Simultaneous Voltammetric Determination of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Using a Modified Carbon Paste Electrode and Chemometrics
Abstract
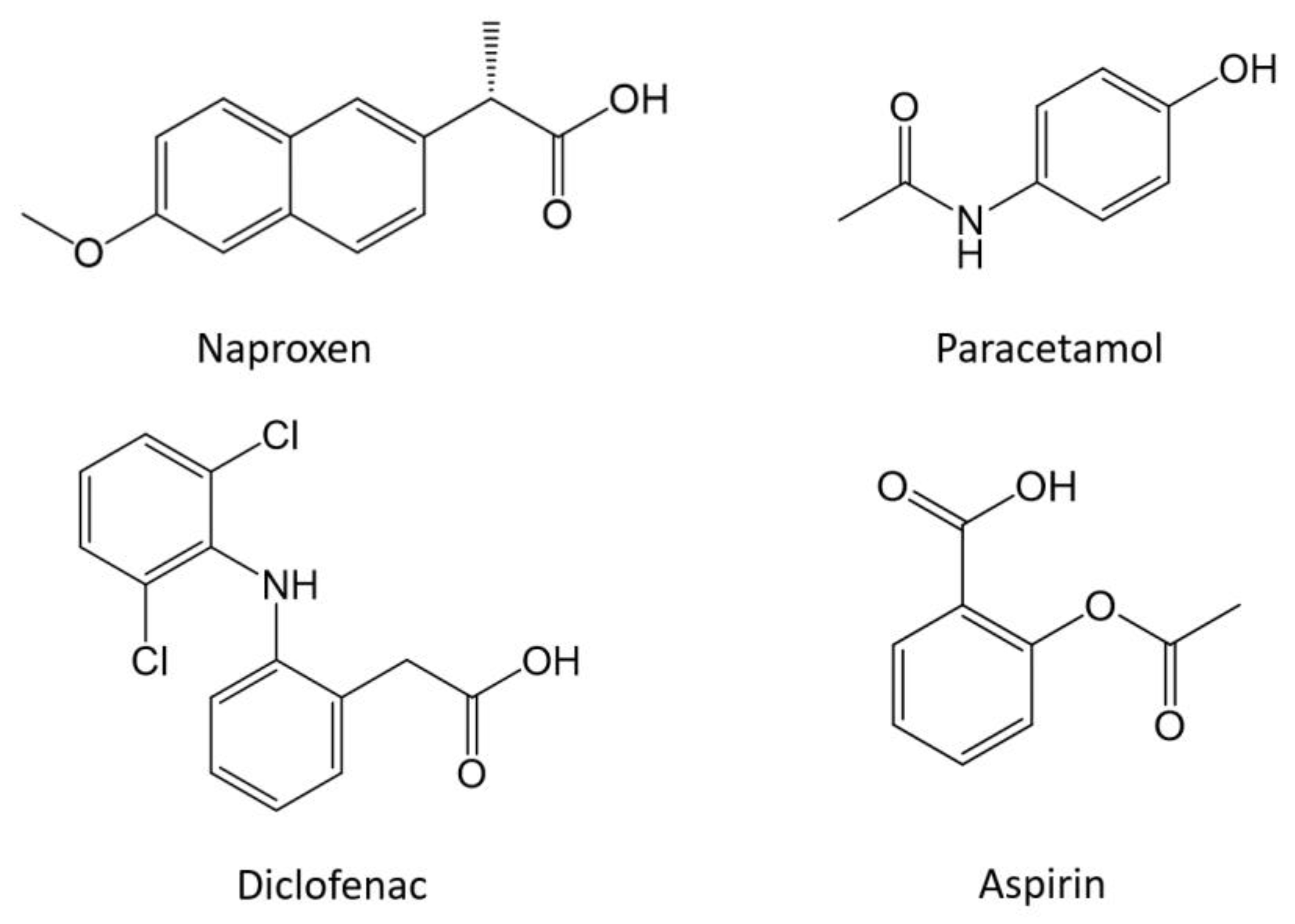
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Instrumentation
2.3. Working Electrode Preparation
2.4. Sample Preparation
2.5. Electrochemical Analysis and Procedure
2.6. Data Processing
3. Results and Discussion
3.1. System Characterization
3.2. Quantification of NSAIDs by Chemometric Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villoria, C.M. Tratamiento de Dolor Crónico, Diagnóstico, Clínica y Tratamiento; Arán Ediciones, S.L.: Madrid, Spain, 2007. [Google Scholar]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic effects of NSAIDs in non-target species: A review from the perspective of the aquatic environment. Environ. Pollut. 2020, 273, 115891. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, K.B.; Weeke, P.; Wissenberg, M.; Olsen, A.-M.S.; Fosbøl, E.; Lippert, F.K.; Torp-Pedersen, C.; Gislason, G.H.; Folke, F. Non-steroidal anti-inflammatory drug use is associated with increased risk of out-of-hospital cardiac arrest: A nationwide case–time–control study. Eur. Heart J.-Cardiovasc. Pharmacother. 2017, 3, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Maric, F. Mitigating the environmental impact of NSAIDs-physiotherapy as a contribution to One Health and the SDGs. Eur. J. Physiother. 2021, 1–5. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Baciu, A.; Vasilie, S.; Pop, A. Highly sensitive and simultaneous electrochemical determinations of non-steroidal anti-inflammatory drugs in water using nanostructured carbon-based paste electrodes. Sci. Total Environ. 2022, 846, 157412. [Google Scholar] [CrossRef]
- Aguilar-Lira, G.Y.; Hernández, A.R.; Rodriguez, J.A.; Hernández, M.E.P.; Romero, G.A.A. Optimized Quantification of Naproxen Based on DPV and a Multiwalled MWCNT-Carbon Paste Electrode. J. Electrochem. Soc. 2020, 167, 166510. [Google Scholar] [CrossRef]
- Bielsa-Fernández, M.; la Cuesta, J.T.-D.; Lizárraga-López, J.; Remes-Troche, J.; Carmona-Sánchez, R.; Aldana-Ledesma, J.; Avendaño-Reyes, J.; Ballesteros-Amozorrutia, M.; De Ariño, M.; de Giau-Triulzi, L.; et al. The Mexican consensus on the diagnosis, treatment, and prevention of NSAID-induced gastropathy and enteropathy. Rev. Gastroenterol. Méx. Engl. Ed. 2020, 85, 190–206. [Google Scholar] [CrossRef]
- Nikolin, B.; Imamović, B.; Medanhodžić-Vuk, S.; Sober, M. High performance liquid chromatography in pharmaceutical analyses. Bosn. J. Basic Med. Sci. 2004, 4, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Gnana Raja, M.; Geetha, G.; Sangaranarayanan, A. Simultaneous, Stability Indicating Method Development and Validation for Related Compounds of Ibuprofen and Paracetamol Tablets by RP-HPLC Method. J. Chromatogr. Sep. Tech. 2012, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Arteaga, K.; Rodriguez, J.; Miranda, J.; Medina, J.; Barrado, E. Determination of non-steroidal anti-inflammatory drugs in wastewaters by magnetic matrix solid phase dispersion–HPLC. Talanta 2010, 80, 1152–1157. [Google Scholar] [CrossRef]
- Cunha, R.R.; Chaves, S.C.; Ribeiro, M.M.A.C.; Torres, L.M.F.C.; Muñoz, R.A.A.; Dos Santos, W.T.P.; Richter, E.M. Simultaneous determination of caffeine, paracetamol, and ibuprofen in pharmaceutical formulations by high-performance liquid chromatography with UV detection and by capillary electrophoresis with conductivity detection. J. Sep. Sci. 2015, 38, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Borahan, T.; Unutkan, T.; Şahin, A.; Bakırdere, S. A rapid and sensitive reversed phase-HPLC method for simultaneous determination of ibuprofen and paracetamol in drug samples and their behaviors in simulated gastric conditions. J. Sep. Sci. 2018, 42, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.B.; Torres, L.M.F.C.; Guimarães, C.F.R.C.; Verly, R.M.; Da Silva, L.M.; Junior, D.C.; Dos Santos, W.T.P. Simultaneous Determination of Paracetamol and Ibuprofen in Pharmaceutical Samples by Differential Pulse Voltammetry Using a Boron-Doped Diamond Electrode. J. Braz. Chem. Soc. 2014, 25, 478–483. [Google Scholar] [CrossRef]
- Rivera-Hernández, S.I.; Álvarez-Romero, G.A.; Corona-Avendaño, S.; Páez-Hernández, M.E.; Galán-Vidal, C.A.; Romero-Romo, M. Voltammetric determination of ibuprofen using a carbon paste – multiwalled carbon nanotube composite electrode. Instrum. Sci. Technol. 2016, 44, 483–494. [Google Scholar] [CrossRef]
- Silah, H.; Erkmen, C.; Demir, E.; Uslu, B. Modified indium tin oxide electrodes: Electrochemical applications in pharmaceutical, biological, environmental and food analysis. TrAC Trends Anal. Chem. 2021, 141, 116289. [Google Scholar] [CrossRef]
- Karadurmus, L.; Sahin, I.F.; Kurbanoglu, S.; Ozkan, S.A. Electrochemical Determination of Non-Steroidal Anti-Inflammatory Drugs. Curr. Anal. Chem. 2019, 15, 485–501. [Google Scholar] [CrossRef]
- Franco-Guzmán, M.; Mendoza-Huizar, L.H.; Roa-Morales, G.; Galán-Vidal, C.A.; Álvarez-Romero, G.A. Voltammetric Quantification of Diclofenac Using a Modified CPE Enriched With TiO2 Nanoparticles and MWCNT. J. Electrochem. Soc. 2021, 168, 037502. [Google Scholar] [CrossRef]
- Wang, J.; Liu, A.J.; Cepra, G. Thermal Stabilization of Enzymes Immobilized within Carbon Paste Electrodes. Anal. Chem. 1997, 69, 3124–3127. [Google Scholar] [CrossRef]
- Gorton, L. Carbon paste electrodes modified with enzymes, tissues, and cells. Electroanalysis 1995, 7, 23–45. [Google Scholar] [CrossRef]
- Bellido-Milla, D.; Cubillana-Aguilera, L.M.; El Kaoutit, M.; Hernández-Artiga, M.P.; De Cisneros, J.L.H.-H.; Naranjo-Rodríguez, I.; Palacios-Santander, J.M. Recent advances in graphite powder-based electrodes. Anal. Bioanal. Chem. 2013, 405, 3525–3539. [Google Scholar] [CrossRef]
- Merkoçi, A. Carbon Nanotubes in Analytical Sciences. Mikrochim. Acta 2005, 152, 157–174. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Fotouhi, L. Carbon paste electrode incorporating multi-walled carbon nanotube/cobalt salophen for sensitive voltammetric determination of tryptophan. Sens. Actuators B Chem. 2007, 123, 942–949. [Google Scholar] [CrossRef]
- Winquist, F.; Bjorklund, R.; Krantz-Rülcker, C.; Lundström, I.; Östergren, K.; Skoglund, T. An electronic tongue in the dairy industry. Sens. Actuators B Chem. 2005, 111–112, 299–304. [Google Scholar] [CrossRef]
- Gil-Sánchez, L.; Soto, J.; Martínez-Máñez, R.; Garcia-Breijo, E.; Ibáñez, J.; Llobet, E. A novel humid electronic nose combined with an electronic tongue for assessing deterioration of wine. Sens. Actuators A Phys. 2011, 171, 152–158. [Google Scholar] [CrossRef]
- Campos, I.; Alcañiz, M.; Aguado, D.; Barat, R.; Ferrer, J.; Gil, L.; Marrakchi, M.; Martínez-Mañez, R.; Soto, J.; Vivancos, J.-L. A voltammetric electronic tongue as tool for water quality monitoring in wastewater treatment plants. Water Res. 2012, 46, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Ivić, M.A.; Antanasijević, J.; Trišović, N.; Antanasijević, D.; Lović, J.; Mijin, D.; Petrović, S. A Chemometrical Analysis of Voltammetric Data for Simultaneous Determination of Phenobarbital Sodium and Paracetamol Obtained at a Gold Electrode. Int. J. Electrochem. Sci. 2016, 11, 5935–5951. [Google Scholar] [CrossRef]
- Alcañiz, M.; Vivancos, J.-L.; Masot, R.; Ibañez, J.; Raga, M.; Soto, J.; Martínez-Máñez, R. Design of an electronic system and its application to electronic tongues using variable amplitude pulse voltammetry and impedance spectroscopy. J. Food Eng. 2012, 111, 122–128. [Google Scholar] [CrossRef]
- Cosio, M.; Ballabio, D.; Benedetti, S.; Gigliotti, C. Evaluation of different storage conditions of extra virgin olive oils with an innovative recognition tool built by means of electronic nose and electronic tongue. Food Chem. 2007, 101, 485–491. [Google Scholar] [CrossRef]
- Gutés, A.; Céspedes, F.; Cartas, R.; Alegret, S.; del Valle, M.; Gutierrez, J.; Muñoz, R. Multivariate calibration model from overlapping voltammetric signals employing wavelet neural networks. Chemom. Intell. Lab. Syst. 2006, 83, 169–179. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, W.; Wang, Y.; Wang, J. Monitoring the fermentation, post-ripeness and storage processes of set yogurt using voltammetric electronic tongue. J. Food Eng. 2017, 203, 41–52. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Li, D.; Wu, M. An Active Feature Selection Strategy for DWT in Artificial Taste. J. Sens. 2018, 2018, 9709505. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.-I.B.; Groves, W.A.; Freivalds, A.; Lee, E.G.; Harper, M. Comparison of artificial neural network (ANN) and partial least squares (PLS) regression models for predicting respiratory ventilation: An exploratory study. Eur. J. Appl. Physiol. 2012, 112, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Beck, M.S.; Bryman, A.; Liao, T.F. Partial least squares regression. In The SAGE Encyclopedia of Social Science Research Methods; SAGE Publications Inc.: Thousand Oaks, CA, USA, 2004; pp. 793–795. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Khatri, P.; Gupta, K.K.; Gupta, R.K. A review of partial least squares modeling (PLSM) for water quality analysis. Model. Earth Syst. Environ. 2021, 7, 703–714. [Google Scholar] [CrossRef]
- Chun, H.; Keleş, S. Sparse partial least squares regression for simultaneous dimension reduction and variable selection. J. R. Stat. Soc. Ser. B Stat. Methodol. 2010, 72, 3–25. [Google Scholar] [CrossRef] [Green Version]
- Zifarelli, A.; Giglio, M.; Menduni, G.; Sampaolo, A.; Patimisco, P.; Passaro, V.M.N.; Wu, H.; Dong, L.; Spagnolo, V. Partial Least-Squares Regression as a Tool to Retrieve Gas Concentrations in Mixtures Detected Using Quartz-Enhanced Photoacoustic Spectroscopy. Anal. Chem. 2020, 92, 11035–11043. [Google Scholar] [CrossRef]
- Wilson, D.; Gutiérrez, J.M.; Alegret, S.; del Valle, M. Simultaneous Determination of Zn(II), Cu(II), Cd(II) and Pb(II) in Soil Samples Employing an Array of Potentiometric Sensors and an Artificial Neural Network Model. Electroanalysis 2012, 24, 2249–2256. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z. Optimization of Stripping Voltammetric Sensor by a Back Propagation Artificial Neural Network for the Accurate Determination of Pb(II) in the Presence of Cd(II). Sensors 2016, 16, 1540. [Google Scholar] [CrossRef] [Green Version]
- Kalambate, P.K.; Noiphung, J.; Rodthongkum, N.; Larpant, N.; Thirabowonkitphithan, P.; Rojanarata, T.; Hasan, M.; Huang, Y.; Laiwattanapaisal, W. Nanomaterials-based electrochemical sensors and biosensors for the detection of non-steroidal anti-inflammatory drugs. TrAC Trends Anal. Chem. 2021, 143, 116403. [Google Scholar] [CrossRef]
- Cetó, X.; Céspedes, F.; Pividori, M.I.; Gutiérrez, J.M.; del Valle, M. Resolution of phenolic antioxidant mixtures employing a voltammetric bio-electronic tongue. Analyst 2012, 137, 349–356. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 501–506. [Google Scholar]
- Mallat, S.G. A theory for multiresolution signal decomposition: The wavelet representation. IEEE Trans. Pattern Anal. Mach. Intell. 1989, 11, 674–693. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Barón, L.; Cartas, R.; Merkoçi, A.; Alegret, S.; del Valle, M.; Leija, L.; Hernandez, P.; Muñoz, R. Application of the wavelet transform coupled with artificial neural networks for quantification purposes in a voltammetric electronic tongue. Sens. Actuators B Chem. 2006, 113, 487–499. [Google Scholar] [CrossRef]
- Švancara, I.; Hvízdalová, M.; Vytřas, K.; Kalcher, K.; Novotný, R. A microscopic study on carbon paste electrodes. Electroanalysis 1996, 8, 61–65. [Google Scholar] [CrossRef]
- Valentini, F.; Amine, A.; Orlanducci, S.; Terranova, M.L.; Palleschi, G. Carbon Nanotube Purification: Preparation and Characterization of Carbon Nanotube Paste Electrodes. Anal. Chem. 2003, 75, 5413–5421. [Google Scholar] [CrossRef]
- De Brito, A.R.; Tavares, I.M.D.C.; de Carvalho, M.S.; de Oliveira, A.J.; Salay, L.C.; Santos, A.S.; dos Anjos, P.N.M.; Oliveira, J.R.; Franco, M. Study of the interaction of the lactase enzyme immobilized in a carbon nanotube matrix for the development of the chemically modified carbon paste electrode. Surf. Interfaces 2020, 20, 100592. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Richtera, L. Preparation and Characterization of Carbon Paste Electrode Bulk-Modified with Multiwalled Carbon Nanotubes and Its Application in a Sensitive Assay of Antihyperlipidemic Simvastatin in Biological Samples. Molecules 2019, 24, 2215. [Google Scholar] [CrossRef] [Green Version]
- Mesa, R.; Khan, S.; Sotomayor, M.D.P.T.; Picasso, G. Using Carbon Paste Electrode Modified with Ion Imprinted Polymer and MWCNT for Electrochemical Quantification of Methylmercury in Natural Water Samples. Biosensors 2022, 12, 376. [Google Scholar] [CrossRef]
- Baghayeri, M.; Sedrpoushan, A.; Mohammadi, A.; Heidari, M. A non-enzymatic glucose sensor based on NiO nanoparticles/functionalized SBA 15/MWCNT-modified carbon paste electrode. Ionics 2017, 23, 1553–1562. [Google Scholar] [CrossRef]
- Todd, P.A.; Clissold, S.P. Naproxen A Reappraisal of its Pharmacology, and Therapeutic Use in Rheumatic Diseases and Pain States. Drugs 1990, 40, 91–137. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Moreno-Barón, L.; Céspedes, F.; Muñoz, R.; del Valle, M. Resolution of Heavy Metal Mixtures from Highly Overlapped ASV Voltammograms Employing a Wavelet Neural Network. Electroanalysis 2009, 21, 445–451. [Google Scholar] [CrossRef]








| pH | Maximum Peak Current Intensity of Drugs (µA) | ||
|---|---|---|---|
| Paracetamol | Diclofenac | Naproxen/Aspirin | |
| 7 | 4.84 | 7.62 | 11.96 |
| 8 | 5.12 | 7.88 | 12.11 |
| 9 | 6.12 | 8.46 | 17.70 |
| 10 | 7.26 | 8.81 | 18.10 |
| NSAID | Training | Testing | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | m | Δm | b | Δb | R | m | Δm | b | Δb | |
| Paracetamol | 0.827 | 0.684 | 0.196 | 1.274 × 10−5 | 9.098 × 10−6 | 0.974 | 0.865 | 0.328 | 8.771 × 10−6 | 1.520 × 10−5 |
| Diclofenac | 0.778 | 0.605 | 0.206 | 1.654 × 10−6 | 9.869 × 10−6 | 0.947 | 0.964 | 0.532 | −3.590 × 10−5 | 3.107 × 10−5 |
| Naproxen | 0.801 | 0.641 | 0.202 | 1.426 × 10−5 | 9.211 × 10−6 | 0.551 | 0.327 | 0.836 | 2.085 × 10−5 | 2.968 × 10−5 |
| Aspirin | 0.741 | 0.549 | 0.210 | 1.762 × 10−5 | 9.515 × 10−6 | 0.538 | 0.327 | 0.836 | 2.085 × 10−5 | 2.968 × 10−5 |
| NSAID | LOD (µmol L−1) | LOQ (µmol L−1) |
|---|---|---|
| Paracetamol | 15.4 | 51.3 |
| Diclofenac | 10.6 | 35.4 |
| Naproxen | 8.89 | 29.5 |
| Aspirin | 13.4 | 44.8 |
| NSAID | Training | Testing | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | m | Δm | b | Δb | R | m | Δm | b | Δb | |
| Paracetamol | 0.976 | 0.919 | 0.044 | 3.238 × 10−6 | 2.025 × 10−6 | 0.979 | 0.989 | 0.166 | 8.897 × 10−7 | 7.692 × 10−6 |
| Diclofenac | 0.982 | 0.929 | 0.038 | 2.987 × 10−6 | 1.820 × 10−6 | 0.985 | 0.930 | 0.134 | 3.569 × 10−6 | 7.801 × 10−6 |
| Naproxen | 0.981 | 0.930 | 0.039 | 2.762 × 10−6 | 1.765 × 10−6 | 0.969 | 0.804 | 0.166 | 6.299 × 10−6 | 6.255 × 10−6 |
| Aspirin | 0.976 | 0.924 | 0.043 | 2.975 × 10−6 | 1.957 × 10−6 | 0.974 | 0.879 | 0.165 | 3.398 × 10−6 | 5.870 × 10−6 |
| NSAID | Ry (%) | |||
|---|---|---|---|---|
| PLS | ANN | |||
| Training | Testing | Training | Testing | |
| Paracetamol | 174.5 | 171.2 | 122.5 | 114.9 |
| Diclofenac | 102.6 | 119.8 | 97.8 | 103.7 |
| Naproxen | 149.5 | 62.7 | 118.4 | 119.9 |
| Aspirin | 96.2 | 71.3 | 100.9 | 103.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Lira, G.Y.; López-Barriguete, J.E.; Hernandez, P.; Álvarez-Romero, G.A.; Gutiérrez, J.M. Simultaneous Voltammetric Determination of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Using a Modified Carbon Paste Electrode and Chemometrics. Sensors 2023, 23, 421. https://doi.org/10.3390/s23010421
Aguilar-Lira GY, López-Barriguete JE, Hernandez P, Álvarez-Romero GA, Gutiérrez JM. Simultaneous Voltammetric Determination of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Using a Modified Carbon Paste Electrode and Chemometrics. Sensors. 2023; 23(1):421. https://doi.org/10.3390/s23010421
Chicago/Turabian StyleAguilar-Lira, Guadalupe Yoselin, Jesús Eduardo López-Barriguete, Prisciliano Hernandez, Giaan Arturo Álvarez-Romero, and Juan Manuel Gutiérrez. 2023. "Simultaneous Voltammetric Determination of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Using a Modified Carbon Paste Electrode and Chemometrics" Sensors 23, no. 1: 421. https://doi.org/10.3390/s23010421
APA StyleAguilar-Lira, G. Y., López-Barriguete, J. E., Hernandez, P., Álvarez-Romero, G. A., & Gutiérrez, J. M. (2023). Simultaneous Voltammetric Determination of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Using a Modified Carbon Paste Electrode and Chemometrics. Sensors, 23(1), 421. https://doi.org/10.3390/s23010421







