An Electrochemical Sensor Based on Electropolymerization of β-Cyclodextrin on Glassy Carbon Electrode for the Determination of Fenitrothion
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of β-CDP/GCE
2.3. Electrochemical Impedance Spectroscopy Measurements
2.4. Preparation and Determination of Cabbage and Tap Water Samples
3. Results and Discussion
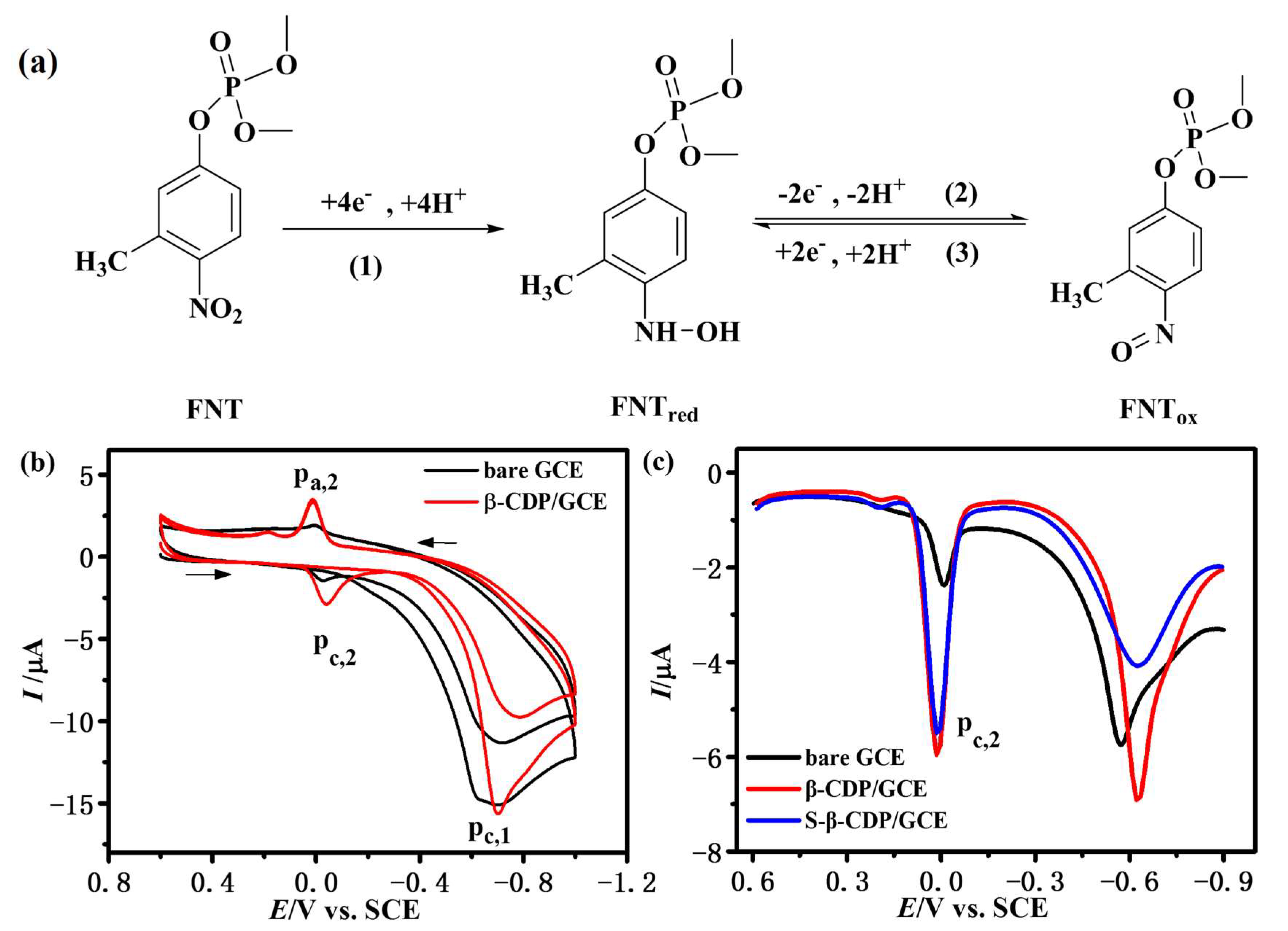
3.1. Electrochemical Behavior of Electropolymerized β-CD on GCE
3.2. Electrochemical Response of FNT
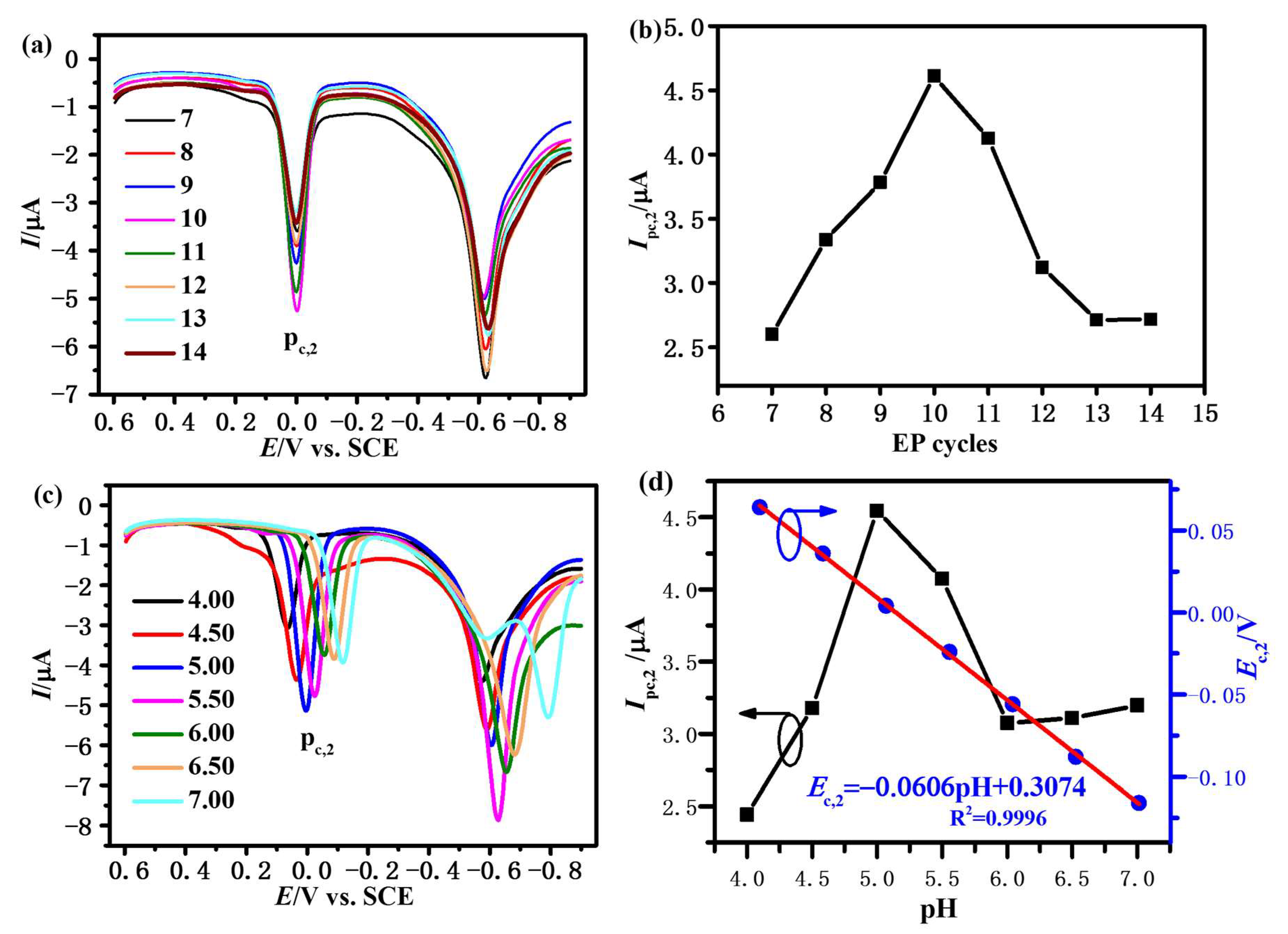
3.3. Optimization of Experimental Conditions
3.3.1. Effect of EP Cycles
3.3.2. Effect of pH
3.3.3. Accumulation Time
3.3.4. Parameters of DPV Method
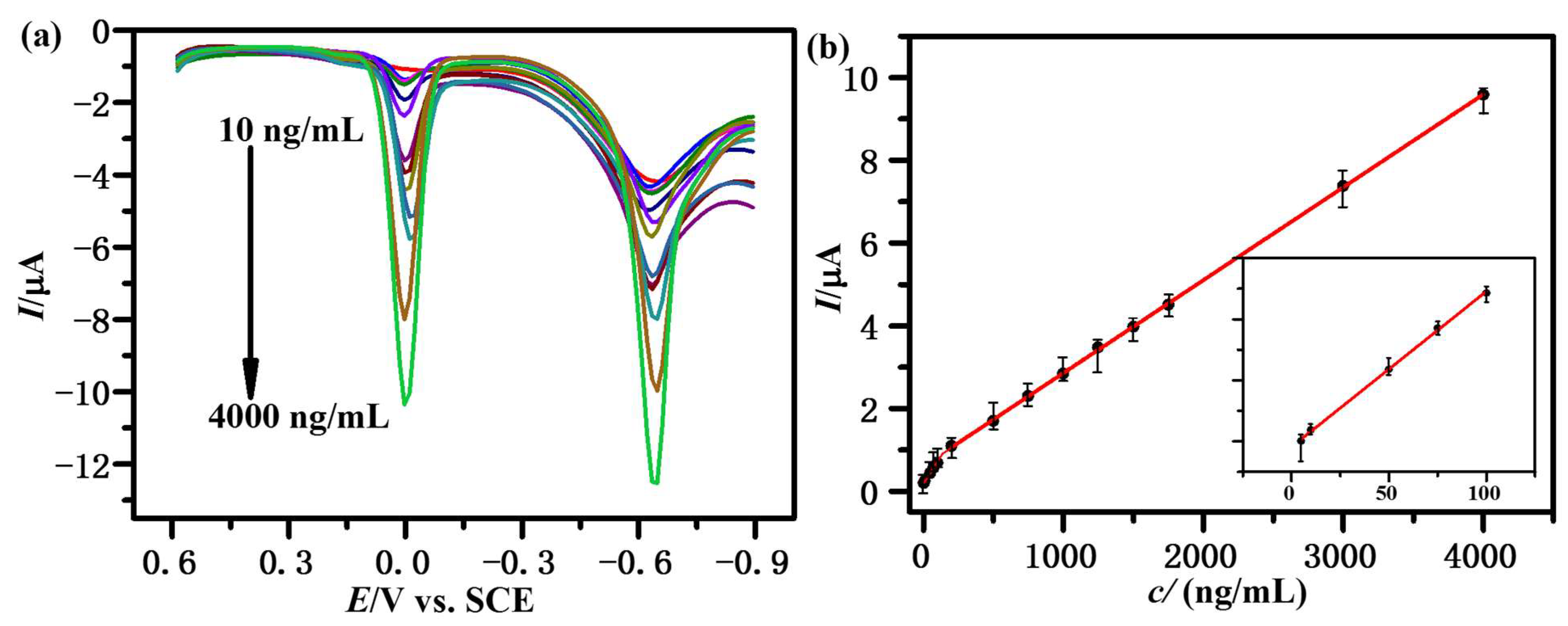
3.4. Calibration Curve
3.5. Repeatability and Interference
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umapathi, R.; Park, B.; Sonwal, S.; Rani, G.M.; Cho, Y.; Huh, Y.S. Advances in optical-sensing strategies for the on-site detection of pesticides in agricultural foods. Trends Food Sci. Technol. 2022, 119, 69–89. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water—A review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef] [PubMed]
- Bolat, G.; Abaci, S.; Vural, T.; Bozdogan, B.; Denkbas, E.B. Sensitive electrochemical detection of fenitrothion pesticide based on self-assembled peptide-nanotubes modified disposable pencil graphite electrode. J. Electroanal. Chem. 2018, 809, 88–95. [Google Scholar] [CrossRef]
- Kumaravel, A.; Chandrasekaran, M. A biocompatible nano TiO2/nafion composite modified glassy carbon electrode for the detection of fenitrothion. J. Electroanal. Chem. 2011, 650, 163–170. [Google Scholar] [CrossRef]
- Cesana, R.; Ferreira, J.H.A.; Gonçalves, J.M.; Gomes, D.; Nakamura, M.; Peres, R.M.; Toma, H.E.; Canevari, T.C. Fluorescent Cdots (N)-Silica composites: Direct synthesis and application as electrochemical sensor of fenitrothion pesticide. Mater. Sci. Eng. B 2021, 267, 115084. [Google Scholar] [CrossRef]
- Dömötörová, M.; Matisová, E. Fast gas chromatography for pesticide residues analysis. J. Chromatogr. A 2008, 1207, 1–16. [Google Scholar] [CrossRef]
- Stachniuk, A.; Fornal, E. Liquid chromatography-mass spectrometry in the analysis of pesticide residues in food. Food Anal. Methods 2016, 9, 1654–1665. [Google Scholar] [CrossRef]
- Almeida, M.O.; Oloris, S.C.S.; Faria, V.H.F.; Ribeiro, M.C.M.; Cantini, D.M.; Soto-Blanco, B. Optimization of method for pesticide detection in honey by using liquid and gas chromatography coupled with mass spectrometric detection. Foods 2020, 9, 1368. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Wang, J.; Fang, G.; Liu, J.; Wang, S. Fluorescent peptide probes for organophosphorus pesticides detection. J. Hazard. Mater. 2020, 389, 122074. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Wang, Z. Micro-/Mesoporous Fluorescent Polymers and Devices for Visual Pesticide Detection with Portability, High Sensitivity, and Ultrafast Response. ACS Appl. Mater. Interfaces 2022, 14, 5815–5824. [Google Scholar] [CrossRef]
- Liao, Y.; Cui, X.; Chen, G.; Wang, Y.; Qin, G.; Li, M.; Zhang, X.; Zhang, Y.; Zhang, C.; Du, P.; et al. Simple and sensitive detection of triazophos pesticide by using quantum dots nanobeads based on immunoassay. Food Agric. Immunol. 2019, 30, 522–532. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Z.; Guo, L.; Xu, X.; Liu, L.; Xu, L.; Song, S.; Xu, C.; Kuang, H. Advances in immunoassays for organophosphorus and pyrethroid pesticides. Trends Anal. Chem. 2020, 131, 116022. [Google Scholar] [CrossRef]
- Bucur, B.; Munteanu, F.-D.; Marty, J.-L.; Vasilescu, A. Advances in enzyme-based biosensors for pesticide detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Fakhri, H.; Hajian, A.; Afkhami, A.; Bagheri, H. High-performance electrochemical enzyme sensor for organophosphate pesticide detection using modified metal-organic framework sensing platforms. Bioelectrochemistry 2019, 130, 107348. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shen, X.; Liu, P.; Wei, Y.; Meng, Y.; Zheng, G.; Diao, G. β-Cyclodextrin polymer as a linker to fabricate ternary nanocomposites AuNPs/pATP-β-CDP/rGO and their electrochemical application. Carbohyd. Polym. 2015, 119, 26–34. [Google Scholar] [CrossRef]
- Devi, K.S.S.; Anusha, N.; Raja, S.; Kumar, A.S. A new strategy for direct electrochemical sensing of a organophosphorus pesticide, triazophos, using a coomassie brilliant-blue dye surface-confined carbon-black-nanoparticle-modified electrode. ACS Appl. Nano Mater. 2018, 1, 4110–4119. [Google Scholar] [CrossRef]
- Ulloa, A.M.; Glassmaker, N.; Oduncu, M.R.; Xu, P.; Wei, A.; Cakmak, M.; Stanciu, L. Roll-to-roll manufactured sensors for nitroaromatic organophosphorus pesticides detection. ACS Appl. Mater. Interfaces 2021, 13, 35961–35971. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.C.; Santana, E.R.; Spinelli, A. Nitrogen and sulfur co-doped graphene quantum dot-modified electrode for monitoring of multivitamins in energy drinks. Talanta 2023, 252, 123836. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Wu, Q.; Si, W.; Gu, Z.; Zhang, Y.; Deng, J.; Hao, Q. Electrochemical determination of imidacloprid using poly (carbazole)/chemically reduced graphene oxide modified glassy carbon electrode. Sens. Actuators B 2013, 183, 102–109. [Google Scholar] [CrossRef]
- Du, D.; Ye, X.; Cai, J.; Liu, J.; Zhang, A. Acetylcholinesterase biosensor design based on carbon nanotube-encapsulated polypyrrole and polyaniline copolymer for amperometric detection of organophosphates. Biosens. Bioelectron. 2010, 25, 2503–2508. [Google Scholar] [CrossRef]
- Kumaravel, A.; Chandrasekaran, M. Electrochemical determination of imidacloprid using nanosilver Nafion®/nanoTiO2 Nafion® composite modified glassy carbon electrode. Sens. Actuators B 2011, 158, 319–326. [Google Scholar] [CrossRef]
- Thangarasu, R.; Victor, V.D.; Alagumuthu, M. MnO2/PANI/rGO-A modified carbon electrode based electrochemical sensor to detect organophosphate pesticide in real food samples. Anal. Bioanal. Electrochem. 2019, 11, 427–447. [Google Scholar]
- Chen, M.; Meng, Y.; Zhang, W.; Zhou, J.; Xie, J.; Diao, G. β-Cyclodextrin polymer functionalized reduced-graphene oxide: Application for electrochemical determination imidacloprid. Electrochim. Acta 2013, 108, 1–9. [Google Scholar] [CrossRef]
- Niu, X.; Mo, Z.; Yang, X.; Sun, M.; Zhao, P.; Li, Z.; Ouyang, M.; Liu, Z.; Gao, H.; Guo, R.; et al. Advances in the use of functional composites of β-cyclodextrin in electrochemical sensors. Microchim. Acta 2018, 185, 328. [Google Scholar] [CrossRef]
- Bae, J.; Shin, K.; Kwon, O.S.; Hwang, Y.; An, J.; Jang, A.; Kim, H.J.; Lee, C.-S. A succinct review of refined chemical sensor systems based on conducting polymer–cyclodextrin hybrids. J. Ind. Eng. Chem. 2019, 79, 19–28. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Bettio, G.B.; Pereira, A.C. An electrochemical sensor based on electropolymerization of β-cyclodextrin and reduced graphene oxide on a glassy carbon electrode for determination of neonicotinoids. Electroanalysis 2018, 30, 1918–1928. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, G.; Zhang, M. Electrochemical sensor based on electro-polymerization of β-cyclodextrin and reduced-graphene oxide on glassy carbon electrode for determination of gatifloxacin. Sens. Actuators B 2016, 228, 59–65. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, X.; Gooding, J.J. Detection of trace nitroaromatic isomers using indium tin oxide electrodes modified using β-cyclodextrin and silver nanoparticles. Anal. Chem. 2012, 84, 8557–8563. [Google Scholar] [CrossRef]
- Qin, Q.; Bai, X.; Hua, Z. Electropolymerization of a conductive β-cyclodextrin polymer on reduced graphene oxide modified screen-printed electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2016, 782, 50–58. [Google Scholar] [CrossRef]
- Wang, R.; Nian, L.; Yao, L.; Liu, L.; Xie, Z.; Ma, Y. Polarized thin layer deposited electrochemically on aluminum-doped zinc oxide as a cathode interlayer for highly efficient organic electronics. ACS Appl. Mater. Interfaces 2016, 8, 26463–26469. [Google Scholar] [CrossRef]
- Salehzadeh, H.; Ebrahimi, M.; Nematollahi, D.; Salarian, A.A. Electrochemical study of fenitrothion and bifenox and their simultaneous determination using multiwalled carbon nanotube modified glassy carbon electrode. J. Electroanal. Chem. 2016, 767, 188–194. [Google Scholar] [CrossRef]
- Akyüz, D.; Keleş, T.; Biyiklioglu, Z.; Koca, A. Electrochemical pesticide sensors based on electropolymerized metallophthalocyanines. J. Electroanal. Chem. 2017, 804, 53–63. [Google Scholar]
- Wang, L.; Dong, J.; Wang, Y.; Cheng, Q.; Yang, M.; Cai, J.; Liu, F. Novel signal-amplified fenitrothion electrochemical assay, based on glassy carbon electrode modified with dispersed graphene oxide. Sci. Rep. 2016, 6, 23409. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Noroozi, R.; Zandi–Atashbar, N.; Rezaei, B. Cerium (IV) oxide decorated on reduced graphene oxide, a selective and sensitive electrochemical sensor for fenitrothion determination. Sens. Actuators B 2017, 245, 980–987. [Google Scholar] [CrossRef]
- Kilele, J.C.; Chokkareddy, R.; Redhi, G.G. Ultra-sensitive electrochemical sensor for fenitrothion pesticide residues in fruit samples using IL@CoFe2O4NPs@MWCNTs nanocomposite. Microchem. J. 2021, 164, 106012. [Google Scholar] [CrossRef]
- Yu, J.; Shendre, S.; Koh, W.-K.; Liu, B.; Li, M.; Hou, S.; Hettiarachchi, C.; Delikanli, S.; Hernández-Martínez, P.; Birowosuto, M.D. Electrically control amplified spontaneous emission in colloidal quantum dots. Sci. Adv. 2019, 5, eaav3140. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Tao, H.; Xu, M.; Zou, J.; Ning, H.; Peng, J.; Cao, Y. Doping-free tandem white organic light-emitting di-odes. Sci. Bull. 2017, 62, 1193–1200. [Google Scholar] [CrossRef]
- Liu, B.; Tao, H.; Su, Y.-J.; Gao, D.-Y.; Lan, L.-F.; Zou, J.-H.; Peng, J.-B. Color-stable, reduced efficiency roll-off hybrid white organic light emitting diodes with ultra high brightness. Chin. Phys. B 2013, 22, 077303. [Google Scholar] [CrossRef]

| Sensors | Linear Ranges (μM) | Detection Limit (μM) | Ref. |
|---|---|---|---|
| MWCNT/GCE | 0.2–60 | 0.08 | [31] |
| CoPc(ma)/GCE | 1.2–42.0 | 0.46 | [32] |
| GO/GCE | 0.0036–1.44 | 0.1 | [33] |
| CeO2@RGO/GCE | 0.025–2.00 | 0.003 | [34] |
| IL@CoFe2O4NPs@MWCNTs/GCE | 0.02–160 | 0.0135 | [35] |
| β-CDP/GCE | 0.036–14.4 | 0.02 | This work |
| Sample | Added (ng/mL) | Detected (ng/mL) | Recovery (%) |
|---|---|---|---|
| Cabbage 1 | 2000 | 2145 | 107.25 |
| Cabbage 2 | 50 | 49.7 | 99.4 |
| Water 1 | 2000 | 2240 | 112 |
| Water 2 | 50 | 49.17 | 98.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, S.; Qin, C.; Nie, Q.; Luo, Y.; Qin, Q.-P.; Wang, R.; Liu, B.; Luo, D. An Electrochemical Sensor Based on Electropolymerization of β-Cyclodextrin on Glassy Carbon Electrode for the Determination of Fenitrothion. Sensors 2023, 23, 435. https://doi.org/10.3390/s23010435
Wang R, Wang S, Qin C, Nie Q, Luo Y, Qin Q-P, Wang R, Liu B, Luo D. An Electrochemical Sensor Based on Electropolymerization of β-Cyclodextrin on Glassy Carbon Electrode for the Determination of Fenitrothion. Sensors. 2023; 23(1):435. https://doi.org/10.3390/s23010435
Chicago/Turabian StyleWang, Rong, Shulong Wang, Caihong Qin, Qiyang Nie, Yougang Luo, Qi-Pin Qin, Ruijuan Wang, Baiquan Liu, and Dongxiang Luo. 2023. "An Electrochemical Sensor Based on Electropolymerization of β-Cyclodextrin on Glassy Carbon Electrode for the Determination of Fenitrothion" Sensors 23, no. 1: 435. https://doi.org/10.3390/s23010435
APA StyleWang, R., Wang, S., Qin, C., Nie, Q., Luo, Y., Qin, Q.-P., Wang, R., Liu, B., & Luo, D. (2023). An Electrochemical Sensor Based on Electropolymerization of β-Cyclodextrin on Glassy Carbon Electrode for the Determination of Fenitrothion. Sensors, 23(1), 435. https://doi.org/10.3390/s23010435









