wu, X.W.; Yang, H. Template-free synthesis of Bi2O2CO3 hierarchical nanotubes self-assembled from ordered nanoplates for promising photocatalytic application. Phys. Chem. Chem. Phys. 2022, 24, 8279–8295. [Google Scholar] [CrossRef]
- Lei, Y.Q.; Wang, G.H.; Song, S.Y.; Fan, W.Q.; Zhang, H.J. Synthesis, characterization and assembly of BiOCl nanostructure and their photocatalytic properties. CrystEngComm 2009, 11, 1857–1862. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X = Cl, Br, I) nanoplate microspheres. J. Phys. Chem. C 2008, 112, 747–753. [Google Scholar] [CrossRef]
- Guan, M.L.; Xiao, C.; Zhang, J.; Fan, S.J.; An, R.; Cheng, Q.M.; Xie, J.F.; Zhou, M.; Ye, B.J.; Xie, Y. Vacancy associates promoting solar–driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets. J. Am. Chem. Soc. 2013, 135, 10411–10417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, L.Z.; Wang, J.J.; Li, Q.X.; He, W.W.; Yin, J.J. Surface structure–dependent molecular oxygen activation of BiOCl single–crystalline nanosheets. J. Am. Chem. Soc. 2013, 135, 15750–15753. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.Q.; Deng, K.J.; Xu, F.; Tian, L.H.; Peng, T.Y.; Zan, L. Increasing visible–light absorption for photocatalysis with black BiOCl. Phys. Chem. Chem. Phys. 2012, 14, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.X.; Chen, B.B.; Xie, L.Y.; Zheng, Z.Y.; Liu, P. Facile in situ synthesis of a Bi/BiOCl nanocomposite with high photocatalytic activity. J. Mater. Chem. A 2013, 1, 3068–3075. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Yan, S.; Wang, H.Q.; Sun, Y.J.; Zhang, Y.X.; Huang, H.W.; Wu, Z.B. Facets and defects cooperatively promote visible light plasmonic photocatalysis with Bi nanowires@BiOCl nanosheets. J. Catal. 2016, 344, 401–410. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.Z.; Li, H.; He, W.W.; Yin, J.J. Self–doping and surface plasmon modification induced visible light photocatalysis of BiOCl. Nanoscale 2013, 5, 10573–10581. [Google Scholar] [CrossRef]
- Tan, C.W.; Zhu, G.Q.; Hojamberdiev, M.; Okada, K.; Liang, J.C.; Luo, X.; Liu, P.; Liu, Y. Co3O4 nanoparticles–loaded BiOCl nanoplates with the dominant {001} facets: Efficient photodegradation of organic dyes under visible light. Appl. Catal. B 2014, 152–153, 425–436. [Google Scholar] [CrossRef]
- Li, T.B.; Chen, G.; Zhou, C.; Shen, Z.Y.; Jin, R.C.; Sun, J.X. New photocatalyst BiOCl/BiOI composites with highly enhanced visible light photocatalytic performances. Dalton Trans. 2011, 40, 6751–6758. [Google Scholar] [CrossRef]
- Yu, L.H.; Zhang, X.Y.; Li, G.W.; Cao, Y.T.; Shao, Y.; Li, D.Z. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye–sensitization. Appl. Catal. B 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Bao, S.Y.; Wang, Z.; Zhang, J.L.; Tian, B.Z. Facet-heterojunction-based Z-Scheme BiVO4{010} microplates decorated with AgBr-Ag nanoparticles for the photocatalytic inactivation of bacteria and the decomposition of organic contaminants. ACS Appl. Nano Mater. 2020, 3, 8604–8617. [Google Scholar] [CrossRef]
- Yuan, Z.; Huang, H.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. All-solid-state WO3/TQDs/In2S3 Z-scheme heterojunctions bridged by Ti3C2 quantum dots for efficient removal of hexavalent chromium and bisphenol A. J. Hazard. Mater. 2021, 409, 125027. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G.M. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Jo, W.K.; Natarajan, T.S. Influence of TiO2 morphology on the photocatalytic efficiency of direct Z-scheme g-C3N4/TiO2 photocatalysts for isoniazid degradation. Chem. Eng. J. 2015, 281, 549–565. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, R.; Wang, H.; Hu, J.; Ren, T. One-step impregnation method to prepare direct Z-scheme LaCoO3/g-C3N4 heterojunction photocatalysts for phenol degradation under visible light. Appl. Surf. Sci. 2019, 491, 432–442. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, J.; Qin, Y.; Song, F.; Du, C.; Su, Y. Direct Z-scheme SnO2/Bi2Sn2O7 photocatalyst for antibiotics removal: Insight on the enhanced photocatalytic performance and promoted charge separation mechanism. J. Photochem. Photobiol. A 2021, 404, 112947. [Google Scholar] [CrossRef]
- Shangguan, X.Y.; Fang, B.L.; Xu, C.X.; Tan, Y.; Chen, Y.G.; Xia, Z.J.; Chen, W. Fabrication of direct Z-scheme FeIn2S4/Bi2WO6 hierarchical heterostructures with enhanced photocatalytic activity for tetracycline hydrochloride photodagradation. Ceram. Int. 2021, 47, 6318–6328. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Zhang, J.; Wang, R.; Liu, H. Rational construction of a direct Z-scheme g-C3N4/CdS photocatalyst with enhanced visible light photocatalytic activity and degradation of erythromycin and tetracycline. Appl. Surf. Sci. 2019, 478, 1056–1064. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, L.Y.; Cheng, B.; Fan, J.J.; Yu, J.G. S-Scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Bao, Y.J.; Song, S.Q.; Yao, G.J.; Jiang, S.J. S-Scheme photocatalytic systems. Sol. RRL 2021, 5, 2100118. [Google Scholar] [CrossRef]
- Luo, Y.S.; Chi, Z.L.; Zhang, J.L.; Tian, B.Z. Photothermocatalytic system designed by facet-heterojunction to enhance the synergistic effect of toluene oxidation. ChemCatChem 2022, 14, e202101958. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Deng, F.; Luo, X.B.; Dionysiou, D.D. Rapid toxicity elimination of organic pollutants by the photocatalysis of environment-friendly and magnetically recoverable step-scheme SnFe2O4/ZnFe2O4 nano-heterojunctions. Chem. Eng. J. 2020, 379, 122264. [Google Scholar] [CrossRef]
- He, R.A.; Liu, H.J.; Liu, H.M.; Xu, D.F.; Zhang, L.Y. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar]
- Gogoi, D.; Makkar, P.; Ghosh, N.N. Solar light-irradiated photocatalytic degradation of model dyes and industrial dyes by a magnetic CoFe2O4–gC3N4 S-scheme heterojunction photocatalyst. ACS Omega 2021, 6, 4831–4841. [Google Scholar] [CrossRef]
- Jia, X.M.; Han, Q.F.; Liu, H.Z.; Li, S.Z.; Bi, H.P. A dual strategy to construct flowerlike S-scheme BiOBr/BiOAc1−xBrx heterojunction with enhanced visible-light photocatalytic activity. Chem. Eng. J. 2020, 399, 125701. [Google Scholar] [CrossRef]
- Dou, L.; Jin, X.Y.; Chen, J.F.; Zhong, J.B.; Li, J.Z.; Zeng, Y.; Duan, R. One-pot solvothermal fabrication of S-scheme OVs-Bi2O3/Bi2SiO5 microsphere heterojunctions with enhanced photocatalytic performance toward decontamination of organic pollutants. Appl. Surf. Sci. 2020, 527, 146775. [Google Scholar] [CrossRef]
- Lian, X.; Xue, W.H.; Dong, S.; Liu, E.Z.; Li, H.; Xu, K.Z. Construction of S-scheme Bi2WO6/g-C3N4 heterostructure nanosheets with enhanced visible-light photocatalytic degradation for ammonium dinitramide. J. Hazard. Mater. 2021, 412, 125217. [Google Scholar] [CrossRef]
- Huang, K.J.; Zhang, J.Z.; Fan, Y. Preparation of layered MoSe2 nanosheets on Ni-foam substrate with enhanced supercapacitor performance. Mater. Lett. 2015, 152, 244–247. [Google Scholar] [CrossRef]
- Eftekhari, A. Molybdenum diselenide (MoSe2) for energy storage, catalysis, and optoelectronics. Appl. Mater. Today 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Zhao, H.; Du, Y. Colloidally synthesized MoSe2/graphene hybrid nanostructures as efficient electrocatalysts for hydrogen evolution. J. Mater. Chem. A 2015, 3, 19706–19710. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, G.G.; Wan, X.; Xu, J.; Tang, H. High-efficiency all-solid-state Z-scheme Ag3PO4/g-C3N4/MoSe2 photocatalyst with boosted visible-light photocatalytic performance for antibiotic elimination. Appl. Surf. Sci. 2020, 530, 147234. [Google Scholar] [CrossRef]
- Zheng, X.T.; Yang, L.M.; Li, Y.B.; Yang, L.X.; Luo, S.L. Direct Z-scheme MoSe2 decorating TiO2 nanotube arrays photocatalyst for water decontamination. Electrochim. Acta 2019, 298, 663–669. [Google Scholar] [CrossRef]
- Tahir, M.B.; Asiri, A.M.; Nabi, G.; Rafique, M.; Sagir, M. Fabrication of heterogeneous photocatalysts for insight role of carbon nanofibre in hierarchical WO3/MoSe2 composite for enhanced photocatalytic hydrogen generation. Ceram. Int. 2019, 45, 5547–5552. [Google Scholar] [CrossRef]
- Zhang, S.M.; Chen, L.; Shen, J.; Li, Z.F.; Wu, Z.H.; Feng, W.H.; Xu, K.Q.; Xu, D.F.; Chen, X.H.; Zhang, S.Y. TiO2@MoSe2 line-to-face heterostructure: An advanced photocatalyst for highly efficient reduction of Cr (VI). Ceram. Int. 2019, 45, 18065–18072. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Xiao, X.Y.; Zhang, L.Z. Synthesis and facet-dependent phoyoreactivity of BiOCl single-crystalline nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef]
- Yang, S.; Shao, C.L.; Zhou, X.J.; Li, X.H.; Tao, R.; Li, X.W.; Liu, S.; Liu, Y.C. MoSe2/TiO2 nanofibers for cycling photocatalytic removing water pollutants under UV-Vis-NIR light. ACS Appl. Nano Mater. 2020, 3, 2278–2287. [Google Scholar] [CrossRef]
- Fan, C.; Wei, Z.; Yang, S.; Li, J. Synthesis of MoSe2 flower-like nanostructures and their photo-responsive properties. RSC Adv. 2014, 4, 775–778. [Google Scholar] [CrossRef]
- Xiao, P.; Lou, J.; Zhang, H.; Song, W.; Wu, X.L.; Lin, H.; Chen, J.; Liu, S.; Wang, X. Enhanced visible-light-driven photocatalysis from WS2 quantum dots coupled to BiOCl nanosheets: Synergistic effect and mechanism insight. Catal. Sci. Technol. 2018, 8, 201–209. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Tian, B.Z.; Zhang, J.L. Core-shell structural CdS@SnO2 nanorods with excellent visible-light photocatalytic activity for the selective oxidation of benzyl alcohol to benzaldehyde. ACS Appl. Mater. Interfaces 2015, 7, 13849–13858. [Google Scholar] [CrossRef]
- Wang, S.; Han, Y.; Luo, Y.; Ma, Y.; Zhang, J.; Tian, B. Au thorn-decorated TiO2 hierarchical microspheres with superior photocatalytic bactericidal activity under red and NIR light irradiation. J. Alloys Compd. 2022, 910, 164485. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Wang, X.; Zhang, J.; Xi, J.; Du, G.; Ji, Z. Mixed 3D/2D dimensional TiO2 nanoflowers/MoSe2 nanosheets for enhanced photoelectrochemical hydrogen generation. J. Am. Chem. Soc. 2019, 103, 1187–1196. [Google Scholar]
- Han, Y.Q.; Li, Q.Y.; Bao, S.Y.; Lu, Y.F.; Guan, Z.P.; Zhang, J.L.; Tian, B.Z. Z-scheme heterostructure BiOCl-Ag-AgBr with enhanced sunlight-driven photocatalytic activity in simultaneous removal of Cr6+ and phenol contaminants. Catal. Today 2021, 376, 151–161. [Google Scholar]
- Guan, Z.P.; Li, Q.Y.; Shen, B.; Bao, S.Y.; Zhang, J.L.; Tian, B.Z. Fabrication of Co3O4 and Au co-modified BiOBr flower-like microspheres with high photocatalytic efficiency for sulfadiazine degradation. Sep. Purif. Technol. 2020, 234, 116100. [Google Scholar] [CrossRef]
- Dion, M.C.L.; Fauzia, V.; Imawan, C. The Effect of deposition of MoSe2 nanosheets on the performance of a ZnO-based UV detector. J. Phys. Conf. Ser. 2021, 1951, 012006. [Google Scholar] [CrossRef]
- Kwon, I.S.; Kwak, I.H.; Debela, T.T.; Abbas, H.G.; Park, Y.C.; Ahn, J.; Park, J.; Kang, H.S. Se-rich MoSe2 nanosheets and their superior electrocatalytic performance for hydrogen evolution reaction. ACS Nano 2020, 14, 6295–6304. [Google Scholar] [CrossRef]
- Wang, G.Y.; Zhang, Y.Z.; You, C.Y.; Liu, B.Y.; Yang, Y.H.; Li, H.J.W.; Cui, A.J.; Liu, D.M.; Yan, H. Two dimensional materials based photodetectors. Infrared Phys. Technol. 2018, 88, 149–173. [Google Scholar] [CrossRef]
- Bao, S.Y.; Wang, Z.Q.; Gong, X.Q.; Zeng, C.Y.; Wu, Q.F.; Tian, B.Z.; Zhang, J.L. AgBr tetradecahedrons with co-exposed {100} and {111} facets: Simple fabrication and enhancing spatial charge separation using facet heterojunctions. J. Mater. Chem. A 2016, 4, 18570–18577. [Google Scholar] [CrossRef]
- Hong, X.D.; Li, Y.; Wang, X.; Long, J.P.; Liang, B. Carbon nanosheet/MnO2/BiOCl ternary composite for degradation of organic pollutants. J. Alloys Compd. 2022, 891, 162090. [Google Scholar] [CrossRef]
- Acharya, L.; Nayak, S.; Pattnaik, S.P.; Acharya, R.; Parida, K. Resurrection of boron nitride in p-n type-II boron nitride/B-doped-g-C3N4 nanocomposite during solid-state Z-scheme charge transfer path for the degradation of tetracycline hydrochloride. J. Colloid Interface Sci. 2020, 566, 211–223. [Google Scholar] [CrossRef]
- Tang, M.L.; Ao, Y.H.; Wang, P.F.; Wang, C. All-solid-state Z-scheme WO3 nanorod/ZnIn2S4 composite photocatalysts for the effective degradation of nitenpyram under visible light irradiation. J. Hazard. Mater. 2020, 387, 121713. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.W.; Fan, J.J.; Cheng, B.; Cao, S.W.; Yu, J.G. An Inorganic/Organic S-scheme heterojunction H2-production photocatalyst and its charge transfer mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.Z.; Fei, X.G.; Yang, Y.; Fan, J.J.; Yu, J.G.; Cheng, B.; Zhang, L.Y. S-scheme heterojunction based on p-type ZnMn2O4 and n-type ZnO with improved photocatalytic CO2 reduction activity. Chem. Eng. J. 2021, 409, 127377. [Google Scholar] [CrossRef]


| Sample | MoSe2 | BiOCl | BiOCl/MoSe2-10 | BiOCl/MoSe2-30 | BiOCl/MoSe2-50 |
|---|---|---|---|---|---|
| MO (min−1) | 0.0020 | 0.0027 | 0.0063 | 0.0307 | 0.0082 |
| SD (h−1) | 0.1246 | 0.3258 | 0.5829 | 0.9323 | 0.4004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chen, F.; Guan, Z.; Luo, Y.; Zhou, L.; Lu, Y.; Tian, B.; Zhang, J. S-Scheme BiOCl/MoSe2 Heterostructure with Enhanced Photocatalytic Activity for Dyes and Antibiotics Degradation under Sunlight Irradiation. Sensors 2022, 22, 3344. https://doi.org/10.3390/s22093344
Huang Y, Chen F, Guan Z, Luo Y, Zhou L, Lu Y, Tian B, Zhang J. S-Scheme BiOCl/MoSe2 Heterostructure with Enhanced Photocatalytic Activity for Dyes and Antibiotics Degradation under Sunlight Irradiation. Sensors. 2022; 22(9):3344. https://doi.org/10.3390/s22093344
Chicago/Turabian StyleHuang, Yan, Fan Chen, Zhipeng Guan, Yusheng Luo, Liang Zhou, Yufeng Lu, Baozhu Tian, and Jinlong Zhang. 2022. "S-Scheme BiOCl/MoSe2 Heterostructure with Enhanced Photocatalytic Activity for Dyes and Antibiotics Degradation under Sunlight Irradiation" Sensors 22, no. 9: 3344. https://doi.org/10.3390/s22093344






