Nanoscale Investigation of DNA Demethylation in Leukemia Cells by Means of Ultrasensitive Vibrational Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colloidal Silver Nanoparticles Preparation
2.2. Cell Culturing
2.3. 5-Azacytidine Treatments
2.4. DNA Extraction
2.5. Raman Spectroscopy Sample Preparation and Measurements
2.6. SERS Sample Preparation and Measurements
3. Results
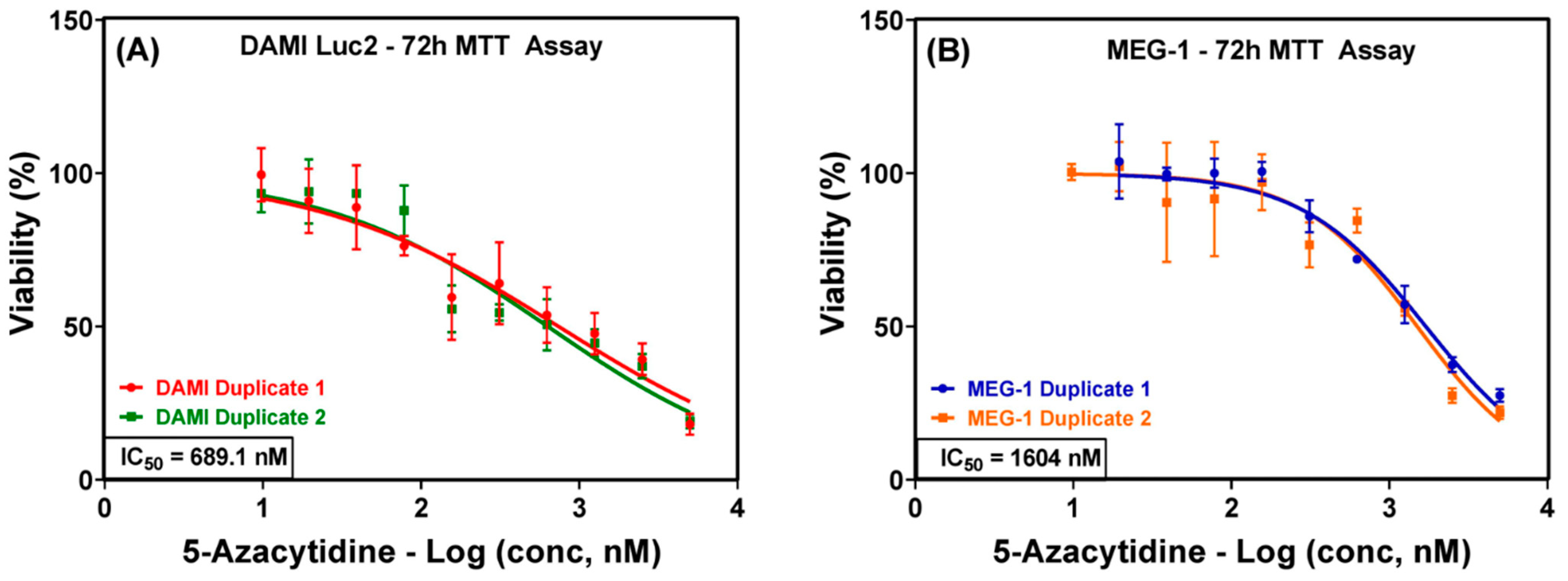
3.1. 5-Azacytidine Treatment Effects
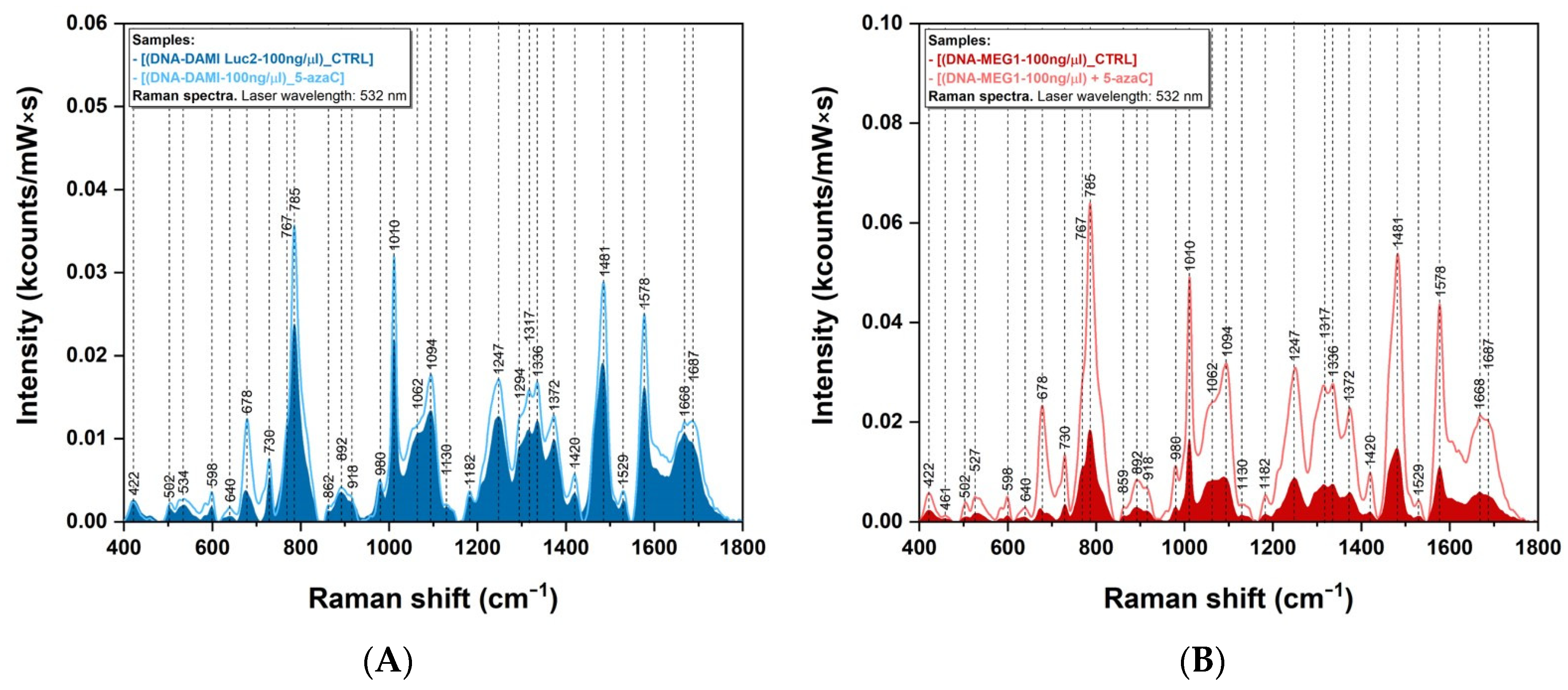
3.2. Raman Spectroscopy Measurements
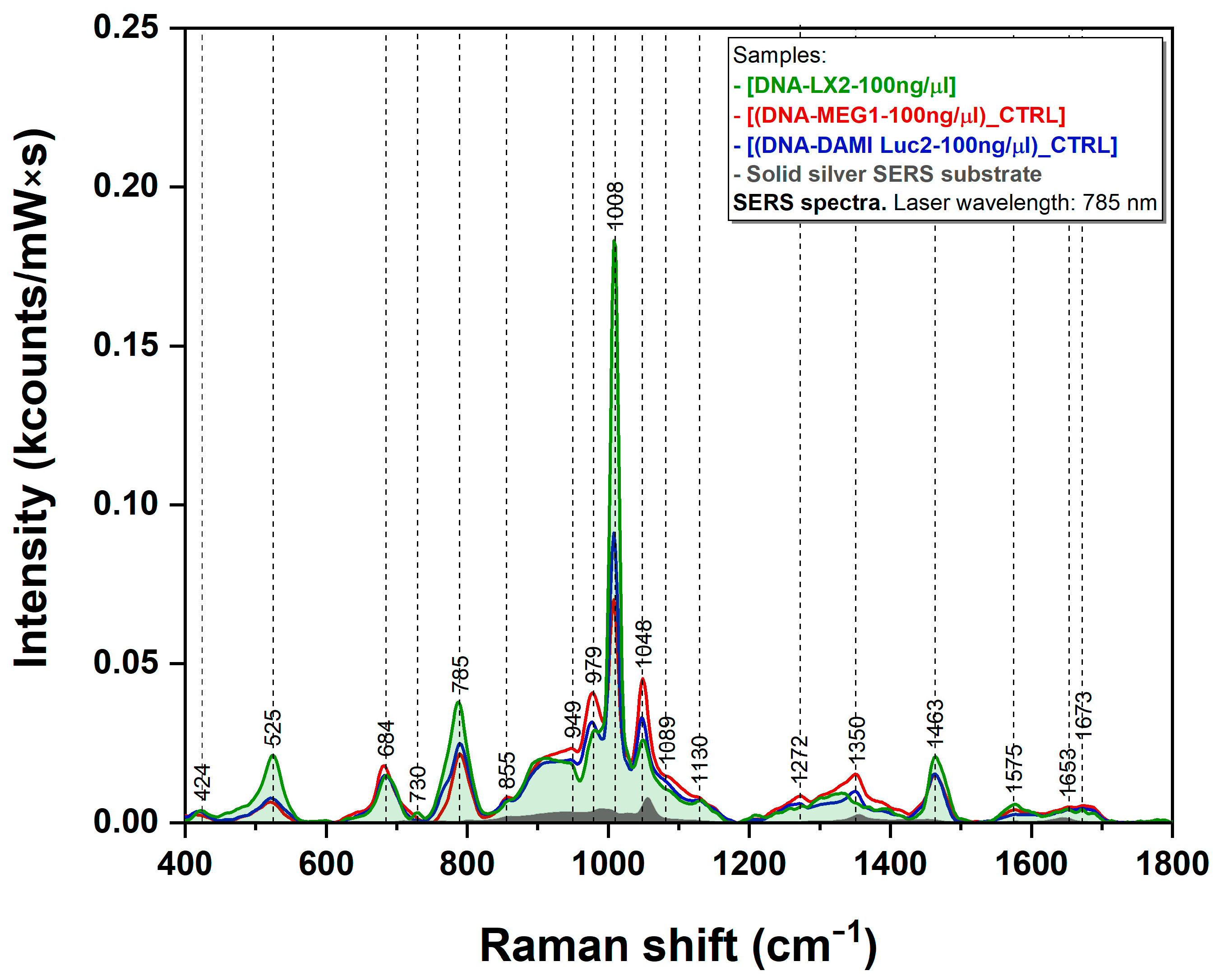
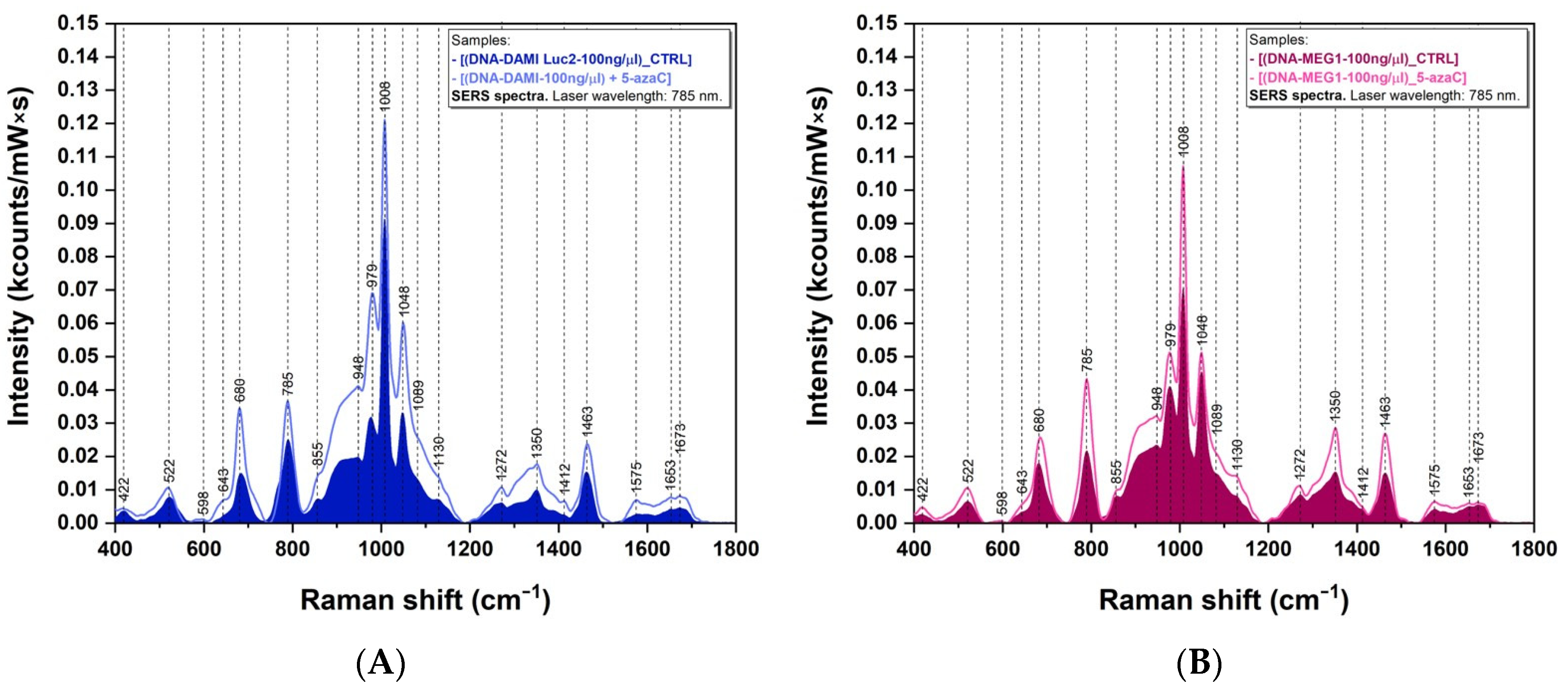
3.3. SERS Measurements
4. Discussion
4.1. Raman Analysis
4.2. SERS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Globocan Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 1 September 2022).
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosavi, A.; Motevalizadeh Ardekani, A. Role of epigenetics in biology and human diseases. Iran. Biomed. J. 2016, 20, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Li, J.; Mao, C.; He, J.; Zhao, X. Role of epigenetic in leukemia: From mechanism to therapy. Chem. Biol. Interact. 2020, 317, 108963. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Bird, A.; Taggart, M.; Frommer, M.; Miller, O.J.; Macleod, D. A fraction of the mouse genome that is derived from islands of nonmethylated, CpG-rich DNA. Cell 1985, 40, 91–99. [Google Scholar] [CrossRef]
- Bird, A.P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980, 8, 1499–1504. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, G.; Ziff, E. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science 1991, 251, 186–189. [Google Scholar] [CrossRef]
- Gnyszka, A.; Jastrzębski, Z.; Flis, S. DNA methyltransferase inhibitors and their emerging role in epigenetic therapy of cancer. Anticancer Res. 2013, 2996, 2989–2996. [Google Scholar]
- Stresemann, C.; Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 2008, 123, 8–13. [Google Scholar] [CrossRef]
- Christman, J.K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef]
- Pískala, A.; Šorm, F. Nucleic acids components and their analogues. LI. Synthesis of 1-glycosyl derivatives of 5-azauracil and 5-azacytosine. Collect. Czechoslov. Chem. Commun. 1964, 29, 2060–2076. [Google Scholar] [CrossRef]
- Šorm, F.; Pískala, A.; Čihák, A.; Veselý, J. 5-Azacytidine, a new, highly effective cancerostatic. Experientia 1964, 20, 202–203. [Google Scholar] [CrossRef]
- Čihák, A. Biological effects of 5-azacytidine in eukaryotes. Oncology 1974, 30, 405–422. [Google Scholar] [CrossRef]
- Karon, M.; Benedict, W.F. Chromatid breakage: Differential effect of inhibitors of DNA synthesis during G2 phase. Science 1972, 178, 62. [Google Scholar] [CrossRef]
- Viegas-Péquignot, E.; Dutrillaux, B. Segmentation of human chromosomes induced by 5-ACR (5-azacytidine). Hum. Genet. 1976, 34, 247–254. [Google Scholar] [CrossRef]
- Halle, S. 5-Azacytidine as a mutagen for arboviruses. J. Virol. 1968, 2, 1228–1229. [Google Scholar] [CrossRef] [Green Version]
- Landolph, J.R.; Jones, P.A. Mutagenicity of 5-azacytidine and related nucleosides in C3H/10T½ clone 8 and V79 cells. Cancer Res. 1982, 42, 817–823. [Google Scholar]
- Li, L.H.; Olin, E.J.; Buskirk, H.H.; Reineke, L.M. Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res. 1970, 30, 2760–2769. [Google Scholar]
- Hollenbach, P.W.; Nguyen, A.N.; Brady, H.; Williams, M.; Ning, Y.; Richard, N.; Krushel, L.; Aukerman, S.L.; Heise, C.; MacBeth, K.J. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS ONE 2010, 5, e9001. [Google Scholar] [CrossRef] [Green Version]
- Momparler, R.L. A perspective on the comparative antileukemic activity of 5-Aza-2′-deoxycytidine (Decitabine) and 5-Azacytidine (Vidaza). Pharmaceuticals 2012, 5, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Weisenberger, D.J.; Velicescu, M.; Cheng, J.C.; Gonzales, F.A.; Liang, G.; Jones, P.A. Role of the DNA methyltransferase variant DNMT3b3 in DNA methylation. Mol. Cancer Res. 2004, 2, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Palii, S.S.; Van Emburgh, B.O.; Sankpal, U.T.; Brown, K.D.; Robertson, K.D. DNA methylation inhibitor 5-Aza-2′-Deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol. Cell. Biol. 2008, 28, 752–771. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; van den Tweel, T.J.J.; de Mul, F.F.M.; Greve, J. Surface-enhanced Raman spectroscopy of DNA bases. J. Raman Spectrosc. 1986, 17, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Prescott, B.; Steinmetz, W.; Thomas, G.J. Characterization of DNA structures by laser Raman spectroscopy. Biopolymers 1984, 23, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, A.; Zhang, D.; Tam, F.; Halas, N.J. Surface-enhanced raman spectroscopy of DNA. J. Am. Chem. Soc. 2008, 130, 5523–5529. [Google Scholar] [CrossRef]
- Colceriu-Șimon, I.M.; Hedeșiu, M.; Toma, V.; Armencea, G.; Moldovan, A.; Știufiuc, G.; Culic, B.; Țărmure, V.; Dinu, C.; Berindan-Neagoe, I.; et al. The effects of low-dose irradiation on human saliva: A surface-enhanced raman spectroscopy study. Diagnostics 2019, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Știufiuc, G.F.; Toma, V.; Buse, M.; Mărginean, R.; Morar-Bolba, G.; Culic, B.; Tetean, R.; Leopold, N.; Pavel, I.; Lucaciu, C.M.C.M.; et al. Solid plasmonic substrates for breast cancer detection by means of SERS analysis of blood plasma. Nanomaterials 2020, 10, 1212. [Google Scholar] [CrossRef]
- Graham, D.; Faulds, K. Surface-enhanced Raman scattering as a detection technique for molecular diagnostics. Expert Rev. Mol. Diagn. 2009, 9, 537–539. [Google Scholar] [CrossRef] [Green Version]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Garcia-Rico, E.; Alvarez-Puebla, R.A.; Guerrini, L. Direct surface-enhanced Raman scattering (SERS) spectroscopy of nucleic acids: From fundamental studies to real-life applications. Chem. Soc. Rev. 2018, 47, 4909–4923. [Google Scholar] [CrossRef]
- Kuhar, N.; Sil, S.; Verma, T.; Umapathy, S. Challenges in application of Raman spectroscopy to biology and materials. RSC Adv. 2018, 8, 25888–25908. [Google Scholar] [CrossRef] [Green Version]
- Huh, Y.S.; Chung, A.J.; Erickson, D. Surface enhanced Raman spectroscopy and its application to molecular and cellular analysis. Microfluid. Nanofluidics 2009, 6, 285–297. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Hao, B.; Wang, K.; Zhou, Y.; Sui, C.; Wang, L.; Bai, R.; Yang, Z. Label-free detecting of the compaction and decompaction of ctDNA molecules induced by surfactants with SERS based on a nanoPAA-ZnCl 2 -AuLs solid substrate. ACS Omega 2020, 5, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Pyrak, E.; Krajczewski, J.; Kowalik, A.; Kudelski, A.; Jaworska, A. Surface enhanced raman spectroscopy for DNA biosensors—How far are we? Molecules 2019, 24, 4423. [Google Scholar] [CrossRef] [Green Version]
- Leopold, N.; Lendl, B. A new method for fast preparation of highly surface-enhanced raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Sina, A.A.I.; Carrascosa, L.G.; Liang, Z.; Grewal, Y.S.; Wardiana, A.; Shiddiky, M.J.A.; Gardiner, R.A.; Samaratunga, H.; Gandhi, M.K.; Scott, R.J.; et al. Epigenetically reprogrammed methylation landscape drives the DNA self-assembly and serves as a universal cancer biomarker. Nat. Commun. 2018, 9, 4915. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lim, S.F.; Puretzky, A.; Riehn, R.; Hallen, H.D. DNA Methylation Detection using resonance and nanobowtie-antenna-enhanced raman spectroscopy. Biophys. J. 2018, 114, 2498–2506. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Ma, R.; Wu, Y.; Zhai, Y.; Li, S. Reciprocal regulation of metabolic reprogramming and epigenetic modifications in cancer. Front. Genet. 2018, 9, 394. [Google Scholar] [CrossRef] [Green Version]
- Villa, E.; Ali, E.; Sahu, U.; Ben-Sahra, I. Cancer cells tune the signaling pathways to empower de novo synthesis of nucleotides. Cancers 2019, 11, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Zeng, Q.Y.; Li, L.F.; Qi, M.N.; Qi, Q.C.; Li, S.X.; Xu, J.F. Label-free rapid identification of tumor cells and blood cells with silver film SERS substrate. Opt. Express 2018, 26, 33044. [Google Scholar] [CrossRef] [PubMed]
- Moisoiu, V.; Stefancu, A.; Gulei, D.; Boitor, R.; Magdo, L.; Raduly, L.; Pasca, S.; Kubelac, P.; Mehterov, N.; Chis, V.; et al. SERS-based differential diagnosis between multiple solid malignancies: Breast, colorectal, lung, ovarian and oral cancer. Int. J. Nanomed. 2019, 14, 6165–6178. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Bloomfield, V.A.; Benevides, J.M.; Thomas, G.J. Dependence of the raman signature of genomic B-DNA on nucleotide base sequence. Biopolymers 1999, 50, 656–666. [Google Scholar] [CrossRef]
- Gu, X.; Tohme, R.; Tomlinson, B.; Sakre, N.; Hasipek, M.; Durkin, L.; Schuerger, C.; Grabowski, D.; Zidan, A.M.; Radivoyevitch, T.; et al. Decitabine- and 5-azacytidine resistance emerges from adaptive responses of the pyrimidine metabolism network. Leukemia 2021, 35, 1023–1036. [Google Scholar] [CrossRef]
- Moskovits, M. Surface selection rules. J. Chem. Phys. 1982, 77, 4408–4416. [Google Scholar] [CrossRef]
- Stiufiuc, R.; Iacovita, C.; Lucaciu, C.M.; Stiufiuc, G.; Nicoara, R.; Oltean, M.; Chis, V.; Bodoki, E. Adsorption geometry of propranolol enantiomers on silver nanoparticles. J. Mol. Struct. 2013, 1031, 201–206. [Google Scholar] [CrossRef]
- Śanchez-Cortés, S.; Garcia-Ramos, J.V. SERS of cytosine and its methylated derivatives on metal colloids. J. Raman Spectrosc. 1992, 23, 61–66. [Google Scholar] [CrossRef]
- Aroca, R.; Bujalski, R. Surface enhanced vibrational spectra of thymine. Vib. Spectrosc. 1999, 19, 11–21. [Google Scholar] [CrossRef]
- Moisoiu, V.; Stefancu, A.; Iancu, S.D.; Moisoiu, T.; Loga, L.; Dican, L.; Alecsa, C.D.; Boros, I.; Jurj, A.; Dima, D.; et al. SERS assessment of the cancer-specific methylation pattern of genomic DNA: Towards the detection of acute myeloid leukemia in patients undergoing hematopoietic stem cell transplantation. Anal. Bioanal. Chem. 2019, 411, 7907–7913. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onaciu, A.; Toma, V.; Moldovan, C.; Țigu, A.B.; Cenariu, D.; Culic, C.; Borșa, R.M.; David, L.; Știufiuc, G.F.; Tetean, R.; et al. Nanoscale Investigation of DNA Demethylation in Leukemia Cells by Means of Ultrasensitive Vibrational Spectroscopy. Sensors 2023, 23, 346. https://doi.org/10.3390/s23010346
Onaciu A, Toma V, Moldovan C, Țigu AB, Cenariu D, Culic C, Borșa RM, David L, Știufiuc GF, Tetean R, et al. Nanoscale Investigation of DNA Demethylation in Leukemia Cells by Means of Ultrasensitive Vibrational Spectroscopy. Sensors. 2023; 23(1):346. https://doi.org/10.3390/s23010346
Chicago/Turabian StyleOnaciu, Anca, Valentin Toma, Cristian Moldovan, Adrian Bogdan Țigu, Diana Cenariu, Carina Culic, Rareș Mario Borșa, Luca David, Gabriela Fabiola Știufiuc, Romulus Tetean, and et al. 2023. "Nanoscale Investigation of DNA Demethylation in Leukemia Cells by Means of Ultrasensitive Vibrational Spectroscopy" Sensors 23, no. 1: 346. https://doi.org/10.3390/s23010346







