Nanosphere Structures Using Various Materials: A Strategy for Signal Amplification for Virus Sensing
Abstract
1. Introduction
2. Polymeric Nanospheres
2.1. Preparation Method
2.2. Electroluminescence Detection
2.3. Colorimetric Detection
2.4. Electrochemical Detection
3. Carbon-Based Nanospheres
3.1. Preparation Method
3.2. Electroluminescence Detection
3.3. Colorimetric Detection
3.4. Electrochemical Detection
4. Silica-Based Nanospheres
4.1. Preparation Method
4.2. Electroluminescence Detection
4.3. Colorimetric Detection
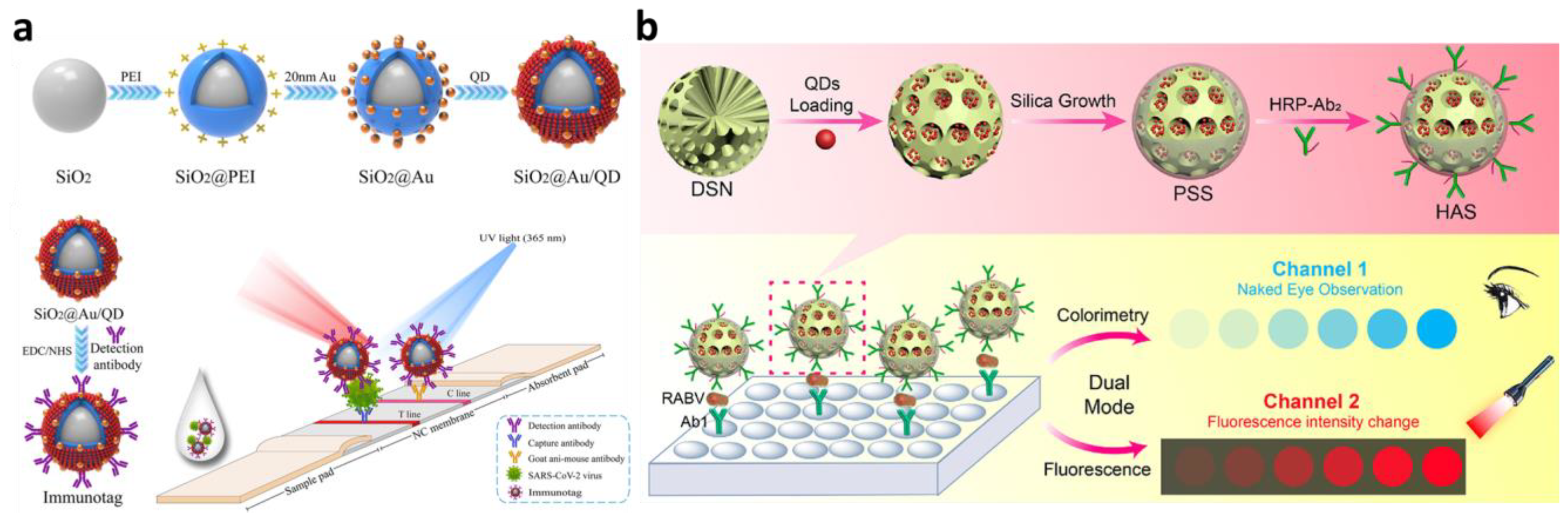
4.4. Electrochemical Detection
5. Metal–Organic Framework-Based Nanospheres
5.1. Preparation Method
5.2. Photoluminescence Detection
5.3. Colorimetric Detection
5.4. Electrochemical Detection
6. Comparison of Nanocarriers and Their Challenges
7. Conclusions and Outlook
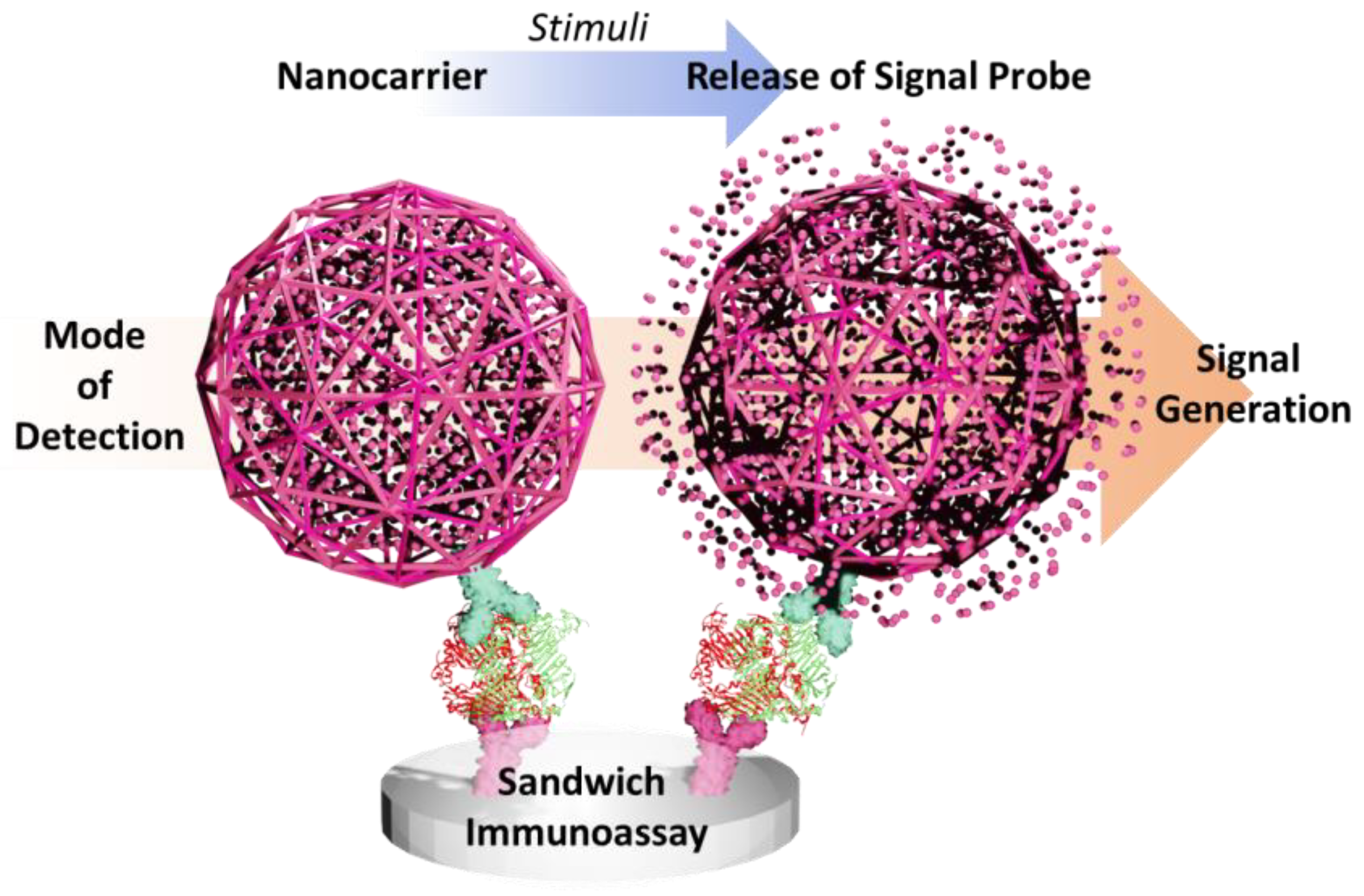
- Supporting material for biorecognition probe. Nanospheres served as a physical platform for immobilizing biomolecules with target recognition functions. This common strategy is simply exploiting the superior nanosphere surface area to enhance the presence of biorecognition moieties, such as antibodies and aptamers. High amounts of anchored antibodies on nanosphere surfaces significantly affect the accessibility of the antibody to effectively capture the antigen, yielding improved accuracy and sensitivity of the sensor.
- Enrichment of the signal producer. Nanospheres can load a higher quantity of signal producers, such as QDs, to magnify the sensing signal. This probe enrichment strategy could be extended to constructing multitarget biosensors. Several sensing probes with different recognition targets could be encapsulated inside a single nanosphere, allowing a multidetection virus platform. In addition, the nanosphere structure loaded with the sensing probe in this second mechanism may be used as a secondary structure added to the detection platform to generate an additional detection signal. Nanospheres functionalized with secondary antibodies can bind to the preformed complex of the primary antibody and the analyte, constructing a sandwich-type immunosensor. Such a signal amplification strategy allows dual detection readout on the constructed biosensor, such as dual naked eye colorimetric and fluorescence detection.
- Protection of the biorecognition probe. Nanospheres may be used for protecting encapsulated sensing probes, such as an enzyme. As some signal elements have some stability issues in their free form, nanosphere encapsulation may assure the stability of the probe and prevent it from leakage to maintain its sensing activity. Moreover, some nanospheres display intrinsic characteristics that could be further utilized to amplify the signal. This may include MOFs with inherent enzyme-like catalytic activity and carbon nanospheres with superior electronic transport properties, endowing the constructed sensor with favorable characteristics for signal augmentation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roco, M.C.; Mirkin, C.A.; Hersam, M.C. Nanotechnology Research Directions for Societal Needs in 2020; Retrospective and Outlook; Springer: Dordredth, The Netherlands, 2011. [Google Scholar]
- Lee, K.-S.; El-Sayed, M.A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Parveen, S.; Panda, J. The present and future of nanotechnology in human health care. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Wang, X.; Marsch, S.; Hunziker, P. Intelligent nanomaterials for medicine: Carrier platforms and targeting strategies in the context of clinical application. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 742–757. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Halsallb, H.B.; Heinemanb, W.B. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Hasan, M.; Hossain, S.; Ahommed, M.; Daizy, M. Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: State of the art. Biosens. Bioelectron. 2020, 166, 112431. [Google Scholar] [CrossRef] [PubMed]
- Reta, N.; Saint, C.P.; Michelmore, A.; Prieto-Simon, B.; Voelcker, N.H. Nanostructured electrochemical biosensors for label-free detection of water-and food-borne pathogens. ACS Appl. Mater. Interfaces 2018, 10, 6055–6072. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Duncan, C.T.; Sharma, K.K. Recent advances in nanostructured chemosensors and biosensors. Analyst 2009, 134, 1980–1990. [Google Scholar] [CrossRef]
- Achadu, O.J.; Nwaji, N.; Lee, D.; Lee, J.; Akinoglu, E.M.; Giersig, M.; Park, E.Y. 3D hierarchically porous magnetic molybdenum trioxide@ gold nanospheres as a nanogap-enhanced Raman scattering biosensor for SARS-CoV-2. Nanoscale Adv. 2022, 4, 871–883. [Google Scholar] [CrossRef]
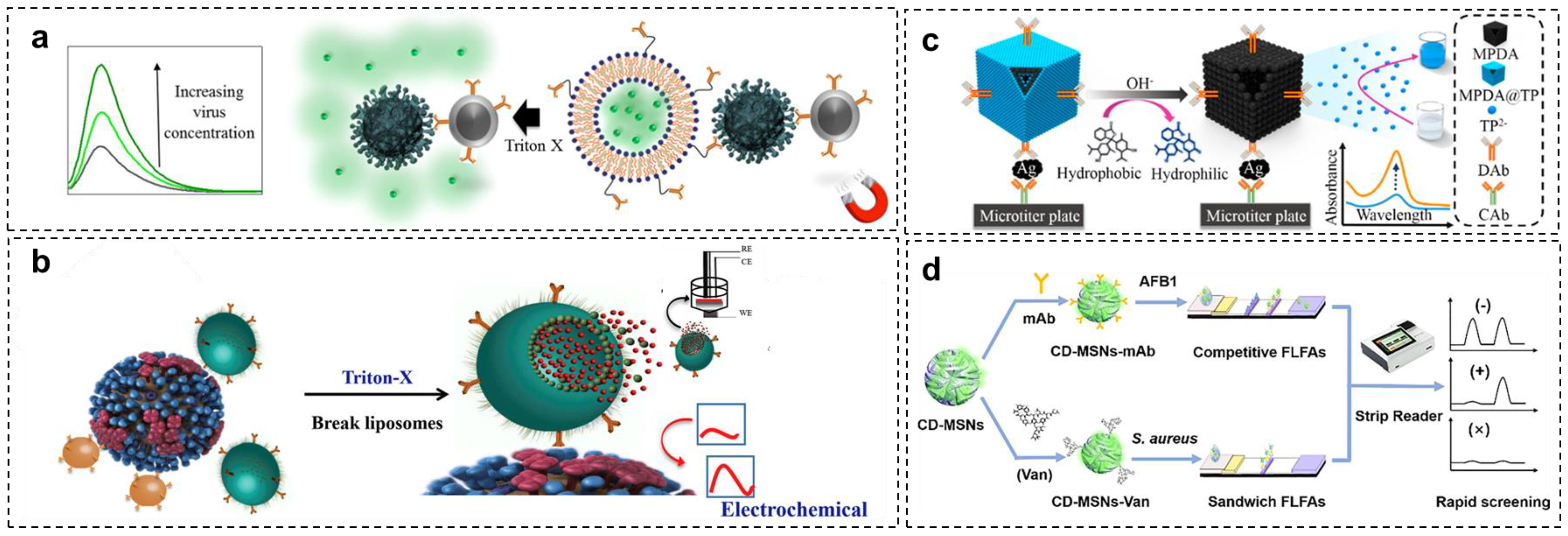
- Ganganboina, A.B.; Chowdhury, A.D.; Khoris, I.M.; Nasrin, F.; Takemura, K.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Dual modality sensor using liposome-based signal amplification technique for ultrasensitive norovirus detection. Biosens. Bioelectron. 2020, 157, 112169. [Google Scholar] [CrossRef]
- Nasrin, F.; Chowdhury, A.D.; Ganganboina, A.B.; Achadu, O.J.; Hossain, F.; Yamazaki, M.; Park, E.Y. Fluorescent and electrochemical dual-mode detection of Chikungunya virus E1 protein using fluorophore-embedded and redox probe-encapsulated liposomes. Microchim. Acta 2020, 187, 674. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Sharmin, S.; Nasrin, F.; Yamazaki, M.; Abe, F.; Suzuki, T.; Park, E.Y. Use of target-specific liposome and magnetic nanoparticle conjugation for the amplified detection of norovirus. ACS Appl. Bio Mater. 2020, 3, 3560–3568. [Google Scholar] [CrossRef] [PubMed]
- Khoris, I.M.; Takemura, K.; Lee, J.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Enhanced colorimetric detection of norovirus using in-situ growth of Ag shell on Au NPs. Biosens. Bioelectron. 2019, 126, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Chowdhury, A.D.; Khoris, I.M.; Doong, R.-A.; Li, T.-C.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Hollow magnetic-fluorescent nanoparticles for dual-modality virus detection. Biosens. Bioelectron. 2020, 170, 112680. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.; Tran, V.T.; Lee, D.K.; Ahmed, S.R.; Hong, J.C.; Lee, J.; Park, E.Y.; Lee, J. Magnetic nanozyme-linked immunosorbent assay for ultrasensitive influenza A virus detection. ACS Appl. Mater. Interfaces 2018, 10, 12534–12543. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Takemura, K.; Kato, C.N.; Suzuki, T.; Park, E.Y. Binary nanoparticle graphene hybrid structure-based highly sensitive biosensing platform for norovirus-like particle detection. ACS Appl. Mater. Interfaces 2017, 9, 27298–27304. [Google Scholar] [CrossRef]
- Campas, M.; Garibo, D.; Prieto-Simon, B. Novel nanobiotechnological concepts in electrochemical biosensors for the analysis of toxins. Analyst 2012, 137, 1055–1067. [Google Scholar] [CrossRef]
- Rebelo, R.; Barbosa, A.I.; Caballero, D.; Kwon, I.K.; Oliveira, J.M.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. 3D biosensors in advanced medical diagnostics of high mortality diseases. Biosens. Bioelectron. 2019, 130, 20–39. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Fei, T.; Zhang, T. Templating synthesis of ZnO hollow nanospheres loaded with Au nanoparticles and their enhanced gas sensing properties. J. Mater. Chem. 2012, 22, 4767–4771. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, F.; Xi, J.; Sun, T.; Xiao, S.; Wang, H.; Wang, S.; Liu, Y. Encapsulating Pd Nanoparticles in Double-Shelled [email protected] Hollow Spheres for Excellent Chemical Catalytic Property. Sci. Rep. 2014, 4, 4053. [Google Scholar] [CrossRef]
- He, L.; Jia, Y.; Meng, F.; Li, M.; Liu, J. Development of sensors based on CuO-doped SnO2 hollow spheres for ppb level H2S gas sensing. J. Mater. Sci. 2009, 44, 4326–4333. [Google Scholar] [CrossRef]
- Kokab, T.; Munir, A.; Shah, A.; Kurbanoglu, S.; Zia, M.A.; Ozkan, S.A. The Effect of Nanomaterials on the Drug Analysis Performance of Nanosensors. In New Developments in Nanosensors for Pharmaceutical Analysis; Academic Press: Cambridge, MA, USA, 2019; pp. 79–118. [Google Scholar]
- Ren, R.; Cai, G.; Yu, Z.; Zeng, Y.; Tang, D. Metal-polydopamine framework: An innovative signal-generation tag for colorimetric immunoassay. Anal. Chem. 2018, 90, 11099–11105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, C.; Qian, S.; Li, H.; Fu, P.; Zhou, H.; Zheng, J. An ultrasensitive lateral flow immunoassay platform for foodborne biotoxins and pathogenic bacteria based on carbon-dots embedded mesoporous silicon nanoparticles fluorescent reporter probes. Food Chem. 2023, 399, 133970. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int. J. Pharm. 1995, 116, 1–9. [Google Scholar] [CrossRef]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Effect of polymer-based nanoparticles on the assay of antimicrobial drug delivery systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Vrignaud, S.; Benoit, J.-P.; Saulnier, P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef]
- Ameller, T.; Marsaud, V.; Legrand, P.; Gref, R.; Barratt, G.; Renoir, J.-M. Polyester-poly(ethylene glycol) nanoparticles loaded with the pure antiestrogen RU 58668: Physicochemical and opsonization properties. Pharm. Res. 2003, 20, 1063–1070. [Google Scholar] [CrossRef]
- Wang, H.; Leeuwenburgh, S.C.; Li, Y.; Jansen, J.A. The use of micro-and nanospheres as functional components for bone tissue regeneration. Tissue Eng. Part B Rev. 2012, 18, 24–39. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Lan, Q.; Shen, H.; Li, J.; Ren, C.; Hu, X.; Yang, Z. Facile synthesis of novel reduced graphene oxide@ polystyrene nanospheres for sensitive label-free electrochemical immunoassay. Chem. Commun. 2020, 56, 699–702. [Google Scholar] [CrossRef]
- Guan, N.; Liu, C.; Sun, D.; Xu, J. A facile method to synthesize carboxyl-functionalized magnetic polystyrene nanospheres. Colloids Surf. Physicochem. Eng. Asp. 2009, 335, 174–180. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Shin, C.H.; Han, S. Dispersion polymerization of polystyrene particles using alcohol as reaction medium. Nanoscale Res. Lett. 2016, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, J.; Zeng, T.; Zhang, Z.-L.; Chen, J.; Wong, G.; Qiu, X.; Liu, W.; Gao, G.F.; Bi, Y. Ultrasensitive ebola virus detection based on electroluminescent nanospheres and immunomagnetic separation. Anal. Chem. 2017, 89, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Luo, F.; Wen, W.; Zhang, X.; Wang, S. Enrichment–stowage–cycle strategy for ultrasensitive electrochemiluminescent detection of HIV-DNA with wide dynamic range. Anal. Chem. 2019, 91, 12238–12245. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, T.; Guo, W.-J.; Bai, Y.-Y.; Pang, D.-W.; Zhang, Z.-L. Digital single virus immunoassay for ultrasensitive multiplex avian influenza virus detection based on fluorescent magnetic multifunctional nanospheres. ACS Appl. Mater. Interfaces 2019, 11, 5762–5770. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, Y.-Z.; Wu, L.-L.; Wu, Z.; Bi, Y.; Wong, G.; Qiu, X.; Chen, J.; Pang, D.-W.; Zhang, Z.-L. Dual-signal readout nanospheres for rapid point-of-care detection of ebola virus glycoprotein. Anal. Chem. 2017, 89, 13105–13111. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, R.; Guo, X.; Liang, J.; Deng, Q.; Li, M.; An, T.; Liu, T.; Wu, Y. Simultaneous quantitation of cytokeratin-19 fragment and carcinoembryonic antigen in human serum via quantum dot-doped nanoparticles. Biosens. Bioelectron. 2017, 91, 60–65. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, K.; Qin, W.; Hou, Y.; Lu, W.; Xu, H.; Wo, Y.; Cui, D. Use of quantum dot beads-labeled monoclonal antibody to improve the sensitivity of a quantitative and simultaneous immunochromatographic assay for neuron specific enolase and carcinoembryonic antigen. Talanta 2017, 164, 463–469. [Google Scholar] [CrossRef]
- Hu, J.; Tang, F.; Jiang, Y.-Z.; Liu, C. Rapid screening and quantitative detection of Salmonella using a quantum dot nanobead-based biosensor. Analyst 2020, 145, 2184–2190. [Google Scholar] [CrossRef]
- Chen, R.; Hu, Y.; Chen, M.; An, J.; Lyu, Y.; Liu, Y.; Li, D. Naked-Eye Detection of Hepatitis B Surface Antigen Using Gold Nanoparticles Aggregation and Catalase-Functionalized Polystyrene Nanospheres. ACS Omega 2021, 6, 9828–9833. [Google Scholar] [CrossRef]
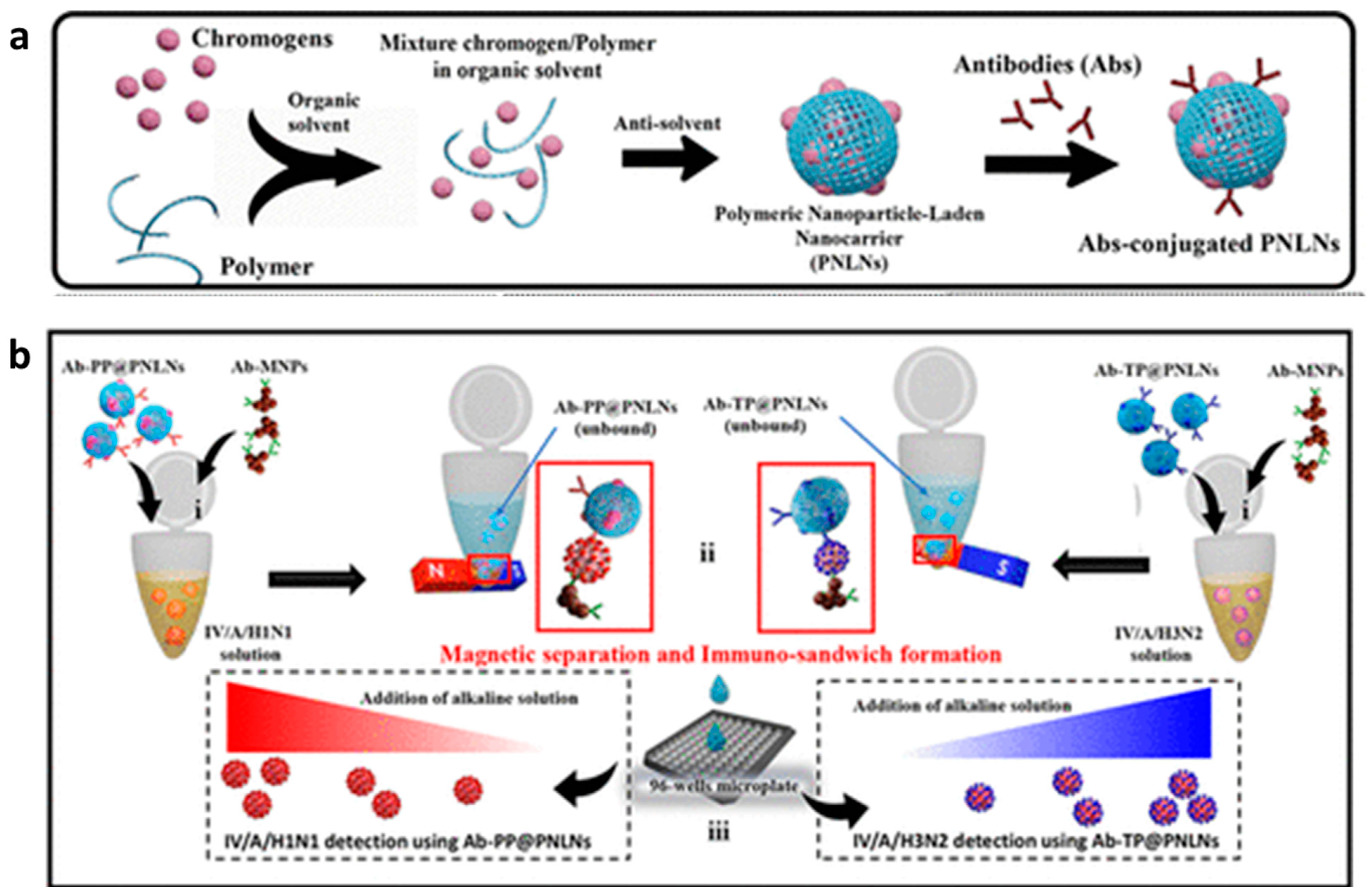
- Khoris, I.M.; Ganganboina, A.B.; Park, E.Y. Self-Assembled Chromogenic Polymeric Nanoparticle-Laden Nanocarrier as a Signal Carrier for Derivative Binary Responsive Virus Detection. ACS Appl. Mater. Interfaces 2021, 13, 36868–36879. [Google Scholar] [CrossRef] [PubMed]
- Khoris, I.M.; Ganganboina, A.B.; Suzuki, T.; Park, E.Y. Self-assembled chromogen-loaded polymeric cocoon for respiratory virus detection. Nanoscale 2021, 13, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Yang, Y.; Tan, Z.; Liu, Q.; Liu, H.; Wang, P.; Chen, L.; Zhang, D.; Li, Y.; Dong, Y. A label-free electrochemical immunosensor based on the novel signal amplification system of AuPdCu ternary nanoparticles functionalized polymer nanospheres. Biosens. Bioelectron. 2018, 103, 151–157. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, B.; Chen, G.; Tang, D. Redox and catalysis ‘all-in-one’infinite coordination polymer for electrochemical immunosensor of tumor markers. Biosens. Bioelectron. 2015, 64, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ling, H.; Lamonier, J.-F.; Jaroniec, M.; Huang, J.; Monteiro, M.J.; Liu, J. A synthetic strategy for carbon nanospheres impregnated with highly monodispersed metal nanoparticles. NPG Asia Mater. 2016, 8, e240. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, H.; Ma, Z.; Han, H. Amperometric biosensor of matrix metalloproteinase-7 enhanced by Pd-functionalized carbon nanocomposites. Nanoscale Res. Lett. 2018, 13, 375. [Google Scholar] [CrossRef]
- Zhang, P.; Qiao, Z.-A.; Dai, S. Recent advances in carbon nanospheres: Synthetic routes and applications. Chem. Commun. 2015, 51, 9246–9256. [Google Scholar] [CrossRef]
- Baccile, N.; Antonietti, M.; Titirici, M.M. One-Step Hydrothermal Synthesis of Nitrogen-Doped Nanocarbons: Albumine Directing the Carbonization of Glucose. ChemSusChem 2010, 3, 246–253. [Google Scholar] [CrossRef]
- Fellinger, T.P.; White, R.J.; Titirici, M.M.; Antonietti, M. Borax—mediated formation of carbon aerogels from glucose. Adv. Funct. Mater. 2012, 22, 3254–3260. [Google Scholar] [CrossRef]
- Wei, Z.; Gong, Y.; Xiong, T.; Zhang, P.; Li, H.; Wang, Y. Highly efficient and chemoselective hydrogenation of α, β-unsaturated carbonyls over Pd/N-doped hierarchically porous carbon. Catal. Sci. Technol. 2015, 5, 397–404. [Google Scholar] [CrossRef]
- White, R.J.; Tauer, K.; Antonietti, M.; Titirici, M.-M. Functional hollow carbon nanospheres by latex templating. J. Am. Chem. Soc. 2010, 132, 17360–17363. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; White, R.J.; Yoshizawa, N.; Antonietti, M.; Titirici, M.-M. Ordered carbohydrate-derived porous carbons. Chem. Mater. 2011, 23, 4882–4885. [Google Scholar] [CrossRef]
- Gao, J.-W.; Chen, M.-M.; Wen, W.; Zhang, X.; Wang, S.; Huang, W.-H. Au-Luminol-decorated porous carbon nanospheres for the electrochemiluminescence biosensing of MUC1. Nanoscale 2019, 11, 16860–16867. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Lou, Y.; Pan, C.; Zhang, Y.; Wang, Z. Aptamer-Based Fluorescence Detection and Selective Disinfection of Salmonella Typhimurium by Using Hollow Carbon Nitride Nanosphere. Biosensors 2022, 12, 228. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 346, 999–1004. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, J.-J. Fabrication of a novel electrochemical immunosensor based on the gold nanoparticles/colloidal carbon nanosphere hybrid material. Electrochim. Acta 2010, 55, 7814–7817. [Google Scholar] [CrossRef]
- Du, D.; Zou, Z.; Shin, Y.; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, I.A.; Lin, Y. Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Anal. Chem. 2010, 82, 2989–2995. [Google Scholar] [CrossRef]
- Xu, Q.; Yan, F.; Lei, J.; Leng, C.; Ju, H. Disposable electrochemical immunosensor by using carbon sphere/gold nanoparticle composites as labels for signal amplification. Chem. Eur. J. 2012, 18, 4994–4998. [Google Scholar] [CrossRef]
- Salimian, R.; Shahrokhian, S.; Panahi, S. Enhanced electrochemical activity of a hollow carbon sphere/polyaniline-based electrochemical biosensor for HBV DNA marker detection. ACS Biomater. Sci. Eng. 2019, 5, 2587–2594. [Google Scholar] [CrossRef]
- Gao, M.; Zeng, J.; Liang, K.; Zhao, D.; Kong, B. Interfacial assembly of mesoporous silica-based optical heterostructures for sensing applications. Adv. Funct. Mater. 2020, 30, 1906950. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous silica nanoparticles for bio-applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Pal, N.; Lee, J.-H.; Cho, E.-B. Recent trends in morphology-controlled synthesis and application of mesoporous silica nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Long, C.; Wu, Z.; Xiong, H.; Chen, M.; Zhang, X.; Wen, W.; Wang, S. Functional silica nanospheres for sensitive detection of H9N2 avian influenza virus based on immunomagnetic separation. Sens. Actuators B Chem. 2020, 310, 127831. [Google Scholar] [CrossRef]
- Han, H.; Wang, C.; Yang, X.; Zheng, S.; Cheng, X.; Liu, Z.; Zhao, B.; Xiao, R. Rapid field determination of SARS-CoV-2 by a colorimetric and fluorescent dual-functional lateral flow immunoassay biosensor. Sens. Actuators B Chem. 2022, 351, 130897. [Google Scholar] [CrossRef]
- Xu, L.-D.; Zhang, Q.; Ding, S.-N.; Xu, J.-J.; Chen, H.-Y. Ultrasensitive detection of severe fever with thrombocytopenia syndrome virus based on immunofluorescent carbon dots/SiO2 nanosphere-based lateral flow assay. ACS Omega 2019, 4, 21431–21438. [Google Scholar] [CrossRef]
- Xu, L.-D.; Du, F.-L.; Zhu, J.; Ding, S.-N. Luminous silica colloids with carbon dot incorporation for sensitive immunochromatographic assay of Zika virus. Analyst 2021, 146, 706–713. [Google Scholar] [CrossRef]
- Ding, L.; Xiang, C.; Zhou, G. Silica nanoparticles coated by poly(acrylic acid) brushes via host-guest interactions for detecting DNA sequence of Hepatitis B virus. Talanta 2018, 181, 65–72. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, M.; Wang, W.; Huang, L.; Lu, Z.; Song, Z.; Foda, M.F.; Zhao, L.; Han, H. Pomegranate-inspired silica nanotags enable sensitive dual-modal detection of rabies virus nucleoprotein. Anal. Chem. 2020, 92, 8802–8809. [Google Scholar] [CrossRef]
- Feng, Y.-G.; Wang, X.-Y.; Wang, Z.-W.; Wang, A.-J.; Mei, L.-P.; Luo, X.; Feng, J.-J. A label-free electrochemical immunosensor based on encapsulated signal molecules in mesoporous silica-coated gold nanorods for ultrasensitive assay of procalcitonin. Bioelectrochemistry 2021, 140, 107753. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Liu, X.; Li, B.; Zhang, C.; Qin, L.; Huang, D.; Yi, H.; Zhang, M.; Li, L. Metal-organic frameworks and their derivatives as signal amplification elements for electrochemical sensing. Coord. Chem. Rev. 2020, 424, 213520. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Lv, X.; Jia, Q. Highly sensitive and selective sensing of free bilirubin using metal–organic frameworks-based energy transfer process. ACS Appl. Mater. Interfaces 2017, 9, 30925–30932. [Google Scholar] [CrossRef]
- Zhao, T.; Han, J.; Jin, X.; Liu, Y.; Liu, M.; Duan, P. Enhanced circularly polarized luminescence from reorganized chiral emitters on the skeleton of a zeolitic imidazolate framework. Angew. Chem. 2019, 131, 5032–5036. [Google Scholar] [CrossRef]
- Hu, Z.; Lustig, W.P.; Zhang, J.; Zheng, C.; Wang, H.; Teat, S.J.; Gong, Q.; Rudd, N.D.; Li, J. Effective detection of mycotoxins by a highly luminescent metal–organic framework. J. Am. Chem. Soc. 2015, 137, 16209–16215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Q.; Qiu, G.-H.; Liang, Z.; Li, M.-M.; Sun, B.; Qin, L.; Yang, S.-P.; Chen, W.-H.; Chen, J.-X. A zinc(II)-based two-dimensional MOF for sensitive and selective sensing of HIV-1 ds-DNA sequences. Anal. Chim. Acta 2016, 922, 55–63. [Google Scholar] [CrossRef]
- Liu, C.; Wang, T.; Ji, J.; Wang, C.; Wang, H.; Jin, P.; Zhou, W.; Jiang, J. The effect of pore size and layer number of metal–porphyrin coordination nanosheets on sensing DNA. J. Mater. Chem. C 2019, 7, 10240–10246. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Li, X.; Hu, N.; Chen, J.; Chen, G.; Wu, Y. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef]
- Wang, C.; Gao, J.; Tan, H. Integrated antibody with catalytic metal–organic framework for colorimetric immunoassay. ACS Appl. Mater. Interfaces 2018, 10, 25113–25120. [Google Scholar] [CrossRef]
- Liu, C.; Dong, J.; Ning, S.; Hou, J.; Waterhouse, G.I.; Cheng, Z.; Ai, S. An electrochemical immunosensor based on an etched zeolitic imidazolate framework for detection of avian leukosis virus subgroup J. Microchim. Acta 2018, 185, 423. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Khoris, I.M.; Chowdhury, A.D.; Li, T.-C.; Park, E.Y. Ultrasensitive Detection of the Hepatitis E Virus by Electrocatalytic Water Oxidation Using Pt-Co3O4 Hollow Cages. ACS Appl. Mater. Interfaces 2020, 12, 50212–50221. [Google Scholar] [CrossRef] [PubMed]
- Khoris, I.M.; Kenta, T.; Ganganboina, A.B.; Park, E.Y. Pt-embodiment ZIF-67-derived nanocage as enhanced immunoassay for infectious virus detection. Biosens. Bioelectron. 2022, 215, 114602. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liang, Z.; Hu, O.; He, Q.; Sun, D.; Chen, Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@ Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta 2021, 387, 138553. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, C.; Cao, X.; Liu, S. Versatile immunosensor using a quantum dot coated silica nanosphere as a label for signal amplification. Anal. Chem. 2010, 82, 6422–6429. [Google Scholar] [CrossRef]
- Wei, W.; Wei, M.; Liu, S. Silica nanoparticles as a carrier for signal amplification. Rev. Anal. Chem 2012, 31, 163–176. [Google Scholar] [CrossRef]
- Li, C.; Zou, Z.; Liu, H.; Jin, Y.; Li, G.; Yuan, C.; Xiao, Z.; Jin, M. Synthesis of polystyrene-based fluorescent quantum dots nanolabel and its performance in H5N1 virus and SARS-CoV-2 antibody sensing. Talanta 2021, 225, 122064. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, S.; Lei, J.; Xu, Q.; Ju, H. Carbon nanospheres enhanced electrochemiluminescence of CdS quantum dots for biosensing of hypoxanthine. Talanta 2011, 85, 2154–2158. [Google Scholar] [CrossRef]
- He, L.; Cui, B.; Liu, J.; Song, Y.; Wang, M.; Peng, D.; Zhang, Z. Novel electrochemical biosensor based on core-shell nanostructured composite of hollow carbon spheres and polyaniline for sensitively detecting malathion. Sensors Actuators B Chem. 2018, 258, 813–821. [Google Scholar] [CrossRef]
- Lai, G.; Zhang, H.; Yu, A.; Ju, H. In situ deposition of Prussian blue on mesoporous carbon nanosphere for sensitive electrochemical immunoassay. Biosens. Bioelectron. 2015, 74, 660–665. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Meng, X.; Shang, K.; Ai, S. One-step “green” preparation of graphene nanosheets and carbon nanospheres mixture by electrolyzing graphite rob and its application for glucose biosensing. Biosens. Bioelectron. 2011, 30, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Su, S.; Li, Y.; He, Q.; Shi, J. Hydrophilic mesoporous carbon nanoparticles as carriers for sustained release of hydrophobic anti-cancer drugs. Chem. Commun. 2011, 47, 2101–2103. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Hernández, M.d.J.; Ricco, R.; Carraro, F.; Limpoco, F.T.; Linares-Moreau, M.; Leitner, E.; Wiltsche, H.; Rattenberger, J.; Schröttner, H.; Frühwirt, P. Degradation of ZIF-8 in phosphate buffered saline media. CrystEngComm 2019, 21, 4538–4544. [Google Scholar] [CrossRef]
- Aguilar-Palma, R.; Malankowska, M.; Coronas, J. Applications of metal-organic frameworks and zeolites to virus detection and control: Biosensors, barriers, and biocomposites. Z. Anorg. Allg. Chem. 2021, 647, 1532–1541. [Google Scholar] [CrossRef]
- Prajapati, D.G.; Kandasubramanian, B. Progress in the development of intrinsically conducting polymer composites as biosensors. Macromol. Chem. Phys. 2019, 220, 1800561. [Google Scholar] [CrossRef]
- Reynolds, A.; Giltrap, M.; Chambers, G. Acute growth inhibition & toxicity analysis of nano-polystyrene spheres on Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2021, 207, 111153. [Google Scholar]
- Dong, C.-D.; Chen, C.-W.; Chen, Y.-C.; Chen, H.-H.; Lee, J.-S.; Lin, C.-H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Cunha, D.; Imbuluzqueta, E.; Ragon, F.; Serre, C.; Blanco-Prieto, M.J.; Horcajada, P. Cytotoxicity of nanoscaled metal–organic frameworks. J. Mater. Chem. B 2014, 2, 262–271. [Google Scholar] [CrossRef]
- Baati, T.; Njim, L.; Neffati, F.; Kerkeni, A.; Bouttemi, M.; Gref, R.; Najjar, M.F.; Zakhama, A.; Couvreur, P.; Serre, C. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(III) metal–organic frameworks. Chem. Sci. 2013, 4, 1597–1607. [Google Scholar] [CrossRef]




| Type of Nanosphere | Function of Nanosphere | Detection Technique and Assay Approach | Type of Analyte and Its Medium | LOD | Magnification of the Amplification Signal | Ref. |
|---|---|---|---|---|---|---|
| Polymeric nanospheres | Signal probe (QDs) cargo, antibody immobilization platform, coupled with magnetic separation | Electroluminescence, sandwich immunoassay | Ebola virus in PBS and chicken-derived matrices | 5.2 pg/mL | 85-fold increase in electroluminescence signal intensity | [36] |
| Signal probe (QDs) cargo, hairpin DNA immobilization platform, coupled with magnetic separation | Electroluminescence, DNA strand displacement assay | HIV-DNA in PBS, milk, urine, FBS, and whole blood | 39.81 fM | 11.3-fold increase in electroluminescence signal intensity | [37] | |
| Signal probe (QDs) cargo, antibody immobilization platform | Colorimetric (LFA), sandwich immunoassay | Ebola virus glycoprotein in PBS and whole blood | 0.18 ng/mL | 294-fold lower LOD than those of the commercial product | [39] | |
| Signal probe (catalase) cargo, antibody immobilization platform | Colorimetric, immunoassay | Hepatitis B virus in PBS and blood serum | 0.1 ng/mL | 6.9-fold lower LOD than those of ELISA assay | [43] | |
| Signal probe (TMB) cargo, antibody immobilization platform | Colorimetric, sandwich immunoassay | H1N1 influenza virus from a clinical sample | 32.37 fg/mL | 3.5-fold increase in the colorimetric detection signal | [45] | |
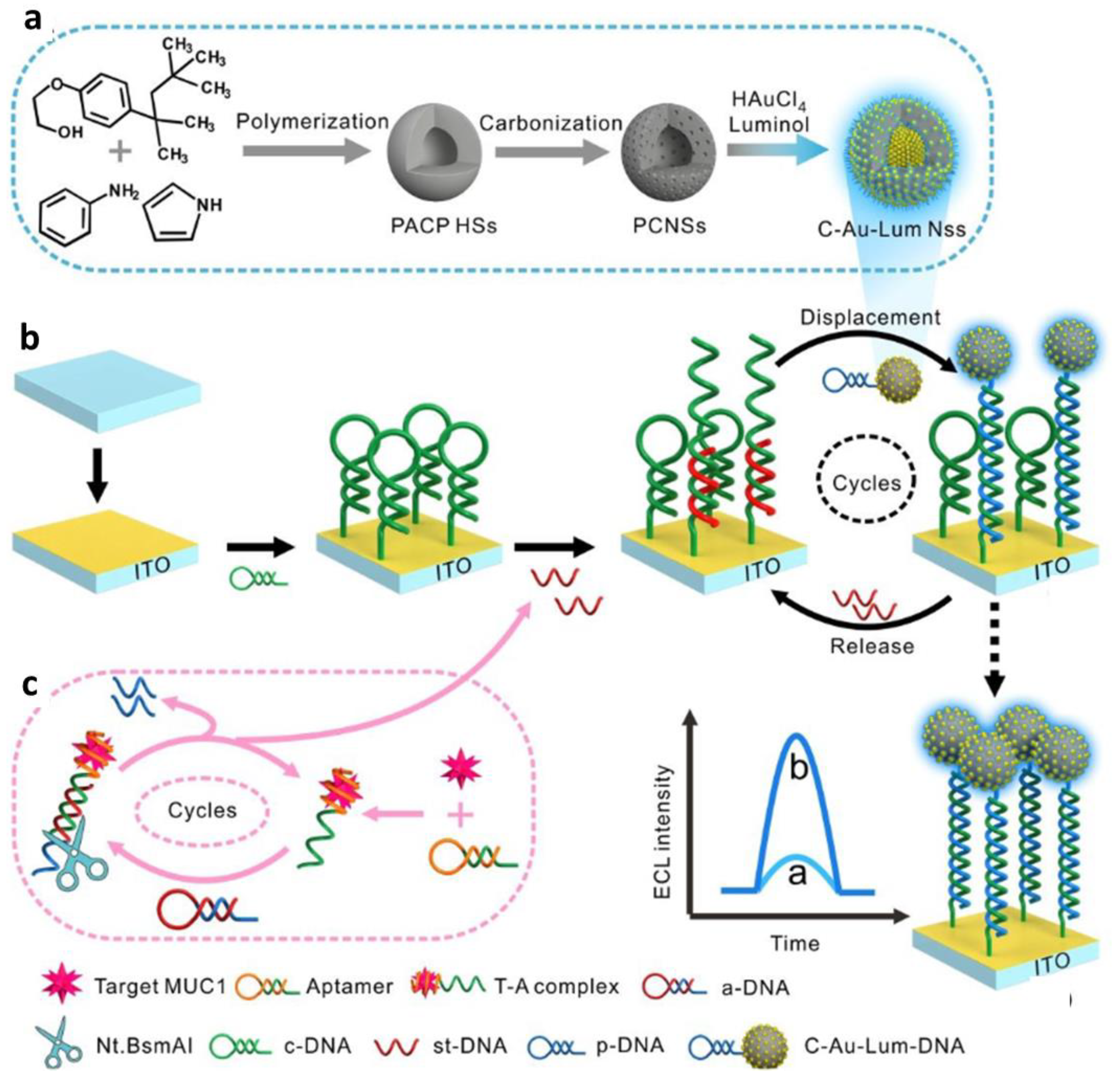
| Carbon-based nanospheres | Signal probe (luminophore Au) cargo, DNA immobilization platform | Electroluminescence, DNA hybridization assay | Mucin1 in PBS and blood serum | 47.6 fg/mL | Not mentioned | [57] |
| Cy5-labeled aptamer immobilization platform | Colorimetric (PET), aptamer assay | Salmonella typhimurium in the milk sample | 13 CFU/mL | Not mentioned | [58] | |
| DNA immobilization platform | Electrochemical (DPV), DNA hybridization assay | Hepatitis B virus (HBV) in buffer and blood serum | 10 fM | Not mentioned | [63] | |
| Silica-based nanospheres | Signal probe (Ru(bpy)32+) cargo, antibody immobilization platform, coupled with magnetic separation | Electroluminescence, sandwich immunoassay | H9N2 avian influenza virus in PBS and chicken-derived matrices | 14 fg/mL | 103-fold increase in electroluminescence signal intensity | [67] |
| Signal probe (QDs) cargo, antibody immobilization platform | Colorimetric (LFA), sandwich immunoassay | SARS-CoV-2 from a clinical sample | 33 pg/mL | 10-fold and 300-fold higher sensitivity of the colorimetric signal than those of Au-based LFA strip, respectively | [68] | |
| Signal probe (CDs) cargo, antibody immobilization platform | Colorimetric (LFA), sandwich immunoassay | Thrombocytopenia syndrome virus (SFTSV) in buffer and blood serum | 10 pg/mL | 2 orders of sensitivity magnitude higher than that of the colloidal gold-based LFA | [69] | |
| Signal probe (glucose oxidase (GOx)) cargo, DNA immobilization platform, coupled with magnetic separation | Colorimetric, DNA hybridization assay | Hepatitis B virus in buffer and blood serum | 3 fM | 4.1-fold higher detection response | [71] | |
| Signal probe (QDs) cargo, HRP-labeled antibody immobilization platform, coupled with magnetic separation | Colorimetric, sandwich immunoassay | Rabies virus nucleoprotein in buffer and mouse brain tissue/fluid | 8 pg/mL | 48-fold signal improvement compared with conventional ELISA assay | [72] | |
| MOF-based nanospheres | Signal probe (FAM-labeled single-stranded DNA) cargo | Photoluminescence (PET), DNA hybridization assay | HIV-DNA in buffer | 10 pM | Increase in the fluorescence anisotropy by a factor of 4.4 | [80] |
| Signal probe cargo (MOF with catalytic activity), antibody immobilization platform | Colorimetric, sandwich immunoassay | mIgG in buffer and blood serum | 0.34 ng/mL | 3-fold lower LOD than that of conventional HRP- labeled capture antibody | [83] | |
| Signal probe cargo (PtNPs, TMB), antibody immobilization platform | Colorimetric, sandwich immunoassay | SARS-CoV-2 in buffer and from a clinical fecal sample | 148.28 fg/mL | 321-fold lower LOD than that of commercial ELISA | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttaqien, S.E.; Khoris, I.M.; Pambudi, S.; Park, E.Y. Nanosphere Structures Using Various Materials: A Strategy for Signal Amplification for Virus Sensing. Sensors 2023, 23, 160. https://doi.org/10.3390/s23010160
Muttaqien SE, Khoris IM, Pambudi S, Park EY. Nanosphere Structures Using Various Materials: A Strategy for Signal Amplification for Virus Sensing. Sensors. 2023; 23(1):160. https://doi.org/10.3390/s23010160
Chicago/Turabian StyleMuttaqien, Sjaikhurrizal El, Indra Memdi Khoris, Sabar Pambudi, and Enoch Y. Park. 2023. "Nanosphere Structures Using Various Materials: A Strategy for Signal Amplification for Virus Sensing" Sensors 23, no. 1: 160. https://doi.org/10.3390/s23010160
APA StyleMuttaqien, S. E., Khoris, I. M., Pambudi, S., & Park, E. Y. (2023). Nanosphere Structures Using Various Materials: A Strategy for Signal Amplification for Virus Sensing. Sensors, 23(1), 160. https://doi.org/10.3390/s23010160







