A Rapid Prototyping Method for Sub-MHz Single-Element Piezoelectric Transducers by Using 3D-Printed Components
Abstract
1. Introduction
2. Materials and Methods
2.1. Transducer Modeling and Simulation
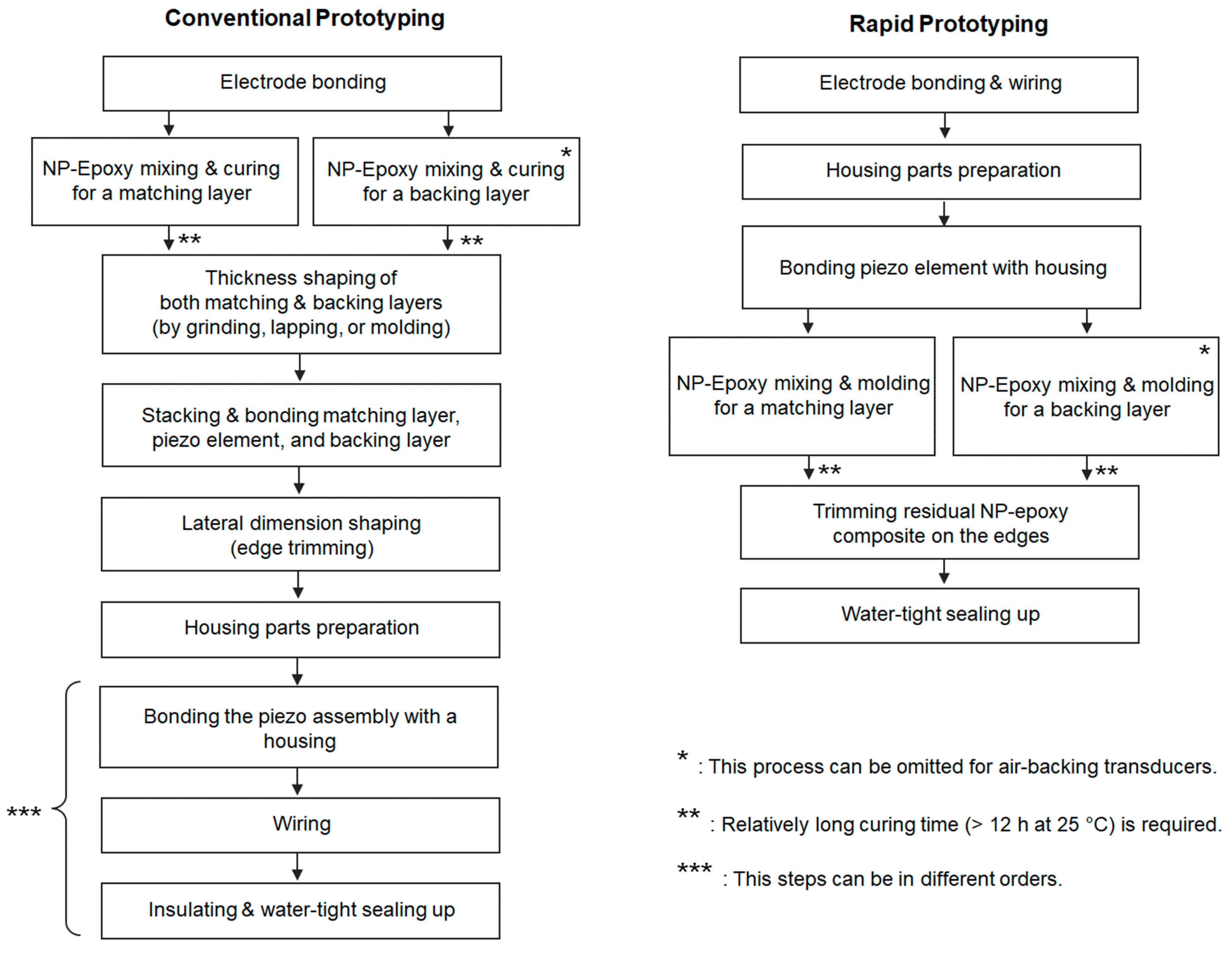
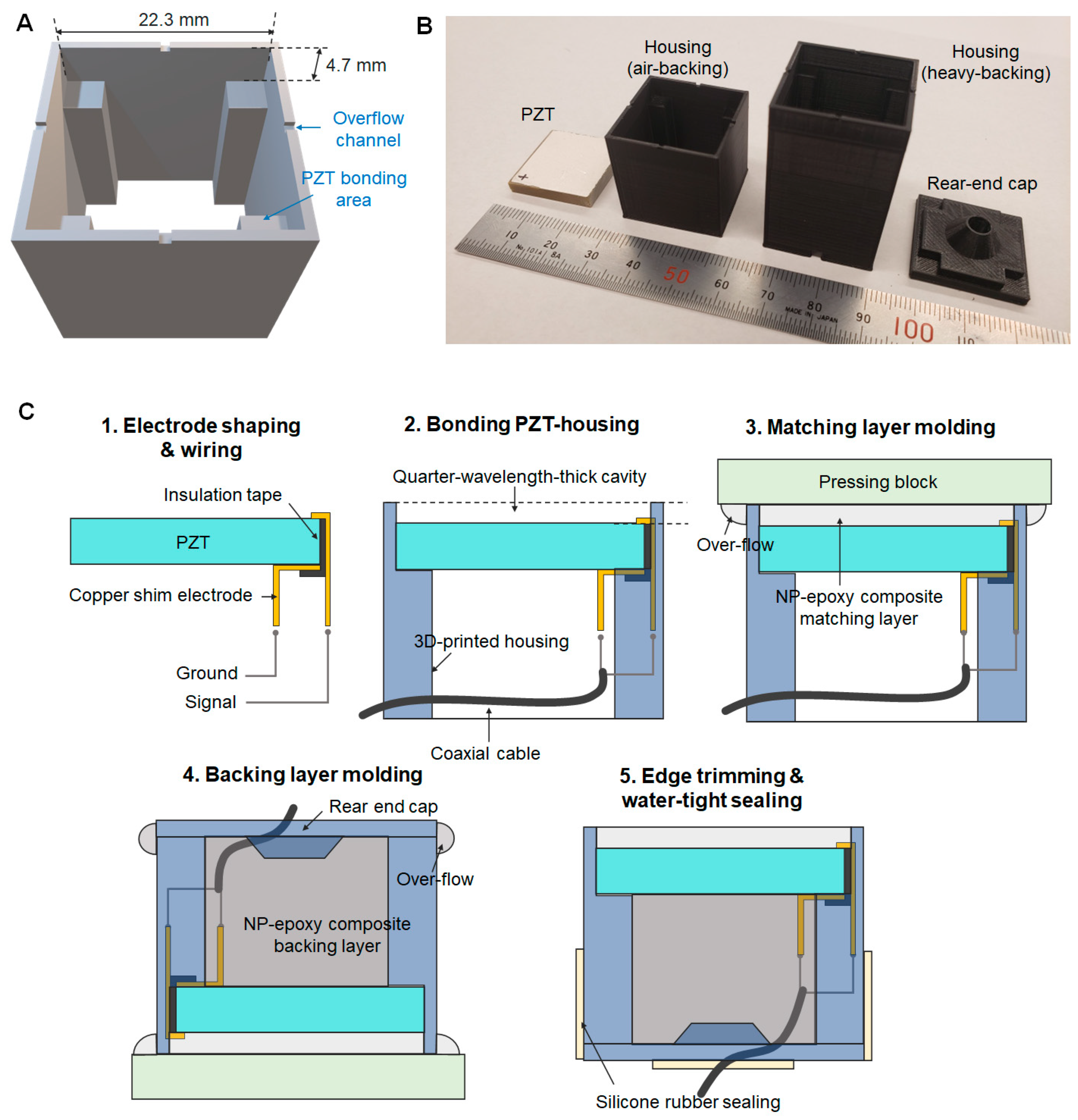
2.2. Transducer Prototyping
2.3. Transducer Characterization
3. Results
3.1. Modeling and Simulation
3.2. Prototype Transducers
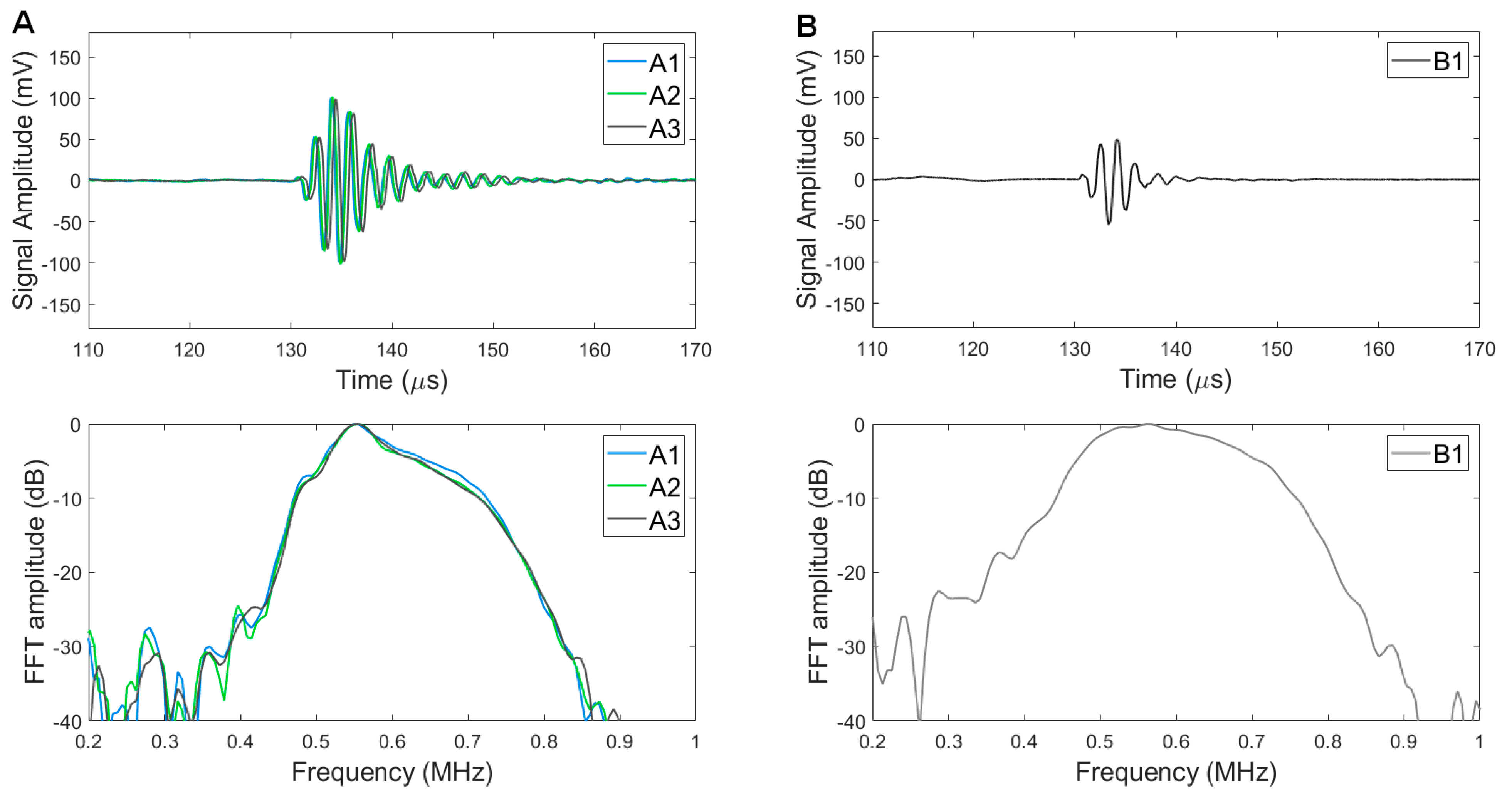
3.3. Pulse Echo Responses
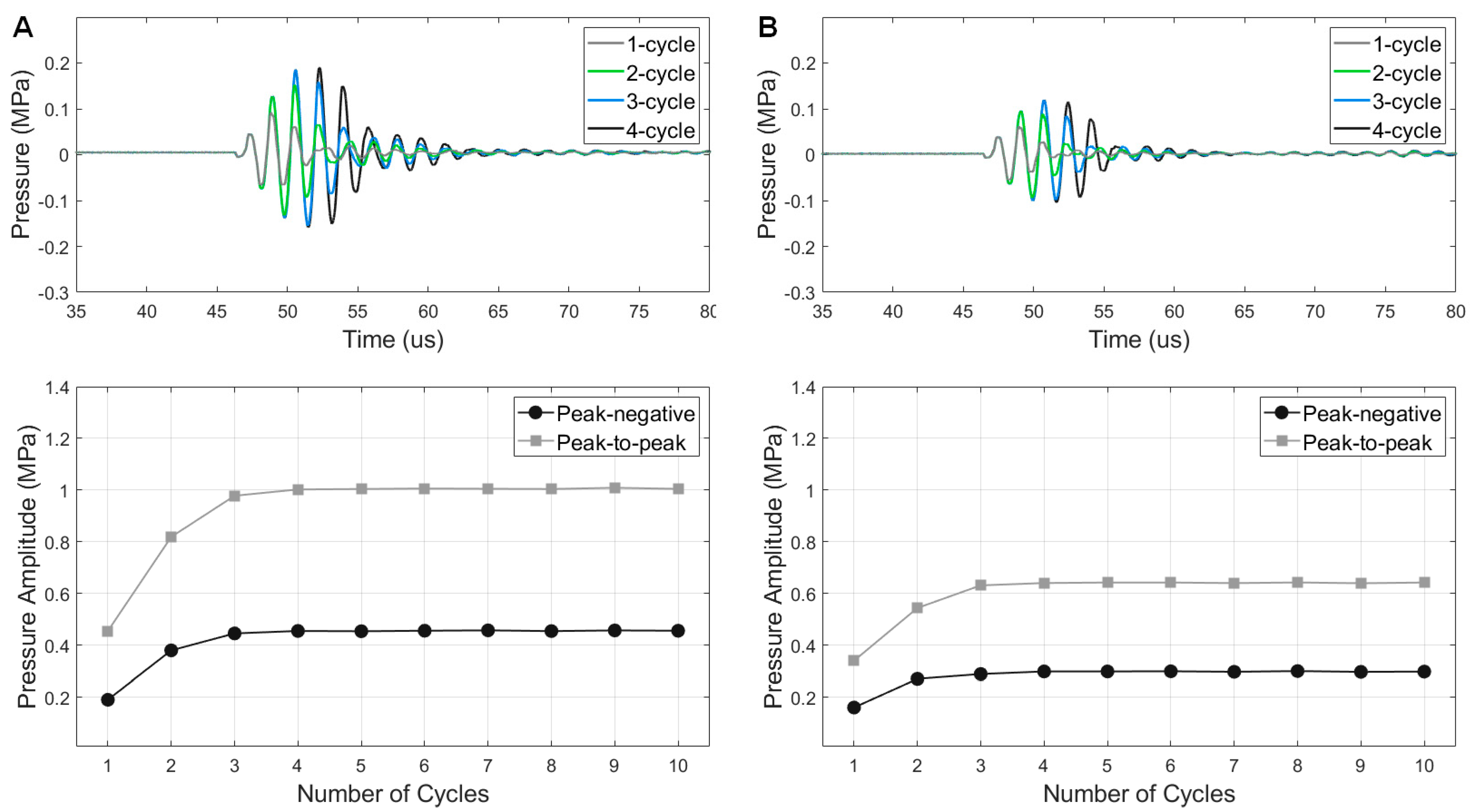
3.4. Pressure Outputs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Safari, A.; Akdogan, E.K. Piezoelectric Transducers and Acoustic Materials for Transducer Aplications; Springer Science & Business Media: New York, NY, USA, 2008; ISBN 978-0-387-76538-9. [Google Scholar]
- Zhou, Q.; Lam, K.H.; Zheng, H.; Qiu, W.; Shung, K.K. Piezoelectric Single Crystal Ultrasonic Transducers for Biomedical Applications. Prog. Mater. Sci. 2014, 66, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.; Martin, K.; Thrush, A. Diagnostic Ultrasound: Physics and Equipment, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Ellens, N.P.K.; Hynynen, K. High-Intensity Focused Ultrasound for Medical Therapy. In Power Ultrasonics; Gallego-Juárez, J.A., Graff, K.F., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 661–693. ISBN 978-1-78242-028-6. [Google Scholar]
- Jiang, X.; Li, S.; Kim, J.; Ma, J. High Frequency Piezo-Composite Micromachined Ultrasound Transducer Array Technology for Biomedical Imaging; ASME Press: New York, NY, USA, 2017; ISBN 9780791860441. [Google Scholar]
- Bouakaz, A.; Ten Cate, F.; de Jong, N. A New Ultrasonic Transducer for Improved Contrast Nonlinear Imaging. Phys. Med. Biol. 2004, 49, 3515–3525. [Google Scholar] [CrossRef] [PubMed]
- Dauba, A.; Delalande, A.; Kamimura, H.A.S.; Conti, A.; Larrat, B.; Tsapis, N.; Novell, A. Recent Advances on Ultrasound Contrast Agents for Blood-Brain Barrier Opening with Focused Ultrasound. Pharmaceutics 2020, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Loskutova, K.; Grishenkov, D.; Ghorbani, M. Review on Acoustic Droplet Vaporization in Ultrasound Diagnostics and Therapeutics. Biomed. Res. Int. 2019, 2019, 9480193. [Google Scholar] [CrossRef]
- Ingram, N.; McVeigh, L.E.; Abou-Saleh, R.H.; Maynard, J.; Peyman, S.A.; McLaughlan, J.R.; Fairclough, M.; Marston, G.; Valleley, E.M.A.; Jimenez-Macias, J.L.; et al. Ultrasound-Triggered Therapeutic Microbubbles Enhance the Efficacy of Cytotoxic Drugs by Increasing Circulation and Tumor Drug Accumulation and Limiting Bioavailability and Toxicity in Normal Tissues. Theranostics 2020, 10, 10973–10992. [Google Scholar] [CrossRef]
- Sung, J.-H.; Chang, J.-H. Mechanically Rotating Intravascular Ultrasound (IVUS) Transducer: A Review. Sensors 2021, 21, 3907. [Google Scholar] [CrossRef]
- Lu, N.; Hall, T.L.; Choi, D.; Gupta, D.; Daou, B.J.; Sukovich, J.R.; Fox, A.; Gerhardson, T.I.; Pandey, A.S.; Noll, D.C.; et al. Transcranial MR-Guided Histotripsy System. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2917–2929. [Google Scholar] [CrossRef]
- Yang, J.; Chérin, E.; Yin, J.; Newsome, I.G.; Kierski, T.M.; Pang, G.; Carnevale, C.A.; Dayton, P.A.; Foster, F.S.; Demore, C.E.M. Characterization of an Array-Based Dual-Frequency Transducer for Superharmonic Contrast Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2419–2431. [Google Scholar] [CrossRef]
- Jani, V.; Shivaram, P.; Porter, T.R.; Kutty, S. Ultrasound Theranostics in Adult and Pediatric Cardiovascular Research. Cardiovasc. Drugs Ther. 2021, 35, 185–190. [Google Scholar] [CrossRef]
- Li, S.; Kim, J.; Wang, Z.; Kasoji, S.; Lindsey, B.D.; Dayton, P.A.; Jiang, X. A Dual-Frequency Co-Linear Array for Acoustic Angiography in Prostate Cancer Evaluation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 2418–2428. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.J.; Le, T.T.; Das, R.; Ke, K.; Santorella, E.M.; Paul, D.; Chorsi, M.T.; Tran, K.T.M.; Baroody, J.; Borges, E.R.; et al. Biodegradable Nanofiber-Based Piezoelectric Transducer. Proc. Natl. Acad. Sci. USA 2020, 117, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Abedi, M.H.; Yao, M.S.; Mittelstein, D.R.; Bar-Zion, A.; Swift, M.B.; Lee-Gosselin, A.; Barturen-Larrea, P.; Buss, M.T.; Shapiro, M.G. Ultrasound-Controllable Engineered Bacteria for Cancer Immunotherapy. Nat. Commun. 2022, 13, 1585. [Google Scholar] [CrossRef] [PubMed]
- Woodacre, J.K.; Landry, T.G.; Brown, J.A. A Low-Cost Miniature Histotripsy Transducer for Precision Tissue Ablation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Wu, H.; Zhang, B.; Dayton, P.A.; Jiang, X. A Multi-Pillar Piezoelectric Stack Transducer for Nanodroplet Mediated Intravascular Sonothrombolysis. Ultrasonics 2021, 116, 106520. [Google Scholar] [CrossRef]
- Kim, Y.; Maxwell, A.D.; Hall, T.L.; Xu, Z.; Lin, K.-W.; Cain, C.A. Rapid Prototyping Fabrication of Focused Ultrasound Transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 1559–1574. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Ma, J.; Qi, X.; Shen, B.; Liu, Y.; Sun, E.; Zhang, R. Enhancement of Ultrasonic Transducer Bandwidth by Acoustic Impedance Gradient Matching Layer. Sensors 2022, 22, 8025. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hossain, M.M.; Kim, H.; Gallippi, C.M.; Jiang, X. A 1.5-D Array for Acoustic Radiation Force (ARF)-Induced Peak Displacement-Based Tissue Anisotropy Assessment With a Row-Column Excitation Method. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1278–1287. [Google Scholar] [CrossRef]
- Lai, J.; Wang, C.; Wang, M. 3D Printing in Biomedical Engineering: Processes, Materials, and Applications. Appl. Phys. Rev. 2021, 8, 21322. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-Curing 3D Printing Technique and Its Challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, S.; Yang, Y.; Gong, Y.; Chen, H. An Affordable and Easy-to-Use Focused Ultrasound Device for Noninvasive and High Precision Drug Delivery to the Mouse Brain. IEEE Trans. Biomed. Eng. 2022, 69, 2723–2732. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Y.; Gong, H.; Lee, Y.-S. Design and 3D Printing of Waveguide-Based Ultrasonic Longitudinal-Torsional Transducers for Medical Needle Insertion. Sens. Actuators A Phys. 2022, 344, 113706. [Google Scholar] [CrossRef]
- Kim, J.; Kasoji, S.; Durham, P.G.; Dayton, P.A. Acoustic Holograms for Directing Arbitrary Cavitation Patterns. Appl. Phys. Lett. 2021, 118, 051902. [Google Scholar] [CrossRef]
- Jones, R.M.; Caskey, C.F.; Dayton, P.A.; Oralkan, Ö.; Pinton, G.F. Transcranial Neuromodulation Array With Imaging Aperture for Simultaneous Multifocus Stimulation in Nonhuman Primates. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, S.; Kasoji, S.; Dayton, P.A.; Jiang, X. Phantom Evaluation of Stacked-Type Dual-Frequency 1–3 Composite Transducers: A Feasibility Study on Intracavitary Acoustic Angiography. Ultrasonics 2015, 63, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, B.D.; Kim, J.; Dayton, P.A.; Jiang, X. Dual-Frequency Piezoelectric Endoscopic Transducer for Imaging Vascular Invasion in Pancreatic Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1078–1086. [Google Scholar] [CrossRef]
- Wang, H.; Ritter, T.A.; Cao, W.; Shung, K.K. High Frequency Properties of Passive Materials for Ultrasonic Transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 78–84. [Google Scholar] [CrossRef]
- Zhou, J.; Bai, J.; Liu, Y. Fabrication and Modeling of Matching System for Air-Coupled Transducer. Micromachines 2022, 13, 781. [Google Scholar] [CrossRef]
- Martin, K.H.; Lindsey, B.D.; Ma, J.; Lee, M.; Li, S.; Foster, F.S.; Jiang, X.; Dayton, P.A. Dual-Frequency Piezoelectric Transducers for Contrast Enhanced Ultrasound Imaging. Sensors 2014, 14, 20825–20842. [Google Scholar] [CrossRef]
- Kim, J.; Wu, H.; Jiang, X. Miniaturized Focused Ultrasound Transducers for Intravascular Therapies. In Proceedings of the International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers Digital Collection, Tampa, FL, USA, 3–9 November 2017. [Google Scholar]
- Liu, D.-L.; Waag, R.C. Propagation and Backpropagation for Ultrasonic Wavefront Design. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997, 44, 1–13. [Google Scholar] [CrossRef]
- Melde, K.; Mark, A.G.; Qiu, T.; Fischer, P. Holograms for Acoustics. Nature 2016, 537, 518–522. [Google Scholar] [CrossRef] [PubMed]





| Structure | Material | Dimensions (mm3) | Acoustic Impedance (MRayl) | VL (m/s) | kt | ε/ε0 | α (dB/cm/MHz) |
|---|---|---|---|---|---|---|---|
| Drive element | PZT-5A | 21 × 21 × 3.5 | 31.2 | 4000 | 0.45 | 850 | - |
| Matching layer | Alumina (25%)-epoxy | 21 × 21 × 1.16 | 4.8 | 2750 | - | - | 5.3 |
| * Backing layer | Tungsten (11%)-epoxy | 21 × 21 × 32 | 5.7 | 1900 | - | - | 25 |
| Test items | Equipment | Parameters |
|---|---|---|
| Pulse-echo |
|
|
| Pressure vs. burst cycles Pressure vs. voltages Pressure beam mapping |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Menichella, B.; Lee, H.; Dayton, P.A.; Pinton, G.F. A Rapid Prototyping Method for Sub-MHz Single-Element Piezoelectric Transducers by Using 3D-Printed Components. Sensors 2023, 23, 313. https://doi.org/10.3390/s23010313
Kim J, Menichella B, Lee H, Dayton PA, Pinton GF. A Rapid Prototyping Method for Sub-MHz Single-Element Piezoelectric Transducers by Using 3D-Printed Components. Sensors. 2023; 23(1):313. https://doi.org/10.3390/s23010313
Chicago/Turabian StyleKim, Jinwook, Bryce Menichella, Hanjoo Lee, Paul A. Dayton, and Gianmarco F. Pinton. 2023. "A Rapid Prototyping Method for Sub-MHz Single-Element Piezoelectric Transducers by Using 3D-Printed Components" Sensors 23, no. 1: 313. https://doi.org/10.3390/s23010313
APA StyleKim, J., Menichella, B., Lee, H., Dayton, P. A., & Pinton, G. F. (2023). A Rapid Prototyping Method for Sub-MHz Single-Element Piezoelectric Transducers by Using 3D-Printed Components. Sensors, 23(1), 313. https://doi.org/10.3390/s23010313







