STIMGRASP: A Home-Based Functional Electrical Stimulator for Grasp Restoration in Daily Activities
Abstract
1. Introduction
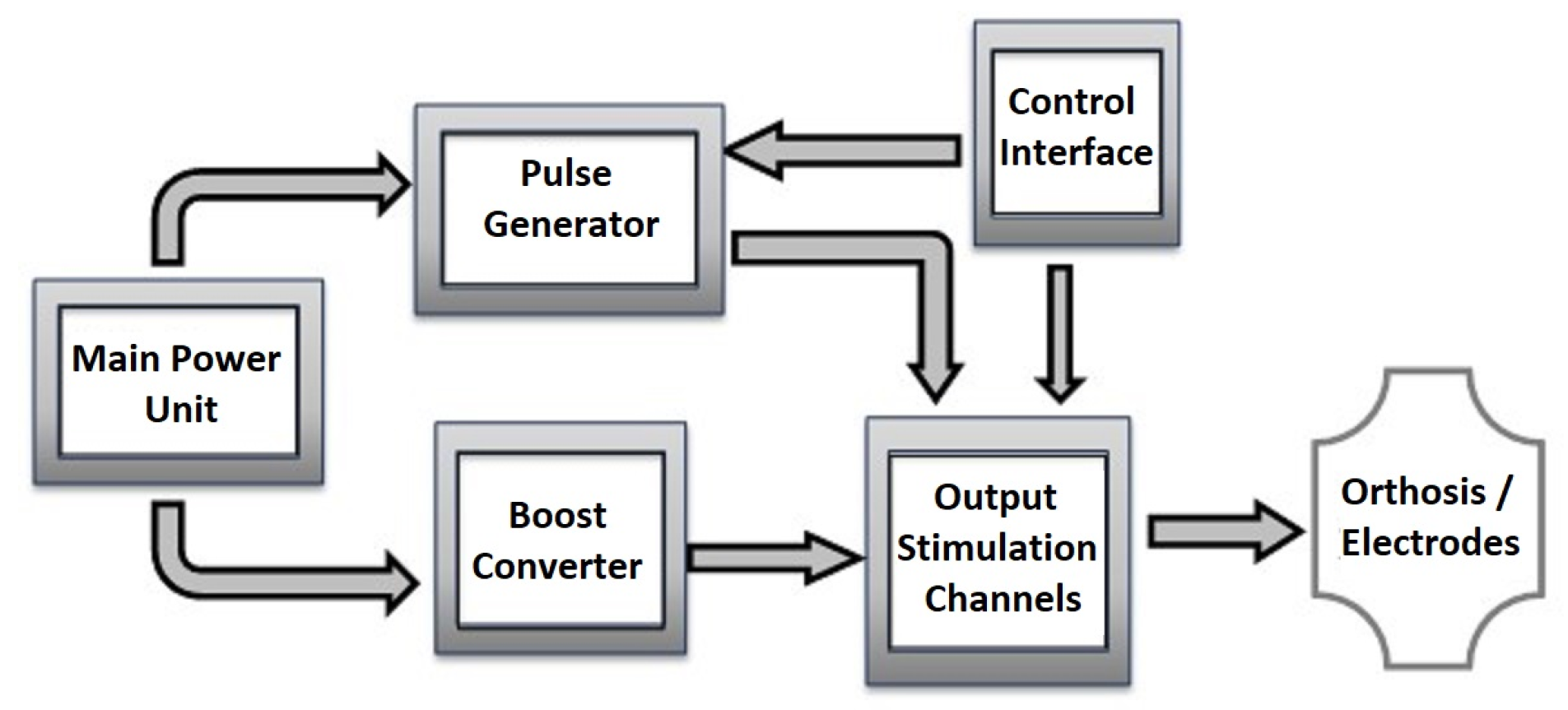
Basic Stimulator Architecture
2. Materials and Methods
2.1. Specifications
- 1.
- Portability
- Miniaturization: the proposed stimulator must have its dimensions and weight reduced to be moved by the user without effort, which is important for functionality and discretion as an assistive device.
- Battery life: the circuit consumption and battery capacity should allow one to use the system during the day and charge it at night.
- 2.
- Wearability
- Easiness to wear the equipment: the more tasks the individual can carry out without aid from other people, the better their self-esteem will be. Thus, there is a need to guarantee user independence to don and doff the system.
- 3.
- Usability
- User-friendliness: the control interface must be user-friendly, considering the user’s motor limitations, especially for people with tetraplegia due to the lack of hand dexterity or even the impossibility of controlling finger movements.
- 4.
- Characteristics
- Microcontrolled 16-bit architecture, PIC24 series system.
- USB or Bluetooth communication (selectable).
- Symmetric biphasic constant current waveform.
- Independent stimulation outputs up to 8 channels.
- Stimulation parameters in commonly used range.
- Powered by rechargeable lithium-ion battery.
- LEDs’ general status indication.
2.2. Circuit Design
2.2.1. Power Sources
2.2.2. Biphasic Pulse Generation
2.2.3. Output Channel Multiplexing
2.2.4. Data Communication Channels
2.2.5. The Microcontroller’s Firmware
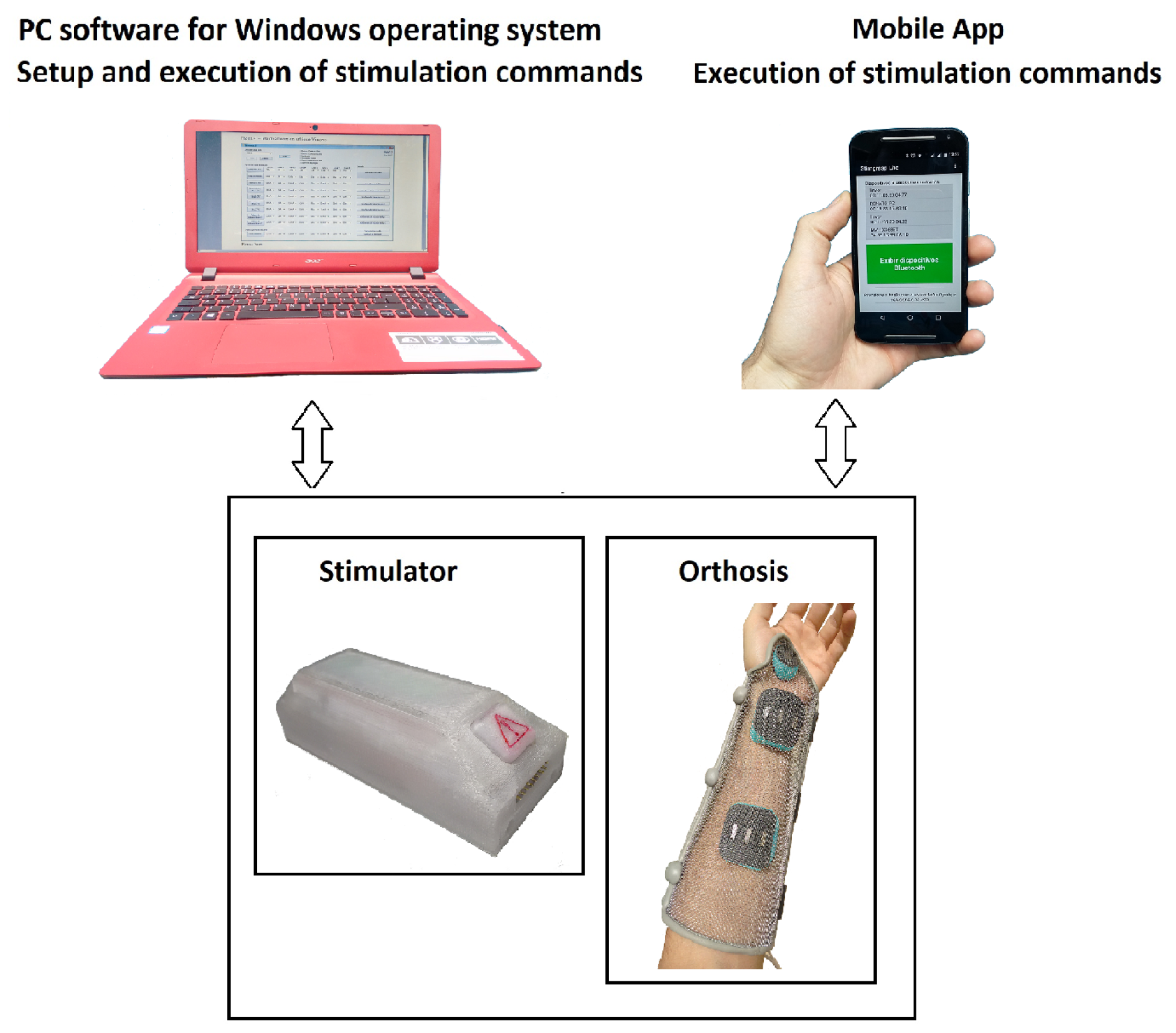
2.3. Configuration and Control Platforms
2.4. Orthosis
2.5. System Validation
- Donning and doffing the orthosis.
- The maintenance of the electrode positioning after configuration by the health professional and orthosis donning.
- Stimulus sequence configuration on the health professional’s platform.
- Mobile application command by the user.
- Generation and application of stimulus sequences for hand opening, palmar and lateral grasping, and forefinger extension.
3. Results
3.1. Hardware Assembly
3.2. Battery Life
- Moderate use: In this situation, the sequences of palmar grasp, lateral grasp, and forefinger extension (each of them with a 20-s duration) were alternated every 40 s, with a stimulation intensity between 4.8 and 6.6 mA. In that case, the battery lasted 14 h and 30 min until the automatic shutdown of the stimulator (when it reached 3.03 V).
- Intense use: In this situation, the sequences of moderate use were repeated with three times more stimulation intensity (from 15 to 20 mA). In that case, the battery lasted 13 h and 30 min until the automatic shutdown of the stimulator.
- Maximum use: In this case, all channels were considered with the maximum output amplitude (40 mA), resulting in a total current battery consumption of 440 mA. In that situation, the battery lasted 5 h and 40 min.
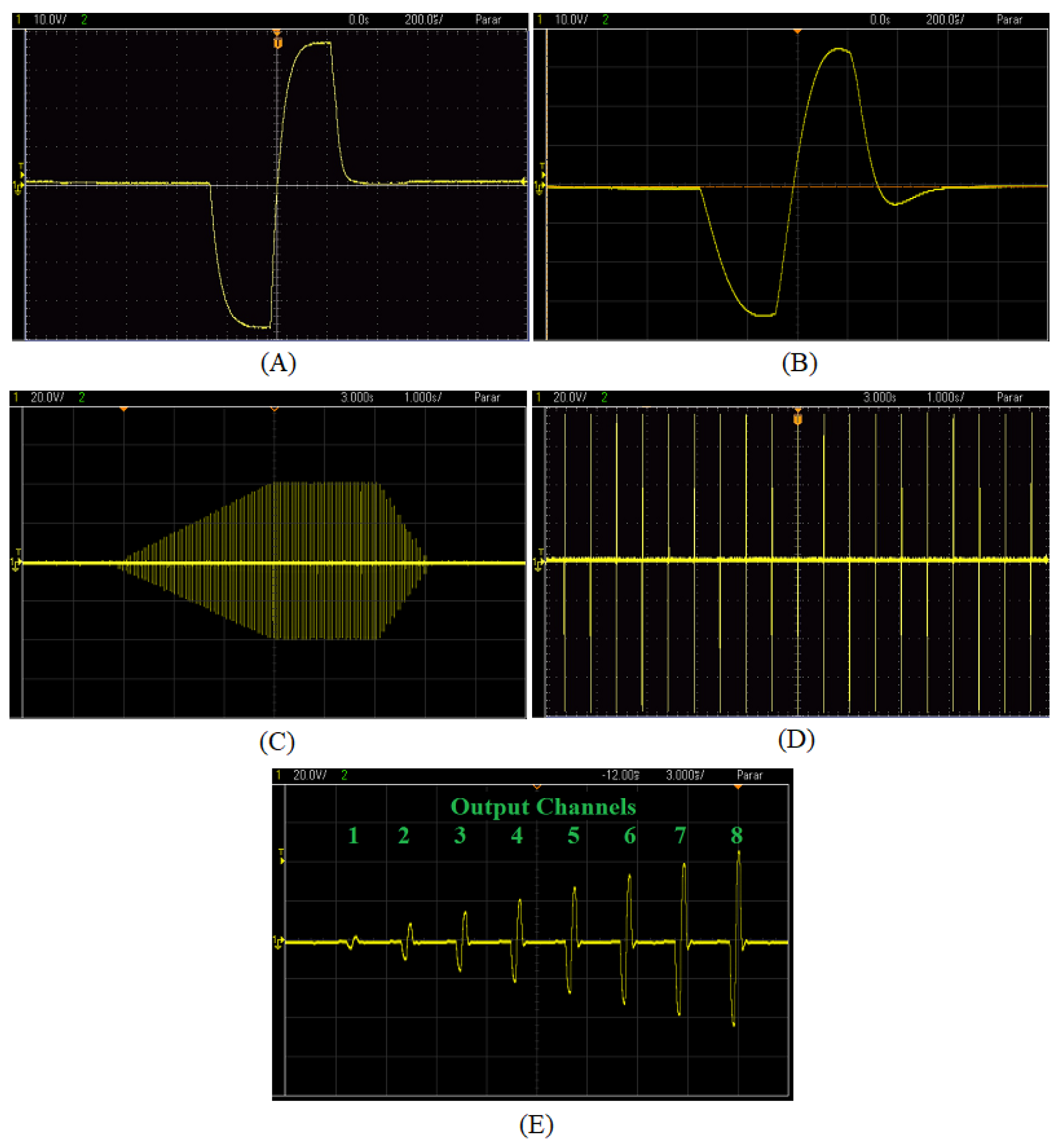
3.3. Output Pulses Generation
3.4. System Validation
4. Discussion
5. Conclusions
- 1.
- Social
- The system aims to reinsert individuals with SCI and hemiplegia in society by increasing their independence to carry out ADLs without aid from other people.
- 2.
- Therapeutic
- Electrical stimulation keeps the muscles conditioned, avoiding muscular atrophy and joint stiffness.
- Although the system is focused on executing functional grasping as an assistive device with a mobile app control platform, it can also be used in therapy sessions with the same software program and a Windows operating system platform for configuration and therapy.
- 3.
- Functional
- The system successfully provided hand opening, palmar and lateral grasping, and forefinger extension movements. However, since it was validated in a single-section pilot experiment, clinical trials must be done to complete system evaluation.
- The USB–serial converter/Bluetooth modules communication allowed other sensors to be connected to the system, increasing the number of control possibilities.
- The orthosis allowed system and electrode fixation, all in one piece, qualifying as an assistive device for ADLs. Although the orthosis was easy to don and doff, the electrodes moved during orthosis donning, requiring improvements.
- The battery life allowed the system to be used during the day and charged at night.
- 4.
- Technical
- The system provided eight stimulation channels, with more efficient circuits and modern components, in a smaller physical size.
- A derivative compensation negative feedback was proposed to accelerate the circuit response considering an RC load, which better represented the electrode–tissue impedance, partially correcting the system response for a critically dampened behavior. However, results showed a small charge imbalance, requiring further adjustments. The compensation is a complex solution since the load value is unknown.
- The developed system was characterized as an open-loop system (there was no feedback for the user regarding the force applied in the generated grasp). However, there are additional circuits expected in the hardware platform, which could transform it into a closed-loop system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Neurological Disorders: Public Health Challenges; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization (WHO). Spinal Cord Injury. Fact Sheets. 2013. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 23 March 2022).
- Bickenbach, J.; Officer, A.; Shakespeare, T.; von Groote, P.; World Health Organization; Society, T.I.S.C. International Perspectives on Spinal Cord Injury; World Health Organization: Geneva, Switzerland, 2013; 231p, Available online: https://apps.who.int/iris/handle/10665/94190 (accessed on 23 March 2022).
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3375668/ (accessed on 23 March 2022). [PubMed]
- Marquez-Chin, C.; Popovic, M.R. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: A review. Biomed. Eng. Online 2020, 19, 34. [Google Scholar] [CrossRef]
- Ragnarsson, K.T. Functional electrical stimulation after spinal cord injury: Current use, therapeutic effects and future directions. Spinal Cord 2008, 46, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Rupp, R.; Rohm, M.; Schneiders, M.; Kreilinger, A.; Müller-Putz, G.R. Functional Rehabilitation of the Paralyzed Upper Extremity After Spinal Cord Injury by Noninvasive Hybrid Neuroprostheses. Proc. IEEE 2015, 103, 954–968. [Google Scholar] [CrossRef]
- Venugopalan, L.; Taylor, P.N.; Cobb, J.E.; Swain, I.D. Upper limb functional electrical stimulation devices and their man–machine interfaces. J. Med. Eng. Technol. 2015, 39, 471–479. [Google Scholar] [CrossRef]
- Kapadia, N.; Moineau, B.; Popovic, M.R. Functional Electrical Stimulation Therapy for Retraining Reaching and Grasping After Spinal Cord Injury and Stroke. Front. Neurosci. 2020, 14, 718. [Google Scholar] [CrossRef]
- Peckham, P.H.; Keith, M.W.; Kilgore, K.L.; Grill, J.H.; Wuolle, K.S.; Thrope, G.B.; Gorman, P.; Hobby, J.; Mulcahey, M.J.; Carroll, S.; et al. Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: A multicenter study. Arch. Phys. Med. Rehabil. 2001, 82, 1380–1388. [Google Scholar] [CrossRef]
- Degnan, G.G.; Wind, T.C.; Jones, E.V.; Edlich, R. A Review on Functional Electrical Stimulation in Tetraplegic Patients to Restore Hand Function. J. Long-Term Eff. Med. Implant. 2017, 27, 257–268. [Google Scholar] [CrossRef]
- Prochazka, A.; Gauthier, M.; Wieler, M.; Kenwell, Z. The bionic glove: An electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch. Phys. Med. Rehabil. 1997, 78, 608–614. [Google Scholar] [CrossRef]
- IJzerman, M.; Stoffers, T.S.; Groen, F.I.T.; Klatte, M.A.P.; Snoek, G.J.; Vorsteveld, J.H.C.; Nathan, R.H.; Hermens, H.J. The NESS handmaster orthosis: Restoration of hand function in C5 and stroke patients by means of electrical stimulation. J. Rehabil. Sci. 2012, 9, 86–89. Available online: https://ris.utwente.nl/ws/portalfiles/portal/6857142/IJzerman96ness.pdf (accessed on 17 June 2022).
- Popovic, D.; Kekker, T. Modular transcutaneous functional electrical stimulation system. Med. Eng. Phys. 2005, 27, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Mangold, S.; Keller, T.; Curt, A.; Dietz, V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord 2005, 43, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rupp, R.; Kreilinger, A.; Rohm, M.; Kaiser, V.; Müller-Putz, G.R. Development of a non-invasive, multifunctional grasp neuroprosthesis and its evaluation in an individual with a high spinal cord injury. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2012, 2012, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhou, Y.; Wang, Y.; Zhang, J.; Lü, X.; Wang, Z. Electrode placement on the forearm for selective stimulation of finger extension/flexion. PLoS ONE 2018, 13, e0190936. [Google Scholar] [CrossRef] [PubMed]
- Malešević, N.M.; Maneski, L.Z.P.; Ilić, V.; Jorgovanović, N.; Bijelić, G.; Keller, T.; Popović, D.B. A multi-pad electrode based functional electrical stimulation system for restoration of grasp. J. Neuroeng. Rehabil. 2012, 9, 66. [Google Scholar] [CrossRef]
- Argentim, L.M.; Castro, M.C.F.; Tomaz, P.A. Human Interface for a Neuroprothesis Remotely Control. In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies—BIODEVICES, INSTICC, SciTePress, Madeira, Portugal, 19–21 January 2018; pp. 247–253. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Piscitelli, D.; Norouzi-Gheidari, N.; Batalla, M.A.P.; Archambault, P.; Levin, M.F. Electromyogram-Related Neuromuscular Electrical Stimulation for Restoring Wrist and Hand Movement in Poststroke Hemiplegia: A Systematic Review and Meta-Analysis. Neurorehabilit. Neural Repair 2019, 33, 96–111. [Google Scholar] [CrossRef]
- Tabernig, C.B.; Lopez, C.A.; Carrere, L.C.; Spaich, E.G.; Ballario, C.H. Neurorehabilitation therapy of patients with severe stroke based on functional electrical stimulation commanded by a brain computer interface. J. Rehabil. Assist. Technol. Eng. 2018, 5, 2055668318789280. [Google Scholar] [CrossRef]
- Jovanovic, L.I.; Desai, N.; Marquez-Chin, C. Restoration of Upper Limb Voluntary Motor Function in Chronic Severe Hemiplegia Using a Brain-Computer Interface-Triggered Functional Electrical Stimulation Therapy. In Proceedings of the 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC), Bari, Italy, 6–9 October 2019; pp. 1292–1297. [Google Scholar] [CrossRef]
- Choi, I.; Kwon, G.H.; Lee, S.; Nam, C.S. Functional Electrical Stimulation Controlled by Motor Imagery Brain-Computer Interface for Rehabilitation. Brain Sci. 2020, 10, 512. [Google Scholar] [CrossRef]
- Chen, L.; Gu, B.; Wang, Z.; Zhang, L.; Xu, M.; Liu, S.; He, F.; Ming, D. EEG-controlled functional electrical stimulation rehabilitation for chronic stroke: System design and clinical application. Front. Med. 2021, 15, 740–749. [Google Scholar] [CrossRef]
- Hernandez-Rojas, L.G.; Cantillo-Negrete, J.; Mendoza-Montoya, O.; Carino-Escobar, R.I.; Leyva-Martinez, I.; Aguirre-Guemez, A.V.; Barrera-Ortiz, A.; Carrillo-Mora, P.; Antelis, J.M. Brain-Computer Interface Controlled Functional Electrical Stimulation: Evaluation With Healthy Subjects and Spinal Cord Injury Patients. IEEE Access 2022, 10, 46834–46852. [Google Scholar] [CrossRef]
- Souza, D.C.; Gaiotto, M.d.C.; Nogueira Neto, G.N.; Castro, M.C.F.; Nohama, P. Power amplifier circuits for functional electrical stimulation systems. Res. Biomed. Eng. 2017, 33, 144–155. [Google Scholar] [CrossRef][Green Version]
- Qu, H.; Wang, T.; Hao, M.; Shi, P.; Zhang, W.; Wang, G.; Lan, N. Development of a network FES system for stroke rehabilitation. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 3119–3122. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Xie, Y.; Liu, X.; He, X.; Hao, M.; Bao, Y.; Xie, Q.; Lan, N. Development of network-based multichannel neuromuscular electrical stimulation system for stroke rehabilitation. J. Rehabil. Res. Dev. 2016, 53, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huang, T.; He, J.; Wang, Y.; Zhou, H. A programmable multi-channel stimulator for array electrodes in transcutaneous electrical stimulation. In Proceedings of the The 2011 IEEE/ICME International Conference on Complex Medical Engineering, Harbin, China, 22–25 May 2011; pp. 652–656. [Google Scholar] [CrossRef]
- Khosravani, S.; Lahimgarzadeh, N.; Maleki, A. Developing a stimulator and an interface for FES-cycling rehabilitation system. In Proceedings of the 18th Iranian Conference of Biomedical Engineering (ICBME), Tehran, Iran, 14–16 December 2011; pp. 175–180. [Google Scholar] [CrossRef]
- Masdar, A.; Ibrahim, B.S.K.K.; Jamil, M.M.A.; Hanafi, D.; Ahmad, M.K.I.; Rahman, K.A.A. Current source with low voltage controlled for surface Electrical Stimulation. In Proceedings of the IEEE 9th International Colloquium on Signal Processing and its Applications, Kuala Lumpur, Malaysia, 8–10 March 2013; pp. 161–164. [Google Scholar] [CrossRef]
- Wang, H.P.; Wang, Z.G.; Lü, X.Y.; Huang, Z.H.; Zhou, Y.X. Design of a pulse-triggered four-channel functional electrical stimulator using complementary current source and time division multiplexing output method. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 1671–1674. [Google Scholar] [CrossRef]
- Patriciu, A.; Yoshida, K.; Struijk, J.J.; DeMonte, T.P.; Joy, M.L.G.; Stødkilde-Jørgensen, H. Current Density Imaging and Electrically Induced Skin Burns Under Surface Electrodes. IEEE Trans. Biomed. Eng. 2005, 52, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Subramanya, K.; Ajithanjaya Kumar, M.K. Design of a programmable flyback based FES system for restoring foot drop in stroke rehabilitation. In Proceedings of the 2015 Annual IEEE India Conference (INDICON), New Delhi, India, 17–20 December 2015; pp. 1–5. [Google Scholar] [CrossRef]
- Bîrlea, S.I.; Breen, P.P.; Corley, G.J.; Bîrlea, N.M.; Quondamatteo, F.; ÓLaighin, G. Changes in the electrical properties of the electrode-skin-underlying tissue composite during a week-long programme of neuromuscular electrical stimulation. Physiol. Meas. 2014, 35, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Vargas Luna, J.L.; Krenn, M.; Cortés Ramírez, J.A.; Mayr, W. Dynamic Impedance Model of the Skin-Electrode Interface for Transcutaneous Electrical Stimulation. PLoS ONE 2015, 10, e0125609. [Google Scholar] [CrossRef]
- Castro, M.C.F.; Cliquet, A., Jr. Artificial Grasping System for the Paralyzed Hand. Artif. Organs 2000, 24, 185–188. [Google Scholar] [CrossRef]
- Castro, M.C.F.; Cliquet, A., Jr. Neuromuscular Electrical Stimulation and Electron-tactile Stimulation in Rehabilitation of Artificial Prehension and Proprioception in Tetraplegic Patients. Acta Ortop. Bras. 2001, 9, 19–28. [Google Scholar] [CrossRef]
- Pribeanu, C.A. Revised Set of Usability Heuristics for the Evaluation of Interactive Systems. Inform. Econ. 2017, 21, 31–38. [Google Scholar] [CrossRef]
- Bioness Inc. Ness H200 Wireless—User’s Guide; Bioness Inc.: Valencia, CA, USA, 2018; Available online: https://www.bioness.com/Documents/H200Consumer/MP612-00964-001%20Rev.%20B%20[efile].pdf (accessed on 10 September 2022).
- Moss, C.W.; Kilgore, K.L.; Peckham, P.H. A Novel Command Signal for Motor Neuroprosthetic Control. Neurorehabilit. Neural Repair 2011, 25, 847–854. [Google Scholar] [CrossRef]
- Balbinot, G.; Li, G.; Wiest, M.J.; Pakosh, M.; Furlan, J.C.; Kalsi-Ryan, S.; Zariffa, J. Properties of the surface electromyogram following traumatic spinal cord injury: A scoping review. Neuroeng. Rehabil 2021, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Simão, C.R.; DE Holanda, L.J.; Urbini, L.F.; Lacerda, M.O.; Fernandes, K.; DA Silva, P.M.; Morya, E.; Lindquist, A.R. Surface electromyography to identify top-down modulation in complete chronic spinal cord injury. Eur. J. Phys. Rehabil. Med. 2022, 58, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Marquez-Chin, C.; Masani, K.; Hirata, M.; Nomura, T.; Popovic, M.R.; Nakazawa, K. Why brain-controlled neuroprosthetics matter: Mechanisms underlying electrical stimulation of muscles and nerves in rehabilitation. BioMed. Eng. OnLine 2020, 19, 81. [Google Scholar] [CrossRef] [PubMed]









| Subphases | Muscles | ||||
|---|---|---|---|---|---|
| Opening | ECR | EDC | AbPB | ||
| Positioning | ECR | AbPB | L | ||
| Grasp | ECR | AbPB | L | FDS | OpP |
| Releasing | ECR | EDC | AbPB | ||
| Subphases | Muscles | |||
|---|---|---|---|---|
| Opening | ECR | EDC | AbPB | |
| Positioning | ECR | AbPB | FDS | |
| Grasp | ECR | AbPB | FDS | OpP |
| Releasing | ECR | EDC | AbPB | |
| Subphases | Muscles | ||
|---|---|---|---|
| Opening | ECR | EDC | AbPB |
| Positioning | L | FDS | |
| Opening | ECR | EDC | AbPB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barelli, R.G.; Avelino, V.F.; Castro, M.C.F. STIMGRASP: A Home-Based Functional Electrical Stimulator for Grasp Restoration in Daily Activities. Sensors 2023, 23, 10. https://doi.org/10.3390/s23010010
Barelli RG, Avelino VF, Castro MCF. STIMGRASP: A Home-Based Functional Electrical Stimulator for Grasp Restoration in Daily Activities. Sensors. 2023; 23(1):10. https://doi.org/10.3390/s23010010
Chicago/Turabian StyleBarelli, Renato G., Valter F. Avelino, and Maria Claudia F. Castro. 2023. "STIMGRASP: A Home-Based Functional Electrical Stimulator for Grasp Restoration in Daily Activities" Sensors 23, no. 1: 10. https://doi.org/10.3390/s23010010
APA StyleBarelli, R. G., Avelino, V. F., & Castro, M. C. F. (2023). STIMGRASP: A Home-Based Functional Electrical Stimulator for Grasp Restoration in Daily Activities. Sensors, 23(1), 10. https://doi.org/10.3390/s23010010






