Photoluminescent Sensor of Scarification Efficiency of Fodder Plants’ Seeds
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
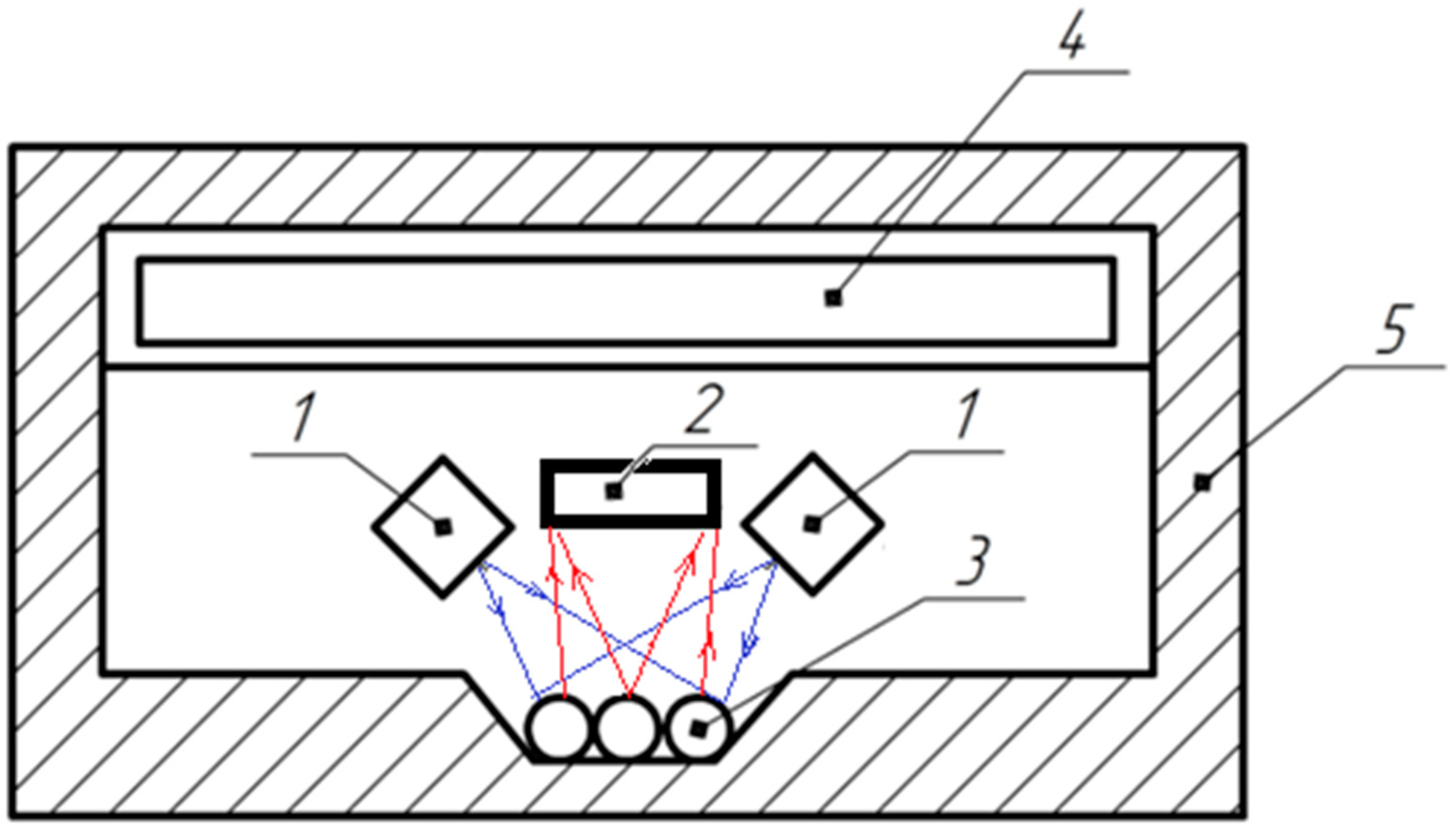
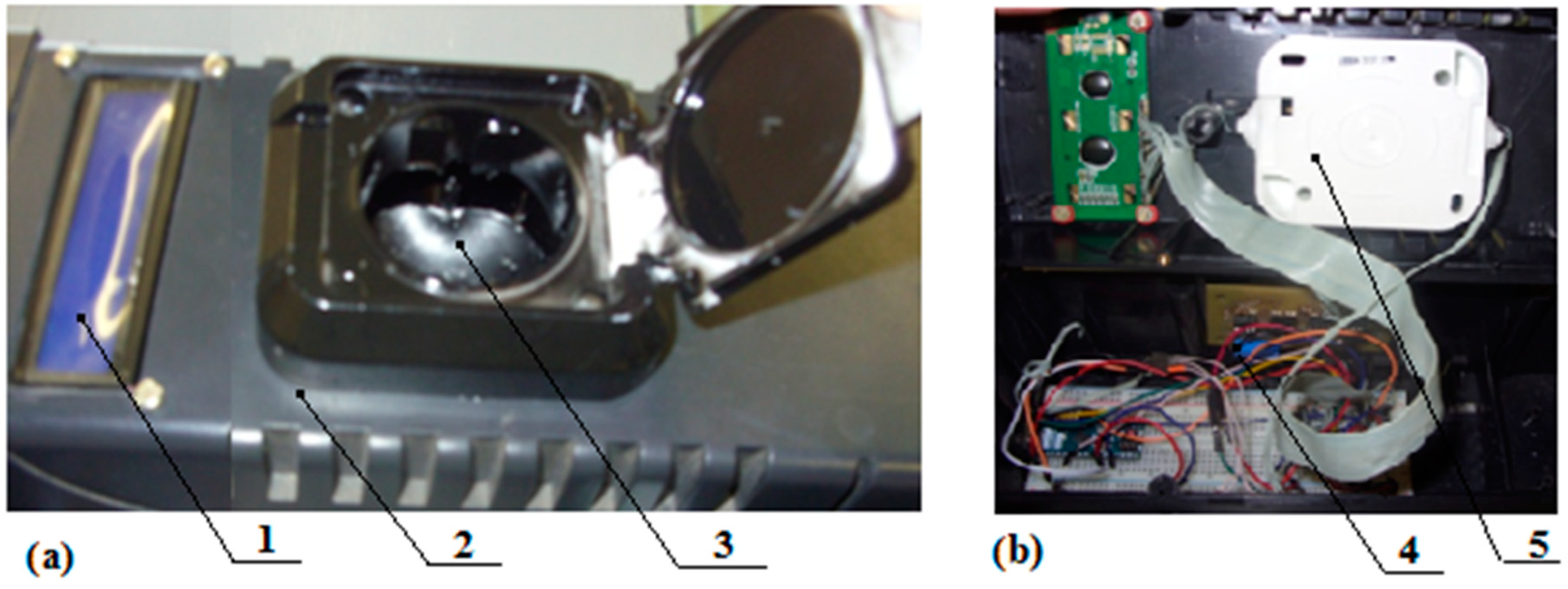
- Forage plants seeds such as galega, clover and alfalfa undergo a process of mechanical scarification. After it, all of the scarified seeds or an experimental sample enter a dark light-tight housing. It is also possible to integrate the technological process of rapid germination diagnostics into the technological processes of scarification for continuous monitoring of its effectiveness.
- Simultaneously with the first stage, the type of seeds (culture, sport) and other seed parameters, such as humidity or clogging, can be measured (or can be set otherwise, for example, according to the supporting documentation), which is necessary to establish the appropriate diagnostic algorithm.
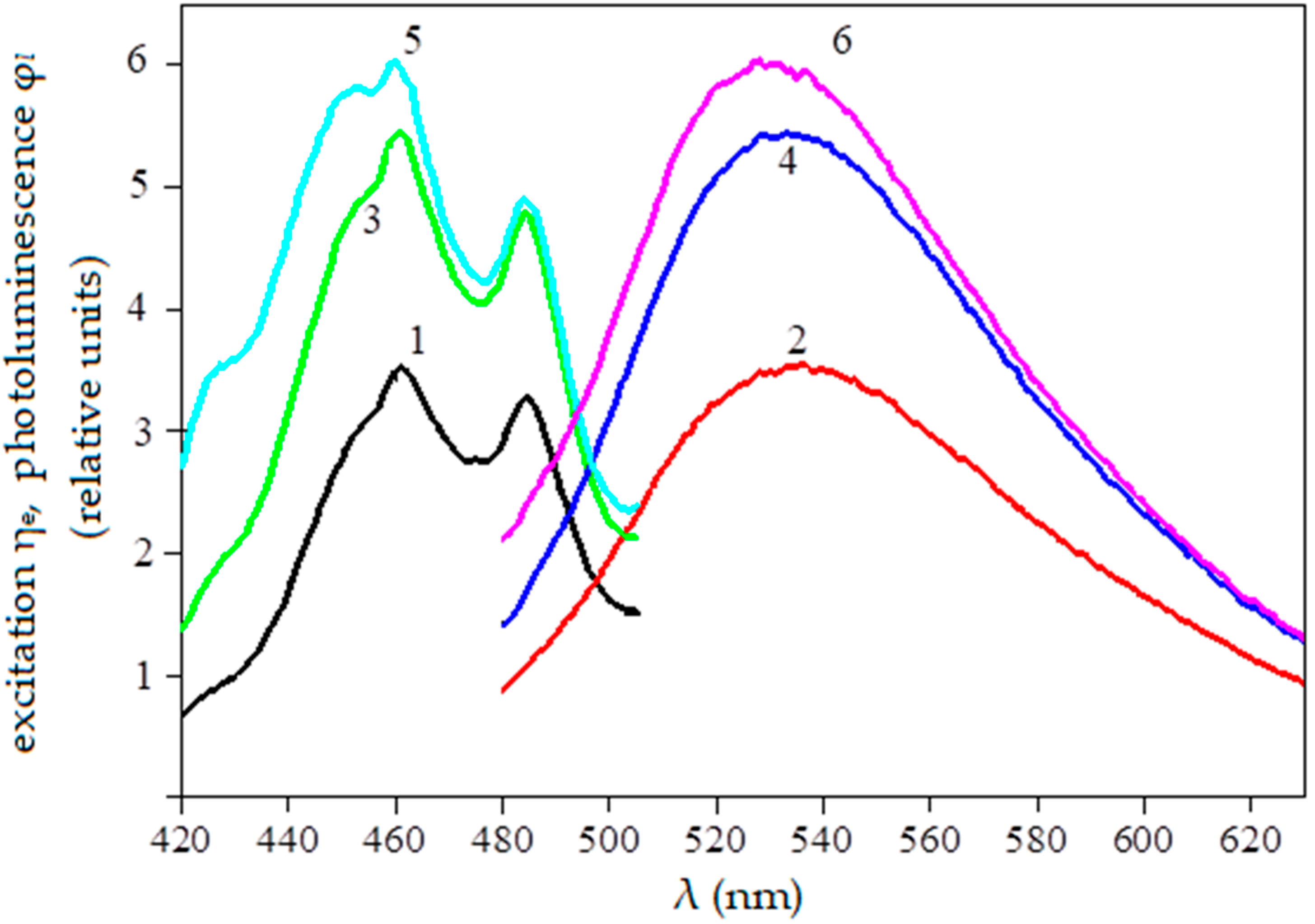
- The photoluminescence of seeds is excited by light of a narrow spectral range with a peak λe ≈ 450 nm (448–460 nm) within 20 μs.
- A signal proportional to the photoluminescence flux Φ in the spectral range from 490 nm to 650 nm is recorded. The process takes 2–3 s with the results averaged.
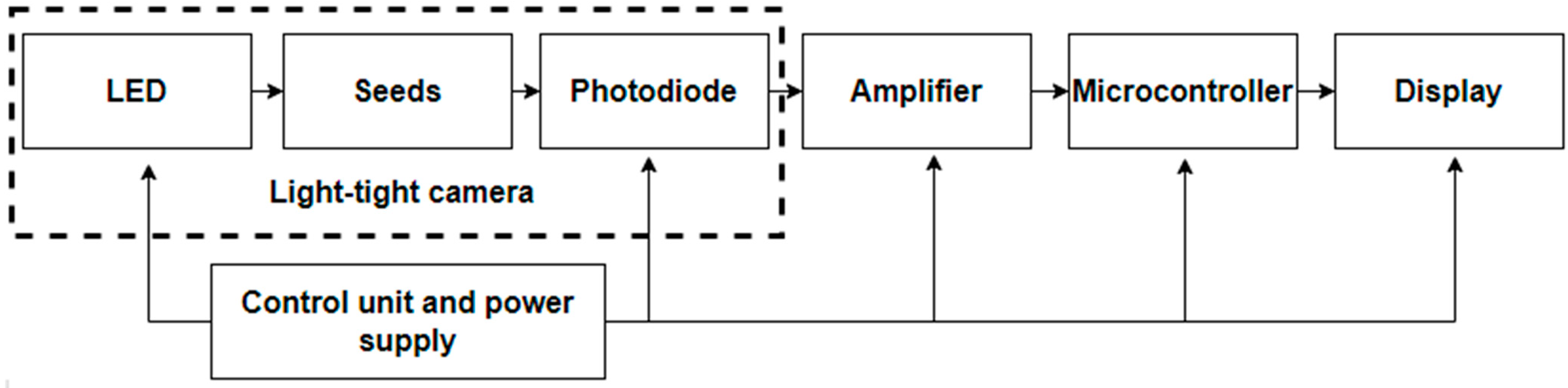
- The received proportional to the photoluminescence flux Φ photosignal (photovoltage U, photocurrent I) is amplified by an amplifier.
- The amplified photo signal enters into the microprocessor. There the signal is processed, taking into account the a priori information available in its memory. This is the linear characteristic B(Φ) obtained for scarified seeds and selected according to the diagnostic algorithm for a specific culture.
- A decision on further actions with seeds is made based on the results of the germination determination. These can be sowing (with sufficient germination value) or repeated scarification to increase germination before sowing with repeated express control.
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belyakov, M.V.; Kondrashova, M.E. Optical-Electronic Technology for Controlling the Effectiveness of Seed Scarification: Monograph; Universum: Smolensk, Russian, 2018; 222p. (In Russian) [Google Scholar]
- Fahey, T.; Pham, H.; Gardi, A.; Sabatini, R.; Stefanelli, D.; Goodwin, I.; Lamb, D.W. Active and Passive Electro-Optical Sensors for Health Assessment in Food Crops. Sensors 2020, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Beć, K.B.; Grabska, J.; Siesler, H.W.; Huck, C.W. Handheld near-infrared spectrometers: Where are we heading? NIR News 2020, 31, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Berzaghi, P.; Cherney, J.; Casler, M. Prediction performance of portable near infrared reflectance instruments using preprocessed dried, ground forage samples. Comput. Electron. Agric. 2021, 182, 106013. [Google Scholar] [CrossRef]
- Makmuang, S.; Nootchanat, S.; Ekgasit, S.; Wongravee, K. Non-destructive method for discrimination of weedy rice using near infrared spectroscopy and modified self-organizing maps (SOMs). Comput. Electron. Agric. 2021, 191, 106522. [Google Scholar] [CrossRef]
- Cherney, J.H.; Digman, M.F.; Cherney, D.J. Handheld NIRS for forage evaluation. Comput. Electron. Agric. 2021, 190, 106469. [Google Scholar] [CrossRef]
- Rego, G.; Ferrero, F.; Valledor, M.; Campo, J.C.; Forcada, S.; Royo, L.J.; Soldado, A. A portable IoT NIR spectroscopic system to analyze the quality of dairy farm forage. Comput. Electron. Agric. 2020, 175, 105578. [Google Scholar] [CrossRef]
- Acosta, J.J.; Castillo, M.S.; Hodge, G.R. Comparison of benchtop and handheld near-infrared spectroscopy devices to determine forage nutritive value. Crop Sci. 2020, 60, 3410–3422. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; Taha, M.F.; Qiu, Z.; He, Y. Determination of Leaf Water Content with a Portable NIRS System Based on Deep Learning and Information Fusion Analysis. Trans. ASABE 2021, 64, 127–135. [Google Scholar] [CrossRef]
- Corrêdo, L.P.; Wei, M.C.; Ferraz, M.N.; Molin, J.P. Near-infrared spectroscopy as a tool for monitoring the spatial variability of sugarcane quality in the fields. Biosyst. Eng. 2021, 206, 150–161. [Google Scholar] [CrossRef]
- Phuphaphud, A.; Saengprachatanarug, K.; Posom, J.; Maraphum, K.; Taira, E. Non-destructive and rapid measurement of sugar content in growing cane stalks for breeding programmes using visible-near infrared spectroscopy. Biosyst. Eng. 2020, 197, 76–90. [Google Scholar] [CrossRef]
- Song, D.; Qiao, L.; Gao, D.; Li, S.; Li, M.; Sun, H.; Ma, J. Development of crop chlorophyll detector based on a type of interference filter optical sensor. Comput. Electron. Agric. 2021, 187, 106260. [Google Scholar] [CrossRef]
- Long, D.S.; McCallum, J.D. Adapting a relatively low-cost reflectance spectrometer for on-combine sensing of grain protein concentration. Comput. Electron. Agric. 2020, 174, 105467. [Google Scholar] [CrossRef]
- Yang, B.; Guo, W.; Huang, X.; Du, R.; Liu, Z. A portable, low-cost and sensor-based detector on sweetness and firmness grades of kiwifruit. Comput. Electron. Agric. 2020, 179, 105831. [Google Scholar] [CrossRef]
- Li, M.; Han, D.; Liu, W. Non-destructive measurement of soluble solids content of three melon cultivars using portable vis-ible/near infrared spectroscopy. Biosyst. Eng. 2019, 188, 31–39. [Google Scholar] [CrossRef]
- Benelli, A.; Cevoli, C.; Ragni, L.; Fabbri, A. In-field and non-destructive monitoring of grapes maturity by hyperspectral imaging. Biosyst. Eng. 2021, 207, 59–67. [Google Scholar] [CrossRef]
- Ramos, R.P.; Gomes, J.S.; Prates, R.M.; Filho, E.F.S.; Teruel, B.J.; Costa, D.S. Non-invasive setup for grape maturation classification using deep learning. J. Sci. Food Agric. 2021, 101, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Casson, A.; Beghi, R.; Giovenzana, V.; Fiorindo, I.; Tugnolo, A.; Guidetti, R. Environmental advantages of visible and near infrared spectroscopy for the prediction of intact olive ripeness. Biosyst. Eng. 2020, 189, 1–10. [Google Scholar] [CrossRef]
- Revelou, P.; Pappa, C.; Kakouri, E.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Discrimination of botanical origin of olive oil from selected Greek cultivars by SPME-GC-MS and ATR-FTIR spectroscopy combined with chemometrics. J. Sci. Food Agric. 2020, 101, 2994–3002. [Google Scholar] [CrossRef]
- Smirnov, A.; Proshkin, Y.; Sokolov, A.; Dorokhov, A. Portable spectral device for monitoring plant stress conditions. E3S Web Conf. 2020, 8, 05016. [Google Scholar] [CrossRef]
- Bashilov, A.M.; Efremenkov, I.Y.; Belyakov, M.V.; Lavrov, A.V.; Gulyaev, A.A.; Gerasimenko, S.A.; Borzenko, S.I.; Boyko, A.A. Determination of Main Spectral and Luminescent Characteristics of Winter Wheat Seeds Infected with Pathogenic Microflora. Photonics 2021, 8, 494. [Google Scholar] [CrossRef]
- Belyakov, M.V. The effect of scarification on the luminescent properties of eastern galega seeds. Bull. NGIEI 2016, 10, 73–82. [Google Scholar]
- Belyakov, M.V.; Malyshkin, V.V.; Kondrashova, M.E.; Rybakov, I.V. Luminescent characteristics of tetraploid clover seeds with double scarifications. In The Strategies of Modern Science Development: Proceedings of the XIII International Scientific–Practical Conference, North Charleston, SC, USA, 3–4 October 2017; CreateSpace: North Charleston, SC, USA, 2017; pp. 25–27. [Google Scholar]
- LED 150353BS74500. Available online: https://ru.mouser.com/datasheet/2/445/150353BS74500-977170.pdf (accessed on 10 February 2022).
- LED ASMT-AB00-NMP01. Available online: https://www.farnell.com/datasheets/529865.pdf (accessed on 10 February 2022).
- LED ASMT-QBB3-NBD0E. Available online: https://ru.mouser.com/datasheet/2/678/olid-State-lighting_060512-AV00-0266EN-1524907.pdf (accessed on 10 February 2022).
- LED KA-3529AQB25Z4S. Available online: https://www.kingbright.com/attachments/file/psearch/000/00/00/KA-3529AQB25Z4S(Ver.7B).pdf (accessed on 10 February 2022).
- LED LZ4-00UA00-00U6. Available online: http://www.farnell.com/datasheets/2360405.pdf (accessed on 10 February 2022).
- Photodiode BPW21R. Available online: http://www.vishay.com/docs/81519/bpw21r.pdf (accessed on 10 February 2022).
- Operational Amplifier AD820. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/AD820.pdf (accessed on 14 February 2022).
- Microcontroller ATmega328P. Available online: https://static.chipdip.ru/lib/549/DOC001549488.pdf (accessed on 14 February 2022).
- Indicator LCD 1602. Available online: https://iarduino.ru/shop/Expansion-payments/1602-lcd-konvertor-v-spi-port.html (accessed on 15 February 2022).
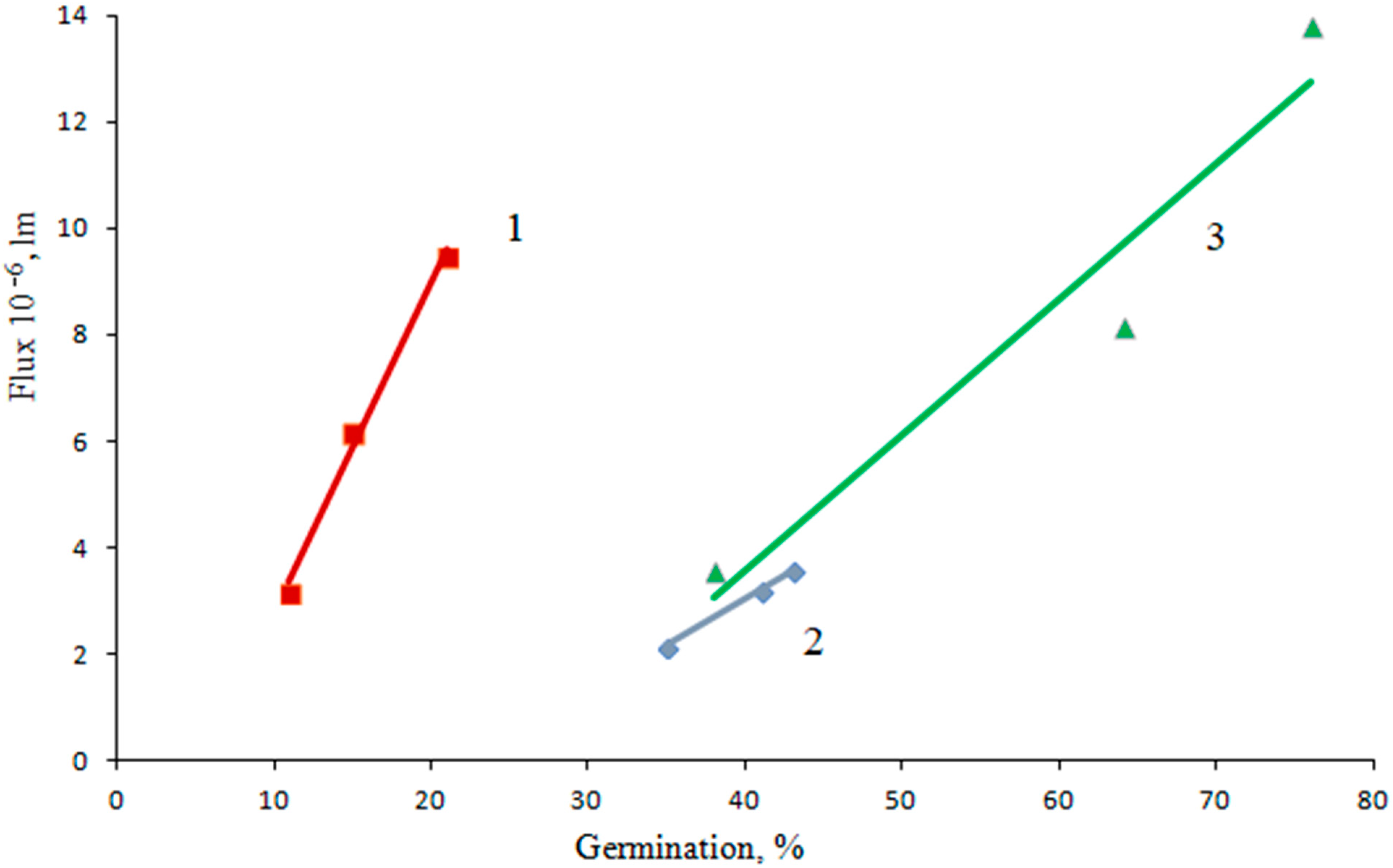

| Scarification | Germination B, % | Φ, r.u. | Φ·10−6, lm |
|---|---|---|---|
| Galega | |||
| Without scarification | 35 | 344 | 2.15 |
| Single scarification | 41 | 518 | 3.23 |
| Double scarification | 43 | 572 | 3.57 |
| Clover | |||
| Without scarification | 11 | 505 | 3.15 |
| Single scarification | 15 | 989 | 6.17 |
| Double scarification | 21 | 1516 | 9.46 |
| Alfalfa | |||
| Without scarification | 38 | 570 | 3.56 |
| Single scarification | 64 | 1313 | 8.19 |
| Double scarification | 76 | 2205 | 13.8 |
| LED Type [Information Source] | Ke,e, % |
|---|---|
| LED 150353BS74500 [24] | 84.4 |
| LED ASMT-AB00-NMP01 [25] | 83.8 |
| LED ASMT-QBB3-NBD0E [26] | 83.5 |
| LED KA-3529AQB25Z4S [27] | 82.5 |
| LED LZ4-00UA00-00U6 [28] | 15.8 |
| № | Name of the Sensor Element | Selected Item | Source of Information |
|---|---|---|---|
| 1 | Light source | LED 150353BS74500 | [24] |
| 2 | Light receiver | BPW21R | [29] |
| 3 | Operational amplifier | AD820ANZ | [30] |
| 4 | Microcontroller | ATmega328P | [31] |
| 5 | Display | LCD1602 | [32] |
| galega | |||
| B, % | 35 | 41 | 43 |
| Uph, mV | 0.78 | 0.85 | 0.86 |
| clover | |||
| B, % | 11 | 15 | 21 |
| Uph, mV | 0.92 | 1.02 | 1.10 |
| alfalfa | |||
| B, % | 38 | 64 | 76 |
| Uph, mV | 0.85 | 1.08 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belyakov, M.V. Photoluminescent Sensor of Scarification Efficiency of Fodder Plants’ Seeds. Sensors 2023, 23, 106. https://doi.org/10.3390/s23010106
Belyakov MV. Photoluminescent Sensor of Scarification Efficiency of Fodder Plants’ Seeds. Sensors. 2023; 23(1):106. https://doi.org/10.3390/s23010106
Chicago/Turabian StyleBelyakov, Mikhail V. 2023. "Photoluminescent Sensor of Scarification Efficiency of Fodder Plants’ Seeds" Sensors 23, no. 1: 106. https://doi.org/10.3390/s23010106





