Electrocatalytic Platform Based on Silver-Doped Sugar Apple-like Cupric Oxide Embedded Functionalized Carbon Nanotubes for Nanomolar Detection of Acetaminophen (APAP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
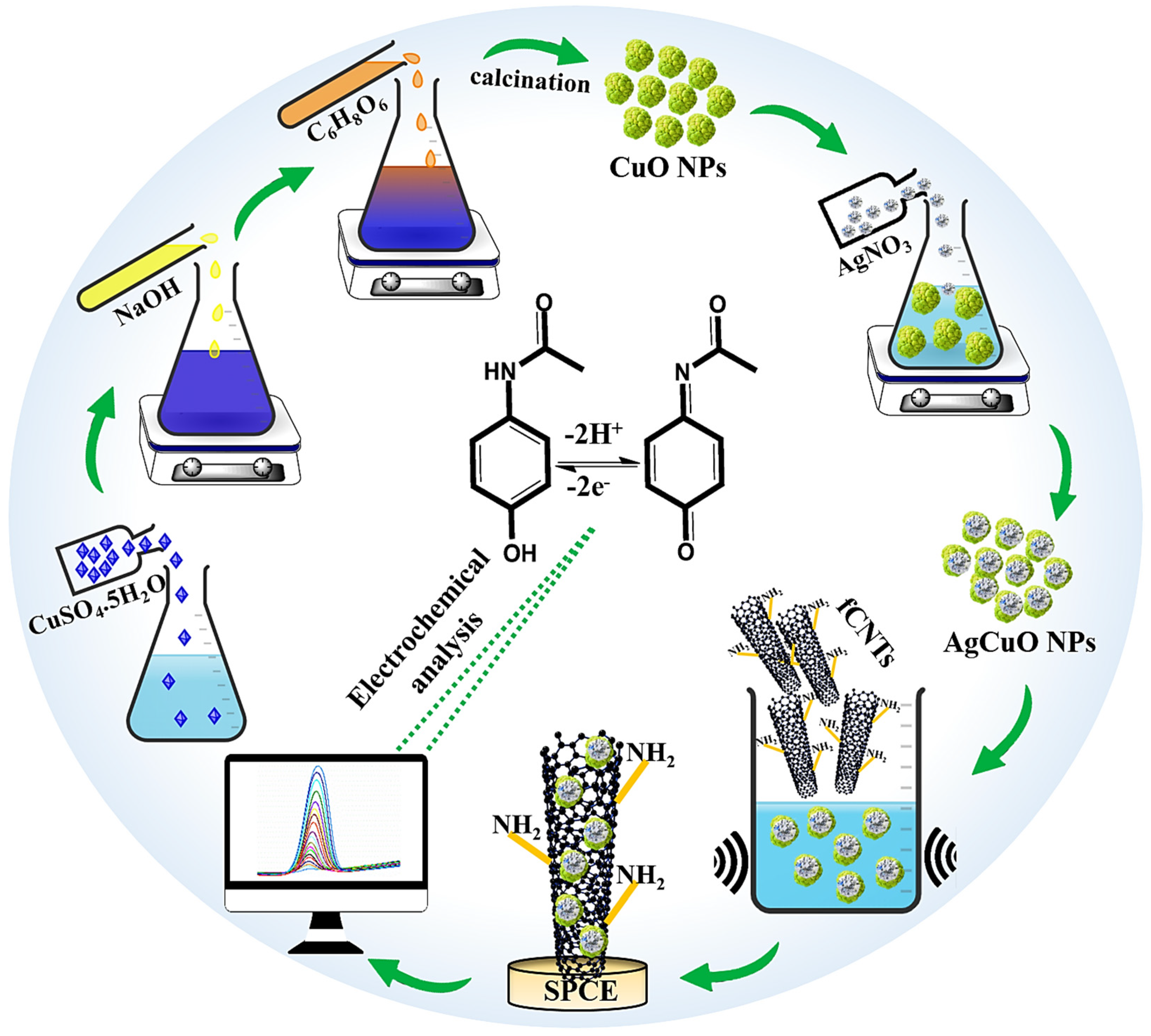
2.2. Ascorbic Acid-Mediated Synthesis of Ag-Doped CuO Nanoparticles
2.3. Synthesis of fCNTs
2.4. Preparation of AgCuO NPs/fCNTs Modified Electrode
3. Results and Discussions
3.1. Characterization of Synthesized Materials
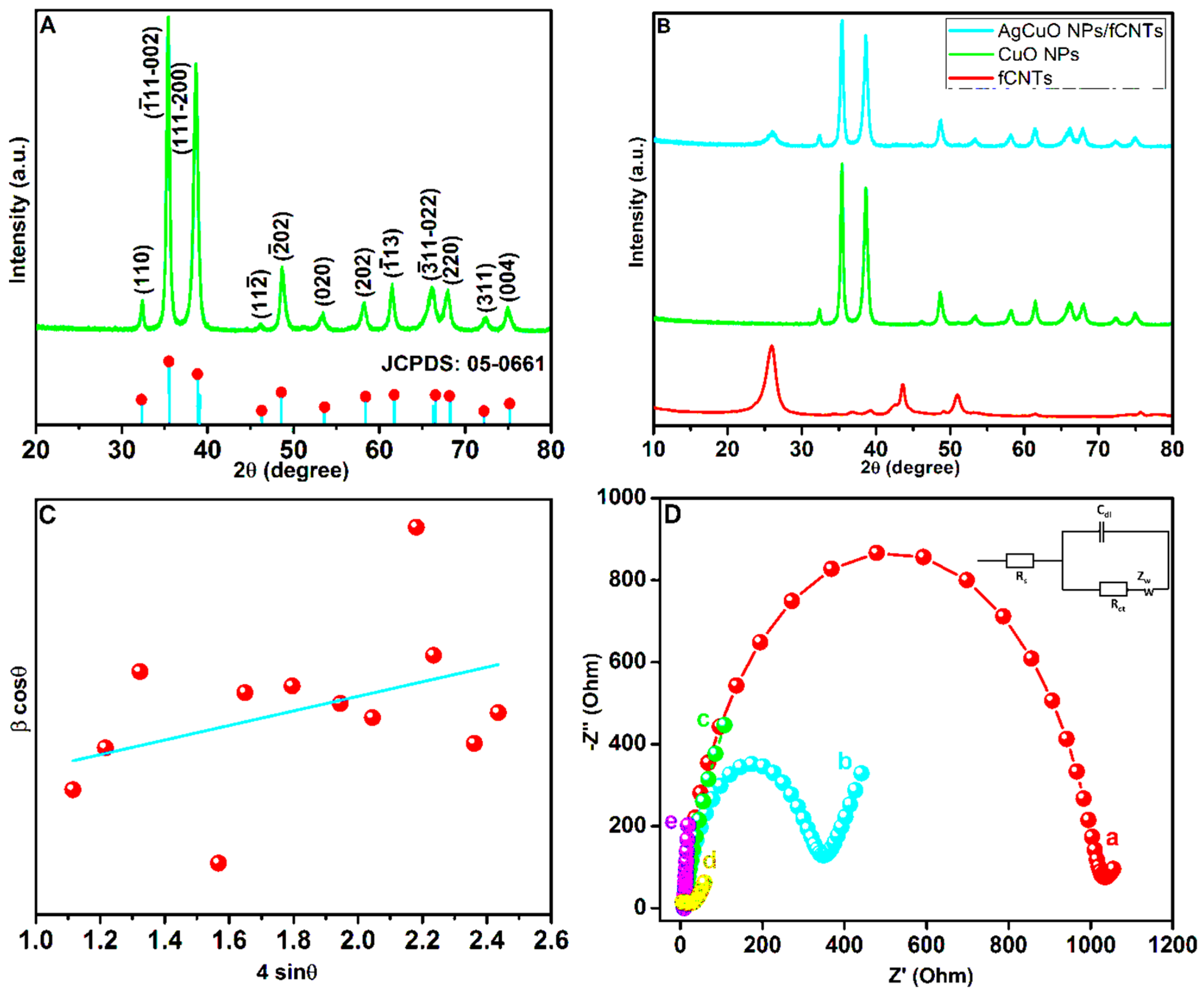
3.1.1. XRD Analysis
3.1.2. Analysis of Electrochemical Impedance Spectroscopy (EIS) Spectra
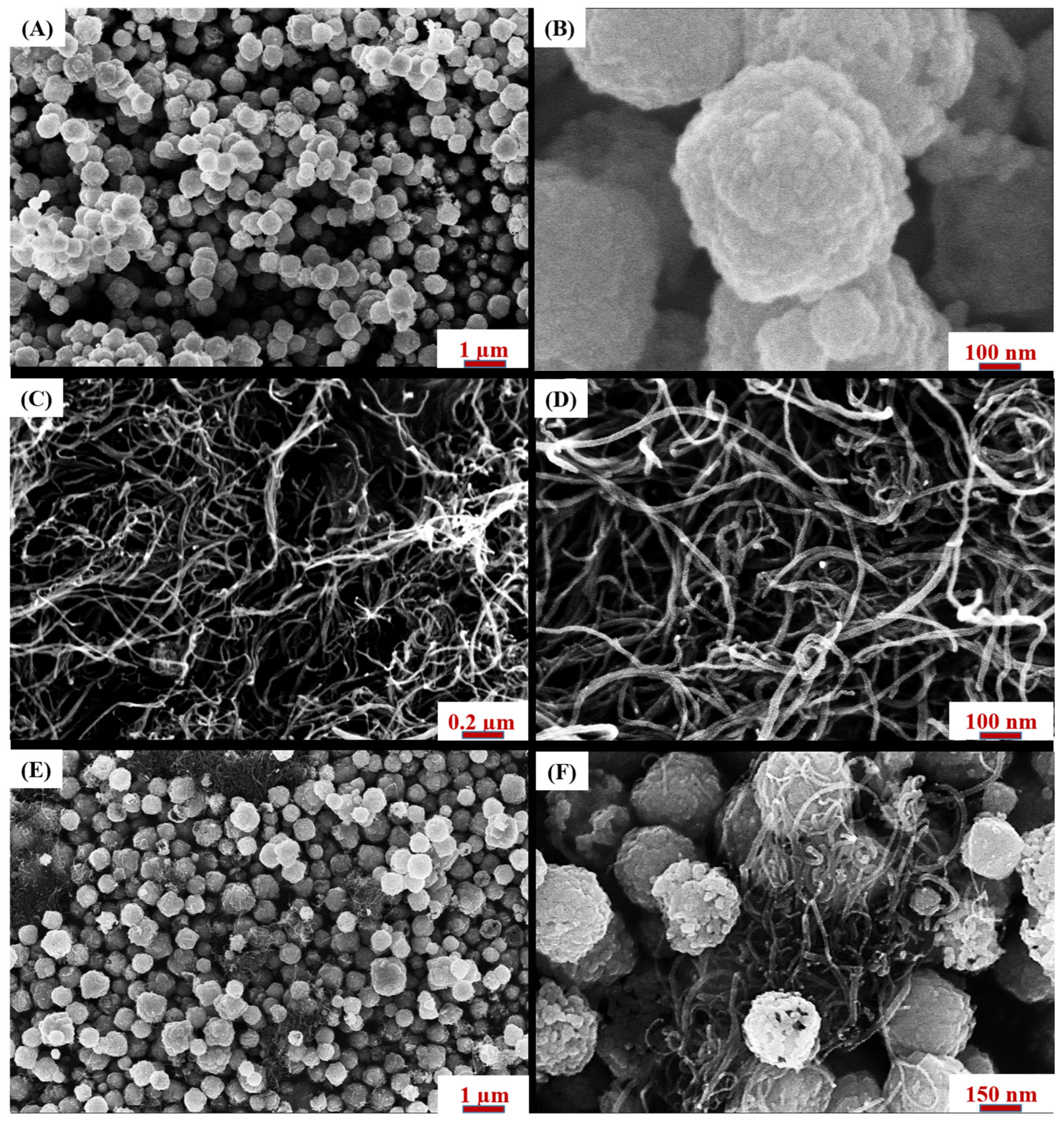
3.1.3. Morphology and Elemental Composition
3.1.4. Analysis of Raman Spectra
3.1.5. Analysis of FTIR Spectra
3.1.6. Analysis of Absorbance Spectra
3.2. Electrochemical Analysis of APAP
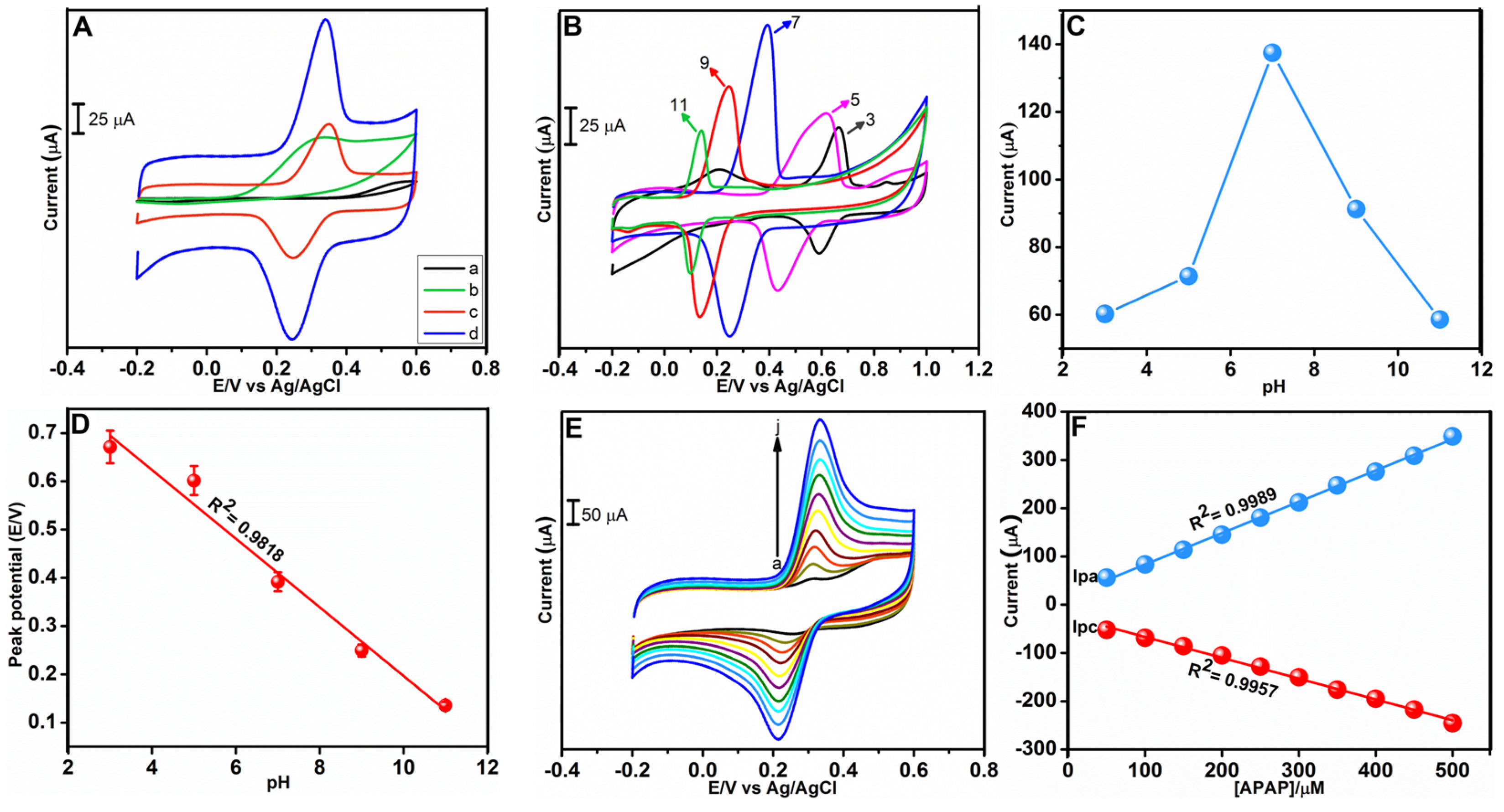
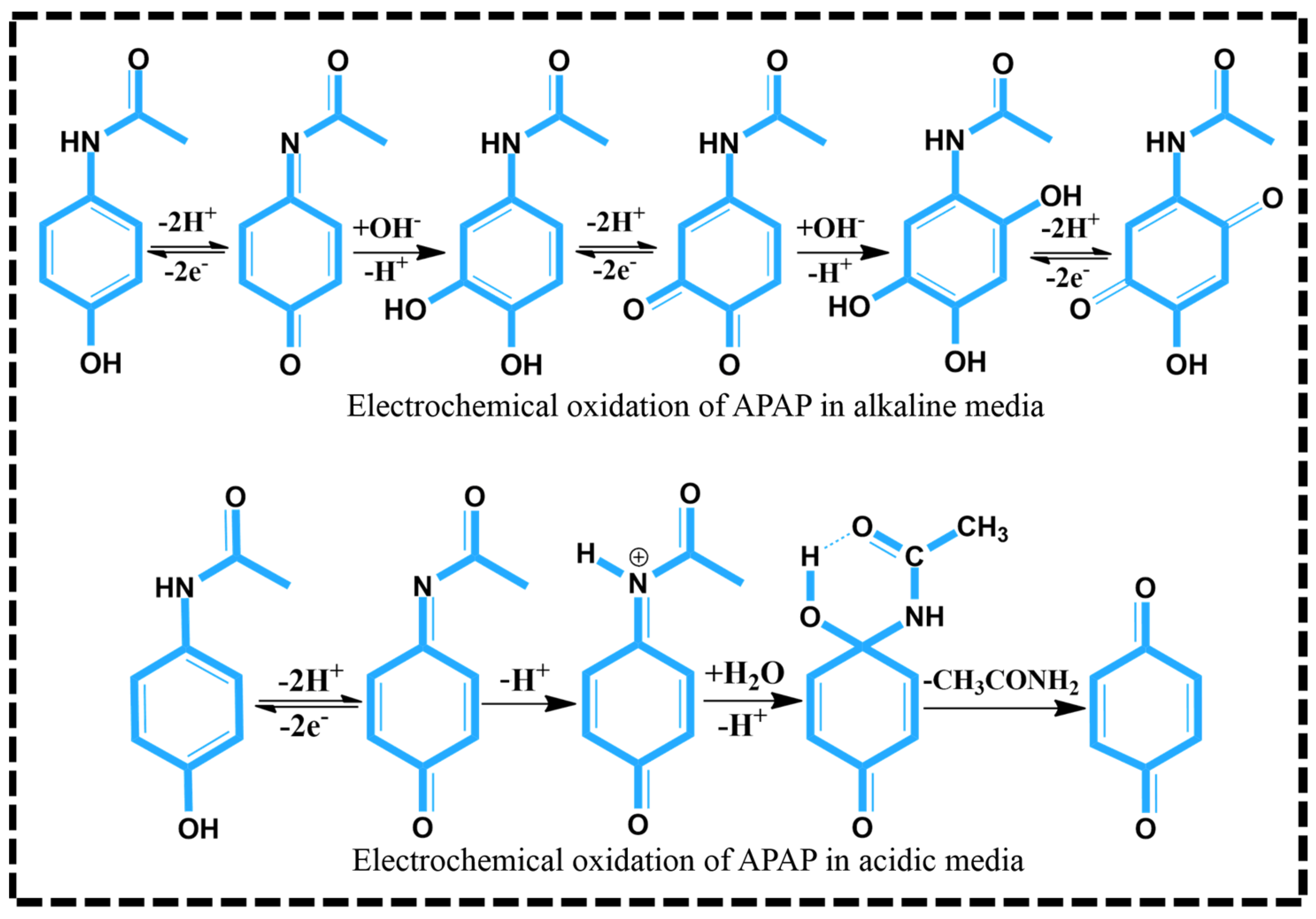
3.2.1. Impact of pH
3.2.2. Effect of Concentration, Scan Rate, Electroactive Surface Area
3.2.3. Electrochemical Determination of APAP
3.2.4. Sensor Performance Evaluation Based on Selectivity, Reproducibility, Stability, and Repeatability
3.2.5. Detection of APAP in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sodhi, M.; Etminan, M. Safety of ibuprofen in patients with COVID-19: Causal or confounded? Chest 2020, 158, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B. COVID-19 and older adult. J. Nutr. Health Aging 2020, 24, 364–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, M. COVID-19: Ibuprofen should not be used for managing symptoms, say doctors and scientists. Br. Med. J. 2020, 368, m1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Apte, U. Liver regeneration after acetaminophen hepatotoxicity: Mechanisms and therapeutic opportunities. Am. J. Pathol. 2019, 189, 719–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, M. Acetaminophen hepatotoxicity. Annu. Rev. Med. 1984, 35, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.M.; Polson, J.; Fontana, R.J.; Davern, T.J.; Lalani, E.; Hynan, L.S.; Reisch, J.S.; Schiødt, F.V.; Ostapowicz, G.; Shakil, A.O. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005, 42, 1364–1372. [Google Scholar] [CrossRef]
- Mofenson, H.C.; Caraccio, T.R.; Nawaz, H.; Steckler, G. Acetaminophen induced pancreatitis. J. Toxicol. Clin. Toxicol. 1991, 29, 223–230. [Google Scholar] [CrossRef]
- Davenport, A.; Finn, R. Paracetamol (acetaminophen) poisoning resulting in acute renal failure without hepatic coma. Nephron 1988, 50, 55–56. [Google Scholar] [CrossRef]
- Shah, A.D.; Wood, D.M.; Dargan, P.I. Understanding lactic acidosis in paracetamol (acetaminophen) poisoning. Br. J. Clin. Pharmacol. 2011, 71, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Choueiri, T.K.; Je, Y.; Cho, E. Analgesic use and the risk of kidney cancer: A meta-analysis of epidemiologic studies. Int. J. Cancer 2014, 134, 384–396. [Google Scholar] [CrossRef]
- Boussetta, K.; Ponvert, C.; Karila, C.; Bourgeois, M.L.; de Blic, J.; Scheinmann, P. Hypersensitivity reactions to paracetamol in children: A study of 25 cases. Allergy 2005, 60, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Ferri, F.F. Ferri’s Clinical Advisor 2017 E-Book: 5 Books in 1; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Daly, F.F.S.; Fountain, J.S.; Murray, L.; Graudins, A.; Buckley, N.A. Guidelines for the management of paracetamol poisoning in Australia and New Zealand-explanation and elaboration. Med. J. Aust. 2008, 188, 296. [Google Scholar] [CrossRef] [PubMed]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Karimi-Maleh, H. A review on magnetic sensors for monitoring of hazardous pollutants in water resources. Sci. Total Environ. 2022, 824, 153844. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Alizadeh, M.; Karaman, O.; Vasseghian, Y.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo dyes using an impressive electrochemical sensor based on carbon paste electrode modified with ZIF-8/g-C3N4/Co and ionic liquid in mouthwash and toothpaste as real samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar] [PubMed]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Raman, P.K.Y.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2022, 287, 132187. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Beitollahi, H.; Kumar, P.S.; Tajik, S.; Jahani, P.M.; Karimi, F.; Karaman, C.; Vasseghian, Y.; Baghayeri, M.; Rouhi, J. Recent advances in carbon nanomaterials-based electrochemical sensors for food azo dyes detection. Food Chem. Toxicol. 2022, 164, 112961. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Balram, D. Electrochemical detection of an antibiotic drug chloramphenicol based on a graphene oxide/hierarchical zinc oxide nanocomposite. Inorg. Chem. Front. 2019, 6, 82–93. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N. A novel electrochemical sensor based on flower shaped zinc oxide nanoparticles for the efficient detection of dopamine. Int. J. Electrochem. Sci. 2018, 13, 1542–1555. [Google Scholar] [CrossRef]
- Buledi, J.A.; Mahar, N.; Mallah, A.; Solangi, A.R.; Palabiyik, I.M.; Qambrani, N.; Karimi, F.; Vasseghian, Y.; Karimi-Maleh, H. Electrochemical quantification of mancozeb through tungsten oxide/reduced graphene oxide nanocomposite: A potential method for environmental remediation. Food Chem. Toxicol. 2022, 161, 112843. [Google Scholar] [CrossRef]
- Wei, C.; Zou, X.; Liu, Q.; Li, S.; Kang, C.; Xiang, W. A highly sensitive non-enzymatic glucose sensor based on CuS nanosheets modified Cu2O/CuO nanowire arrays. Electrochim. Acta 2020, 334, 135630. [Google Scholar] [CrossRef]
- Yang, L.; Chu, D.; Wang, L. Porous hexapod CuO nanostructures: Precursor-mediated fabrication, characterization, and visible-light induced photocatalytic degradation of phenol. Mater. Lett. 2015, 160, 246–249. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly monodisperse Cu2O and CuO nanospheres: Preparation and applications for sensitive gas sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Yuan, W.; Qiu, Z.; Chen, Y.; Zhao, B.; Liu, M.; Tang, Y. A binder-free composite anode composed of CuO nanosheets and multi-wall carbon nanotubes for high-performance lithium-ion batteries. Electrochim. Acta 2018, 267, 150–160. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, L.; Wang, Z. Nonenzymatic amperometric determination of glucose by CuO nanocubes–graphene nanocomposite modified electrode. Bioelectrochemistry 2012, 88, 156–163. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Vadla, S.S.; Roy, S.C. High photoelectrochemical activity of CuO nanoflakes grown on Cu foil. Electrochim. Acta 2019, 319, 390–399. [Google Scholar] [CrossRef]
- Zhang, Z.; Zada, A.; Cui, N.; Liu, N.; Liu, M.; Yang, Y.; Jiang, D.; Jiang, J.; Liu, S. Synthesis of ag loaded ZnO/BiOCl with high photocatalytic performance for the removal of antibiotic pollutants. Crystals 2021, 11, 981. [Google Scholar] [CrossRef]
- Li, Y.; Wan, M.; Yan, G.; Qiu, P.; Wang, X. A dual-signal sensor for the analysis of parathion-methyl using silver nanoparticles modified with graphitic carbon nitride. J. Pharm. Anal. 2021, 11, 183–190. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Balram, D. Ultrasensitive Electrochemical Detection and Plasmon-Enhanced Photocatalytic Degradation of Rhodamine B Based on Dual-Functional, 3D, Hierarchical Ag/ZnO Nanoflowers. Sensors 2022, 22, 5049. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Balram, D.; Noman, M.T.; Amor, N. Silver doped dodecahedral metal-organic framework anchored RGO nanosheets for nanomolar quantification of priority toxic pollutant in aquatic environment. J. Alloys Compd. 2022, 922, 166180. [Google Scholar] [CrossRef]
- Inam, A.K.M.S.; Angeli, M.A.C.; Shkodra, B.; Douaki, A.; Avancini, E.; Magagnin, L.; Lugli, P. Flexible screen-printed amperometric sensors functionalized with spray-coated carbon nanotubes and electrodeposited Cu nanoclusters for nitrate detection. IEEE Sens. J. 2022, 1. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Barka, N. Chemically modified carbon-based electrodes for the determination of paracetamol in drugs and biological samples. J. Pharm. Anal. 2021, 11, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Khadem, E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Flahaut, E.; Peigney, A.; Laurent, C.; Marliere, C.; Chastel, F.; Rousset, A. Carbon nanotube–metal–oxide nanocomposites: Microstructure, electrical conductivity and mechanical properties. Acta Mater. 2000, 48, 3803–3812. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, N.; Yu, W.-C.; Balram, D. Synthesis of amine-functionalized multi-walled carbon nanotube/3D rose flower-like zinc oxide nanocomposite for sensitive electrochemical detection of flavonoid morin. Anal. Chim. Acta 2020, 1095, 71–81. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Hu, Y.-C.; Balram, D.; Yu, Y.-H. Sonochemical synthesis of iron-graphene oxide/honeycomb-like ZnO ternary nanohybrids for sensitive electrochemical detection of antipsychotic drug chlorpromazine. Ultrason. Sonochem. 2019, 59, 104696. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Balzar, D.; Ledbetter, H. Voigt-function modeling in Fourier analysis of size-and strain-broadened X-ray diffraction peaks. J. Appl. Crystallogr. 1993, 26, 97–103. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, V.T. Copper oxide nanomaterials prepared by solution methods, some properties, and potential applications: A brief review. Int. Sch. Res. Not. 2014, 2014, 856592. [Google Scholar] [CrossRef] [Green Version]
- Amirian, M.; Chakoli, A.N.; Cai, W.; Sui, J. Effect of functionalized multiwalled carbon nanotubes on thermal stability of poly (L-LACTIDE) biodegradable polymer. Sci. Iran. 2013, 20, 1023–1027. [Google Scholar]
- Kaur, A.; Mann, S.; Goyal, B.; Pal, B.; Goyal, D. CuO nanostructures of variable shapes as an efficient catalyst for [3 + 2] cycloaddition of azides with terminal alkyne. RSC Adv. 2016, 6, 102733–102743. [Google Scholar] [CrossRef]
- Miner, D.J.; Rice, J.R.; Riggin, R.M.; Kissinger, P.T. Voltammetry of acetaminophen and its metabolites. Anal. Chem. 1981, 53, 2258–2263. [Google Scholar] [CrossRef]
- Beitollahi, H.; Ardakani, M.M.; Ganjipour, B.; Naeimi, H. Novel 2, 2′-[1,2-ethanediylbis (nitriloethylidyne)]-bis-hydroquinone double-wall carbon nanotube paste electrode for simultaneous determination of epinephrine, uric acid and folic acid. Biosens. Bioelectron. 2008, 24, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Shahbakhsh, M.; Noroozifar, M. Copper polydopamine complex/multiwalled carbon nanotubes as novel modifier for simultaneous electrochemical determination of ascorbic acid, dopamine, acetaminophen, nitrite and xanthine. J. Solid State Electrochem. 2018, 22, 3049–3057. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Hatami, M.; Moradi, R.; Khalilzadeh, M.A.; Amiri, S.; Sadeghifar, H. Synergic effect of Pt-Co nanoparticles and a dopamine derivative in a nanostructured electrochemical sensor for simultaneous determination of N-acetylcysteine, paracetamol and folic acid. Microchim. Acta 2016, 183, 2957–2964. [Google Scholar] [CrossRef]
- Li, M.; Jing, L. Electrochemical behavior of acetaminophen and its detection on the PANI–MWCNTs composite modified electrode. Electrochim. Acta 2007, 52, 3250–3257. [Google Scholar] [CrossRef]
- Cao, F.; Dong, Q.; Li, C.; Chen, J.; Ma, X.; Huang, Y.; Song, D.; Ji, C.; Lei, Y. Electrochemical sensor for detecting pain reliever/fever reducer drug acetaminophen based on electrospun CeBiOx nanofibers modified screen-printed electrode. Sens. Actuators B Chem. 2018, 256, 143–150. [Google Scholar] [CrossRef]
- Liu, W.; Shi, Q.; Zheng, G.; Zhou, J.; Chen, M. Electrocatalytic oxidation toward dopamine and acetaminophen based on AuNPs@ TCnA/GN modified glassy carbon electrode. Anal. Chim. Acta 2019, 1075, 81–90. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Z.; Liu, Z. An electrochemical sensor for paracetamol based on an electropolymerized molecularly imprinted o-phenylenediamine film on a multi-walled carbon nanotube modified glassy carbon electrode. Anal. Methods 2014, 6, 5673–5681. [Google Scholar] [CrossRef]
- Baccarin, M.; Santos, F.A.; Vicentini, F.C.; Zucolotto, V.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical sensor based on reduced graphene oxide/carbon black/chitosan composite for the simultaneous determination of dopamine and paracetamol concentrations in urine samples. J. Electroanal. Chem. 2017, 799, 436–443. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Waterhouse, G.I.N.; Xu, L.; Zhang, H.; Qiao, X.; Xu, Z. A selective molecularly imprinted electrochemical sensor with GO@ COF signal amplification for the simultaneous determination of sulfadiazine and acetaminophen. Sens. Actuators B Chem. 2019, 300, 126993. [Google Scholar] [CrossRef]
- Mao, A.; Li, H.; Jin, D.; Yu, L.; Hu, X. Fabrication of electrochemical sensor for paracetamol based on multi-walled carbon nanotubes and chitosan–copper complex by self-assembly technique. Talanta 2015, 144, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Shaikshavali, P.; Reddy, T.M.; Palakollu, V.N.; Karpoormath, R.; Rao, Y.S.; Venkataprasad, G.; Gopal, T.V.; Gopal, P. Multi walled carbon nanotubes supported CuO-Au hybrid nanocomposite for the effective application towards the electrochemical determination of acetaminophen and 4-aminophenol. Synth. Met. 2019, 252, 29–39. [Google Scholar] [CrossRef]
- Foroughi, M.M.; Jahani, S.; Nadiki, H.H. Lanthanum doped fern-like CuO nanoleaves/MWCNTs modified glassy carbon electrode for simultaneous determination of tramadol and acetaminophen. Sens. Actuators B Chem. 2019, 285, 562–570. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Howlader, M.M.R.; Hu, N.-X.; Deen, M.J. Electrochemical sensing of acetaminophen using multi-walled carbon nanotube and β-cyclodextrin. Sens. Actuators B Chem. 2018, 254, 896–909. [Google Scholar] [CrossRef]
- Kenarkob, M.; Pourghobadi, Z. Electrochemical sensor for acetaminophen based on a glassy carbon electrode modified with ZnO/Au nanoparticles on functionalized multi-walled carbon nano-tubes. Microchem. J. 2019, 146, 1019–1025. [Google Scholar] [CrossRef]




| Modified Electrode | Detection Technique | LOD (µM) | Linear Range (µM) | pH | References |
|---|---|---|---|---|---|
| GCE/Cu2+@PDA-MWCNTs | DPV | 0.87 | 5–75 | 2 | [44] |
| Pt-Co/NPs/3,4,DHPID/CPE | SWV | 0.6 | 1–850 | 7 | [45] |
| PANI/MWCNTs/GCE | SWV | 0.25 | 1–100 | 5.5 | [46] |
| CeBiOx NFs/SPE | DPV | 0.2 | 2.5–130 | 7.4 | [47] |
| AuNPs@TC8A/GN/GCE | DPV | 0.1 | 0.5–120 | 7 | [48] |
| MIP–MWCNT/GCE | LSV | 0.08 | 0.2–40 | 7 | [49] |
| RGO-CB-CTS/GCE | SWV | 0.053 | 2.8–19 | 6.2 | [50] |
| MIP/GO@COF/GCE | DPV | 0.032 | 0.05–20 | 7 | [51] |
| MWCNTs/CTS–Cu/GCE | DPV | 0.024 | 0.1–200 | 7 | [52] |
| CuO-Au/MWCNTs/GCE | DPV | 0.016 | 0.2–6.0 | 7.5 | [53] |
| La3+-CuO/MWCNTs/GCE | DPV | 0.014 | 0.5–900 | 7 | [54] |
| MWCNT-βCD/GCE | DPV | 0.011 | 0.05–1, 1–300 | 7.4 | [55] |
| MWCNT/ZnO-Au/GCE | SWV | 0.009 | 0.05–20 | 7 | [56] |
| AgCuO NPs/fCNTs/SPCE | DPV | 0.004 | 0.02–3.77, 3.77–90.02 | 7 | This work |
| Sample | Added (µM) | Found (µM) | Recovery (%) | RSD k |
|---|---|---|---|---|
| Acetaminophen tablet | 5 | 5.02 | 100.4 | 2.34 |
| 10 | 10.09 | 102.93 | 2.04 | |
| 20 | 20.4 | 102.01 | 1.6 | |
| Human urine | 5 | 4.84 | 96.86 | 3.67 |
| 10 | 9.69 | 96.93 | 2.66 | |
| 20 | 19.82 | 99.11 | 2.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balram, D.; Lian, K.-Y.; Sebastian, N. Electrocatalytic Platform Based on Silver-Doped Sugar Apple-like Cupric Oxide Embedded Functionalized Carbon Nanotubes for Nanomolar Detection of Acetaminophen (APAP). Sensors 2023, 23, 379. https://doi.org/10.3390/s23010379
Balram D, Lian K-Y, Sebastian N. Electrocatalytic Platform Based on Silver-Doped Sugar Apple-like Cupric Oxide Embedded Functionalized Carbon Nanotubes for Nanomolar Detection of Acetaminophen (APAP). Sensors. 2023; 23(1):379. https://doi.org/10.3390/s23010379
Chicago/Turabian StyleBalram, Deepak, Kuang-Yow Lian, and Neethu Sebastian. 2023. "Electrocatalytic Platform Based on Silver-Doped Sugar Apple-like Cupric Oxide Embedded Functionalized Carbon Nanotubes for Nanomolar Detection of Acetaminophen (APAP)" Sensors 23, no. 1: 379. https://doi.org/10.3390/s23010379








