Sensitive Biosensor Based on Shape-Controlled ZnO Nanostructures Grown on Flexible Porous Substrate for Pesticide Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of ZnO Nanostructures via Electrodeposition
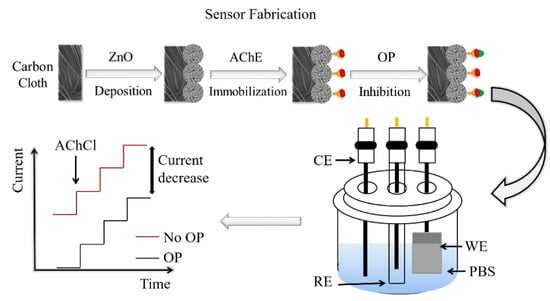
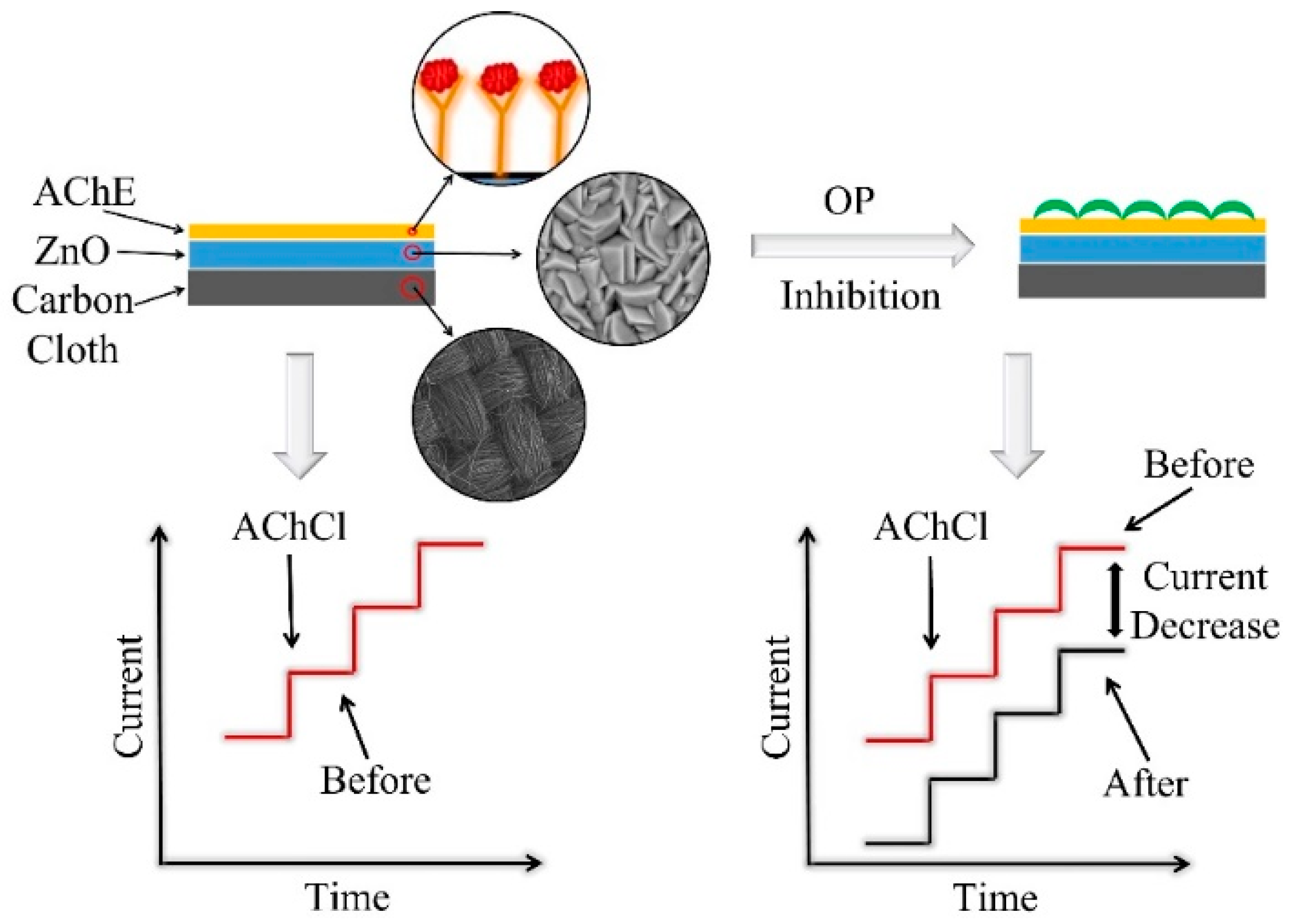
2.3. Fabrication of ZnO-Based Biosensors
2.4. Amperometric Measurements on ZnO-Based Biosensors
2.5. Characterization of ZnO Nanostructures
3. Results and Discussion
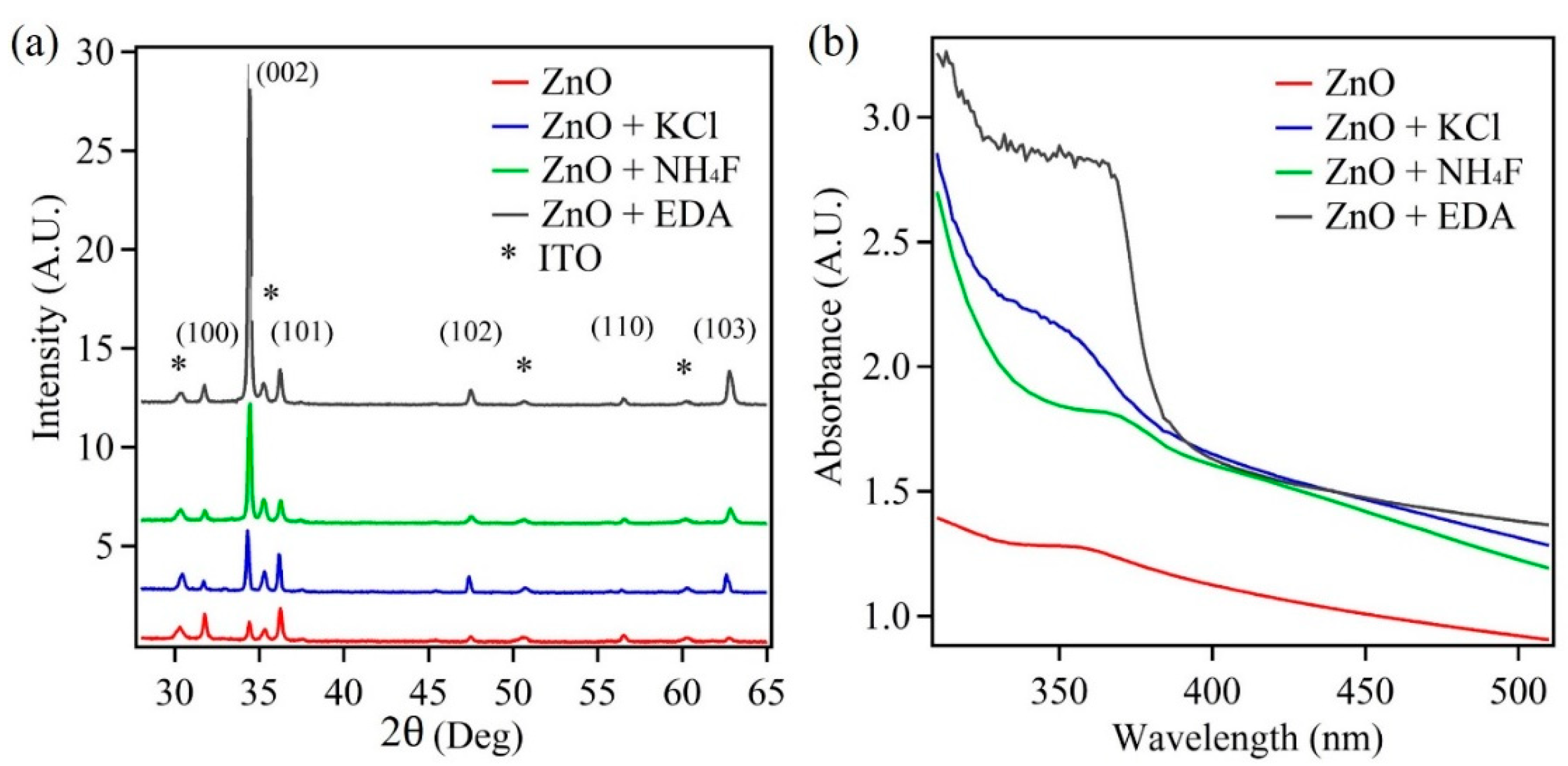
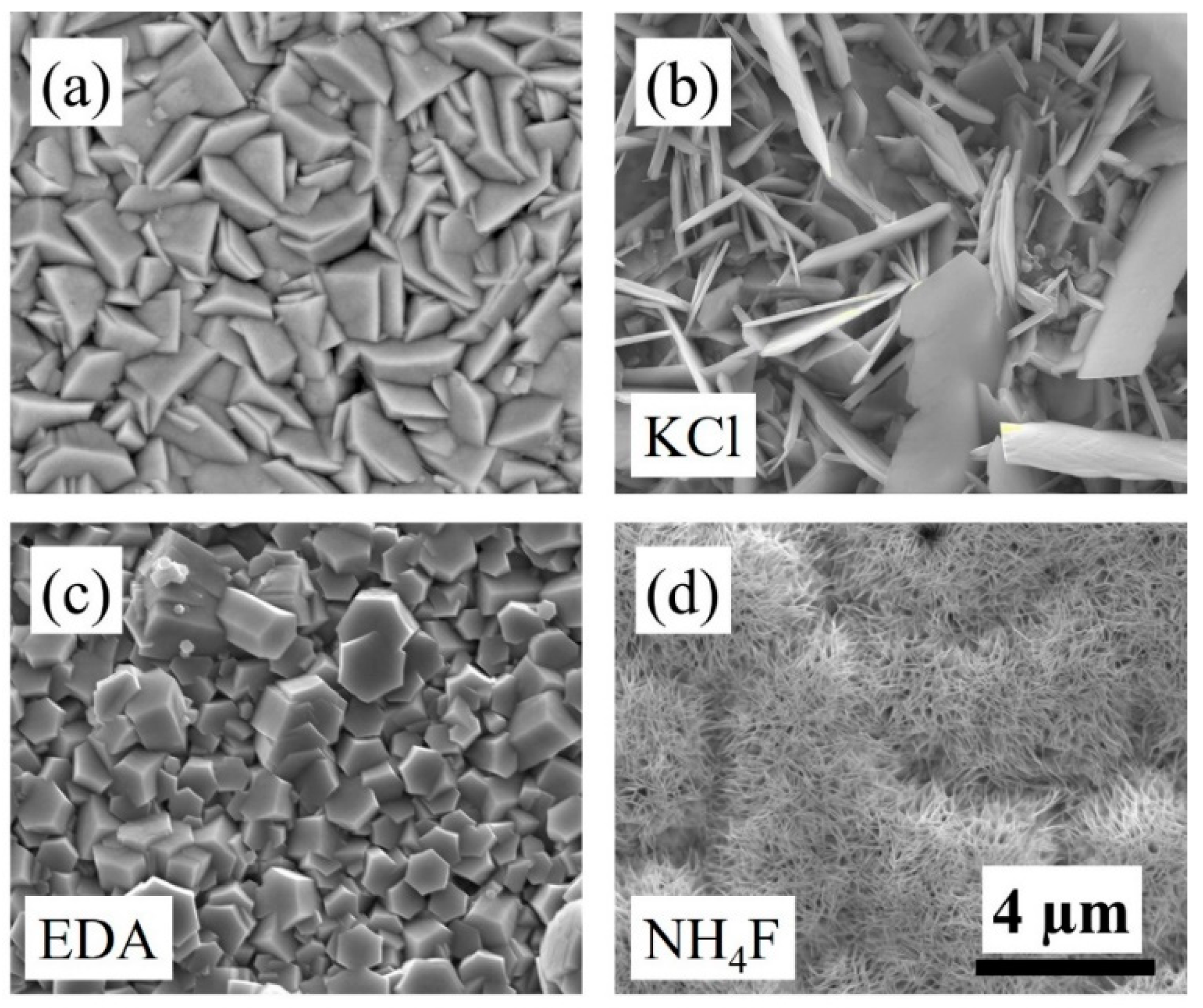
3.1. Crystal Structure, Composition, Absorption, and Morphology Studies of ZnO Nanostructures
3.2. Amperometric Response of ZnO Biosensors to AChCl: Influence of ZnO Morphology
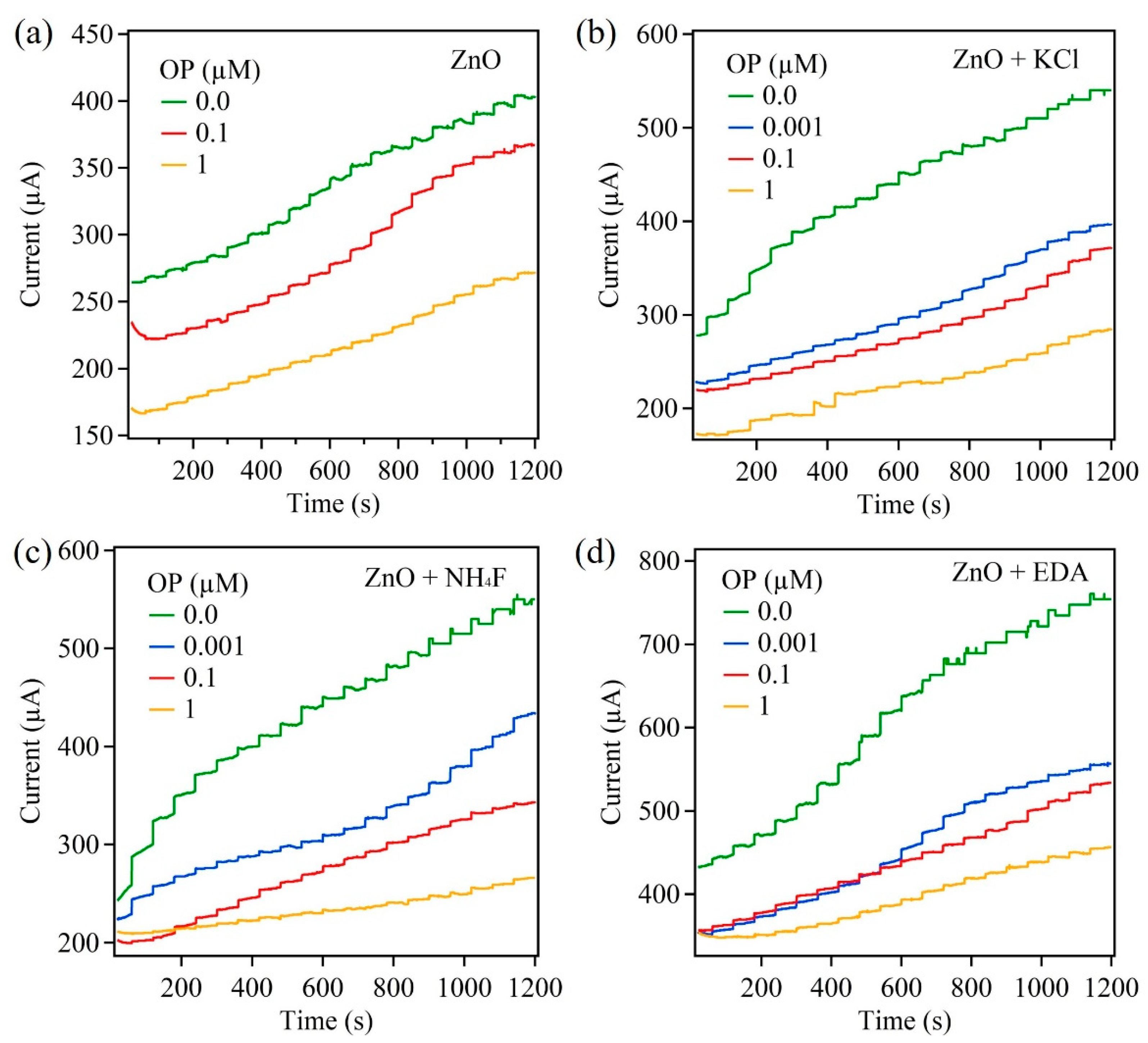
3.3. Amperometric Response of ZnO Biosensors to AChCl: Influence of Underlying Substrate
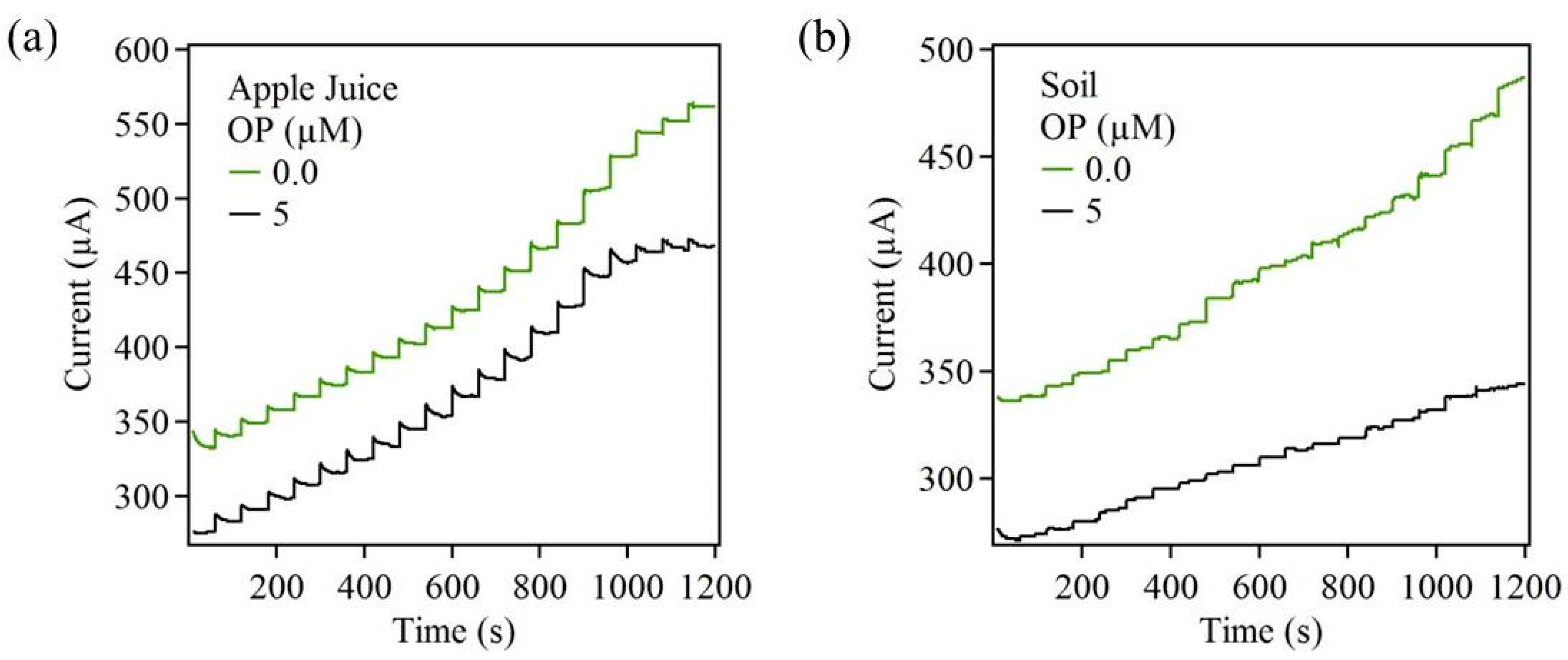
3.4. Performance Evaluation of ZnO-Based Biosensors for Detection of OP in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Kumar, P. Pesticides in agriculture and environment: Impacts on human health. Contam. Agric. Environ. Health Risks Remediat. 2019, 1, 76. [Google Scholar]
- Davydov, R.; Sokolov, M.; Hogland, W.; Glinushkin, A.; Markaryan, A. The application of pesticides and mineral fertilizers in agriculture. MATEC Web Conf. 2018, 245, 11003. [Google Scholar] [CrossRef] [Green Version]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Jamal, G. Neurological syndromes of organophosphorus compounds. Advers. Drug React. Toxicol. Rev. 1997, 16, 133–170. [Google Scholar]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- Sharma, N.; Singhvi, R. Effects of chemical fertilizers and pesticides on human health and environment: A review. Int. J. Agric. Environ. Biotechnol. 2017, 10, 675–680. [Google Scholar] [CrossRef]
- Masiá, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Picó, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef]
- Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Gaweł, M.; Borzęcka, M.; Posyniak, A. Multi-residue method for the determination of pesticides and pesticide metabolites in honeybees by liquid and gas chromatography coupled with tandem mass spectrometry—Honeybee poisoning incidents. J. Chromatogr. A 2016, 1435, 100–114. [Google Scholar] [CrossRef]
- Arjmand, M.; Saghafifar, H.; Alijanianzadeh, M.; Soltanolkotabi, M. A sensitive tapered-fiber optic biosensor for the label-free detection of organophosphate pesticides. Sens. Actuators B Chem. 2017, 249, 523–532. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Soares, M.S.; Marques, C.; Zhang, B.; Kumar, S. Convex fiber-tapered seven core fiber-convex fiber (CTC) structure-based biosensor for creatinine detection in aquaculture. Opt. Express 2022, 30, 13898–13914. [Google Scholar] [CrossRef]
- Cennamo, N.; Zeni, L.; Tortora, P.; Regonesi, M.E.; Giusti, A.; Staiano, M.; D’Auria, S.; Varriale, A. A High Sensitivity Biosensor to detect the presence of perfluorinated compounds in environment. Talanta 2018, 178, 955–961. [Google Scholar] [CrossRef]
- Leitão, C.; Leal-Junior, A.; Almeida, A.R.; Pereira, S.O.; Costa, F.M.; Pinto, J.L.; Marques, C. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol. Rep. 2021, 29, e00587. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Cheng, N.; Luo, Y.; Lin, Y.; Xu, W.; Du, D. Recent advances in nanomaterials-based electrochemical (bio) sensors for pesticides detection. TrAC Trends Anal. Chem. 2020, 132, 116041. [Google Scholar] [CrossRef]
- Kalyani, N.; Goel, S.; Jaiswal, S. On-site sensing of pesticides using point-of-care biosensors: A review. Environ. Chem. Lett. 2021, 19, 345–354. [Google Scholar] [CrossRef]
- Kanoun, O.; Lazarević-Pašti, T.; Pašti, I.; Nasraoui, S.; Talbi, M.; Brahem, A.; Adiraju, A.; Sheremet, E.; Rodriguez, R.D.; Ben Ali, M. A review of nanocomposite-modified electrochemical sensors for water quality monitoring. Sensors 2021, 21, 4131. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.-T.; Liu, Y.; Qi, X.-M.; Jin, H.-G.; Yang, C.; Liu, J.; Li, G.-L.; He, Q.-G. Recent advances in black phosphorus-based electrochemical sensors: A review. Anal. Chim. Acta 2021, 1170, 338480. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Zhang, G.; Wang, S.; Li, K.; Wu, J.; Wan, X.; Liu, Y.; Li, Q. Low-cost Voltammetric Sensors for Robust Determination of Toxic Cd (II) and Pb (II) in Environment and Food Based on Shuttle-like α-Fe2O3 Nanoparticles Decorated β-Bi2O3 Microspheres. Microchem. J. 2022, 179, 107515. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, M.; Mishra, P.; Jahan, N.; Ahsan, M.A.; Ahmad, I.; Khan, M.R.; Watanabe, Y.; Syed, M.A.; Furukawa, H. Engineered hierarchical CuO nanoleaves based electrochemical nonenzymatic biosensor for glucose detection. J. Electrochem. Soc. 2021, 168, 017501. [Google Scholar] [CrossRef]
- Ge, C.; Ramachandran, R.; Wang, F. CeO2-Based Two-Dimensional Layered Nanocomposites Derived from a Metal–Organic Framework for Selective Electrochemical Dopamine Sensors. Sensors 2020, 20, 4880. [Google Scholar] [CrossRef]
- Li, F.; Ni, B.; Zheng, Y.; Huang, Y.; Li, G. A simple and efficient voltammetric sensor for dopamine determination based on ZnO nanorods/electro-reduced graphene oxide composite. Surf. Interfaces 2021, 26, 101375. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Zhang, X.; Cheng, X.; Xu, Y.; Zhao, H.; Gao, S.; Huo, L. Morphology control of ZnO by adjusting the solvent and non-enzymatic nitrite ions electrochemical sensor constructed with stir bar-shaped ZnO/Nafion nanocomposite. Sens. Actuators B Chem. 2021, 346, 130525. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K. ZnO nanoparticles: Synthesis and adsorption study. Nat. Sci. 2009, 1, 129. [Google Scholar] [CrossRef] [Green Version]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Vanathi, P.; Rajiv, P.; Narendhran, S.; Rajeshwari, S.; Rahman, P.K.; Venckatesh, R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater. Lett. 2014, 134, 13–15. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Liewhiran, C.; Phanichphant, S. Improvement of flame-made ZnO nanoparticulate thick film morphology for ethanol sensing. Sensors 2007, 7, 650–675. [Google Scholar] [CrossRef] [Green Version]
- Yakimova, R.; Selegård, L.; Khranovskyy, V.; Pearce, R.; Lloyd Spetz, A.; Uvdal, K. ZnO materials and surface tailoring for biosensing. Front. Biosci. 2012, 4, 254–278. [Google Scholar] [CrossRef]
- Mulchandani, A.; Chen, W.; Mulchandani, P.; Wang, J.; Rogers, K.R. Biosensors for direct determination of organophosphate pesticides. Biosens. Bioelectron. 2001, 16, 225–230. [Google Scholar] [CrossRef]
- Dhull, V.; Gahlaut, A.; Dilbaghi, N.; Hooda, V. Acetylcholinesterase biosensors for electrochemical detection of organophosphorus compounds: A review. Biochem. Res. Int. 2013, 2013, 731501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-Z.; Su, F.-H.; Wang, K.; Jiang, W.; Men, X.-H.; Liu, W.-M. Study on the friction and wear properties of carbon fabric composites reinforced with micro-and nano-particles. Mater. Sci. Eng. A 2005, 404, 251–258. [Google Scholar] [CrossRef]
- Padalkar, S.; Schroeder, K.; Won, Y.; Jang, H.; Stanciu, L. Biotemplated silica and titania nanowires: Synthesis, characterization and potential applications. J. Nanosci. Nanotechnol. 2012, 12, 227–235. [Google Scholar] [CrossRef]
- Bel Hadj Tahar, N.; Bel Hadj Tahar, R.; Ben Salah, A.; Savall, A. Effects of individual layer thickness on the microstructure and optoelectronic properties of sol–gel-derived zinc oxide thin films. J. Am. Ceram. Soc. 2008, 91, 846–851. [Google Scholar] [CrossRef]
- Rogers, K.; Daniels, P. An X-ray diffraction study of the effects of heat treatment on bone mineral microstructure. Biomaterials 2002, 23, 2577–2585. [Google Scholar] [CrossRef]
- Asakuma, N.; Hirashima, H.; Imai, H.; Fukui, T.; Toki, M. Crystallization and Reduction of Sol-Gel-Derived Zinc Oxide Films by Irradiation with Ultraviolet Lamp. J. Sol-Gel Sci. Technol. 2003, 26, 181–184. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanoparticle Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Marie, M.; Mandal, S.; Manasreh, O. An Electrochemical Glucose Sensor Based on Zinc Oxide Nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef]
- Bao, J.; Huang, T.; Wang, Z.; Yang, H.; Geng, X.; Xu, G.; Samalo, M.; Sakinati, M.; Huo, D.; Hou, C. 3D graphene/copper oxide nano-flowers based acetylcholinesterase biosensor for sensitive detection of organophosphate pesticides. Sens. Actuators B Chem. 2019, 279, 95–101. [Google Scholar] [CrossRef]
- Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Electrochemical acetylcholinesterase biosensor based on ZnO nanocuboids modified platinum electrode for the detection of carbosulfan in rice. Biosens. Bioelectron. 2016, 77, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tan, X.; Zhou, Q.; Liu, Y.; Wang, M.; Yang, L. Synthesis of highly dispersed zinc oxide nanoparticles on carboxylic graphene for development a sensitive acetylcholinesterase biosensor. Sens. Actuators B Chem. 2014, 190, 730–736. [Google Scholar] [CrossRef]
- Guan, H.; Jiang, J.; Chen, D.; Wang, W.; Wang, Y.; Xin, J. Acetylcholinesterase biosensor based on chitosan/ZnO nanocomposites modified electrode for amperometric detection of pesticides. In Proceedings of the International Conference on Materials, Environmental and Biological Engineering, Guilin, China, 28–30 March 2015; Atlantis Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Lang, Q.; Han, L.; Hou, C.; Wang, F.; Liu, A. A sensitive acetylcholinesterase biosensor based on gold nanorods modified electrode for detection of organophosphate pesticide. Talanta 2016, 156–157, 34–41. [Google Scholar] [CrossRef]
- Di Tuoro, D.; Portaccio, M.; Lepore, M.; Arduini, F.; Moscone, D.; Bencivenga, U.; Mita, D. An acetylcholinesterase biosensor for determination of low concentrations of Paraoxon and Dichlorvos. New Biotechnol. 2011, 29, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Cyclic voltammetric acetylcholinesterase biosensor for the detection of captan in apple samples with the aid of chemometrics. Anal. Bioanal. Chem. 2015, 407, 4863–4868. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Du, D.; Lin, Y. Acetylcholinesterase biosensor based on a gold nanoparticle–polypyrrole–reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst 2014, 139, 3055–3060. [Google Scholar] [CrossRef]




| Sample (S) | ZnO (Ω) | ZnO + KCl (Ω) | ZnO + NH4F (Ω) | ZnO + EDA (Ω) |
|---|---|---|---|---|
| S | 9.35 ± 1.28 | 4.27 ± 0.69 | 3.56 ± 0.66 | 2.90 ± 0.61 |
| S + AChE | 9.56 ± 2.30 | 10.73 ± 0.81 | 9.85 ± 1.21 | 9.42 ± 0.39 |
| S + AChE + ATCl | 11.36 ± 1.54 | 10.55 ± 0.83 | 10.52 ± 1.37 | 9.60 ± 0.44 |
| S + AChE + OP + ATCl | 18.77 ± 2.47 | 15.24 ± 2.67 | 13.88 ± 2.38 | 11.4 ± 0.69 |
| Sample (S) | ZnO (Ω) | ZnO + KCl (Ω) | ZnO + NH4F (Ω) | ZnO + EDA (Ω) |
|---|---|---|---|---|
| S | 3.67 ± 0.67 | 2.20 ± 0.37 | 2.51 ± 0.42 | 1.10 ± 0.18 |
| S + AChE | 10.65 ± 0.29 | 8.42 ± 0.42 | 9.09 ± 0.32 | 8.74 ± 0.18 |
| S + AChE + ATCl | 10.38 ± 0.27 | 8.23 ± 0.64 | 10.99 ± 0.33 | 9.90 ± 0.11 |
| S + AChE + OP + ATCl | 14.96 ± 1.27 | 12.3 ± 1.29 | 12.81 ± 5.69 | 10.08 ± 0.84 |
| Working Electrode | LOD (nM) | Linear Range (µM) | Reference |
|---|---|---|---|
| ZnO + EDA/AChE (Paper) | 0.5 | 0.001–1.0 | Present work |
| ZnO + EDA/AChE (Cloth) | 0.4 | 0.001–1.0 | Present work |
| Pt/ZnO/AChE/Chitosan | 0.24 | ------ | [43] |
| ZnO NPs-CGR-NF/GCE | 100 | 0.2–30.0 | [44] |
| ZnO/CHI/AChE | 10 | 1.75–10.0 | [45] |
| AChE/AuNRs/GCE | 0.7 | 0.001–5.0 | [46] |
| AChE-Carbon Paste/CuE | 3.1 | 0.0–0.12 | [47] |
| Pt/ZnO/AChE | 107 | 0.05–25.0 | [48] |
| Au–PPy–rGO | 0.5 | 0.001–5.0 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallatah, A.; Kuperus, N.; Almomtan, M.; Padalkar, S. Sensitive Biosensor Based on Shape-Controlled ZnO Nanostructures Grown on Flexible Porous Substrate for Pesticide Detection. Sensors 2022, 22, 3522. https://doi.org/10.3390/s22093522
Fallatah A, Kuperus N, Almomtan M, Padalkar S. Sensitive Biosensor Based on Shape-Controlled ZnO Nanostructures Grown on Flexible Porous Substrate for Pesticide Detection. Sensors. 2022; 22(9):3522. https://doi.org/10.3390/s22093522
Chicago/Turabian StyleFallatah, Ahmad, Nicolas Kuperus, Mohammed Almomtan, and Sonal Padalkar. 2022. "Sensitive Biosensor Based on Shape-Controlled ZnO Nanostructures Grown on Flexible Porous Substrate for Pesticide Detection" Sensors 22, no. 9: 3522. https://doi.org/10.3390/s22093522
APA StyleFallatah, A., Kuperus, N., Almomtan, M., & Padalkar, S. (2022). Sensitive Biosensor Based on Shape-Controlled ZnO Nanostructures Grown on Flexible Porous Substrate for Pesticide Detection. Sensors, 22(9), 3522. https://doi.org/10.3390/s22093522