A Flexible Pressure Sensor with a Mesh Structure Formed by Lost Hair for Human Epidermal Pulse Wave Monitoring
Abstract
1. Introduction
2. Materials and Methods
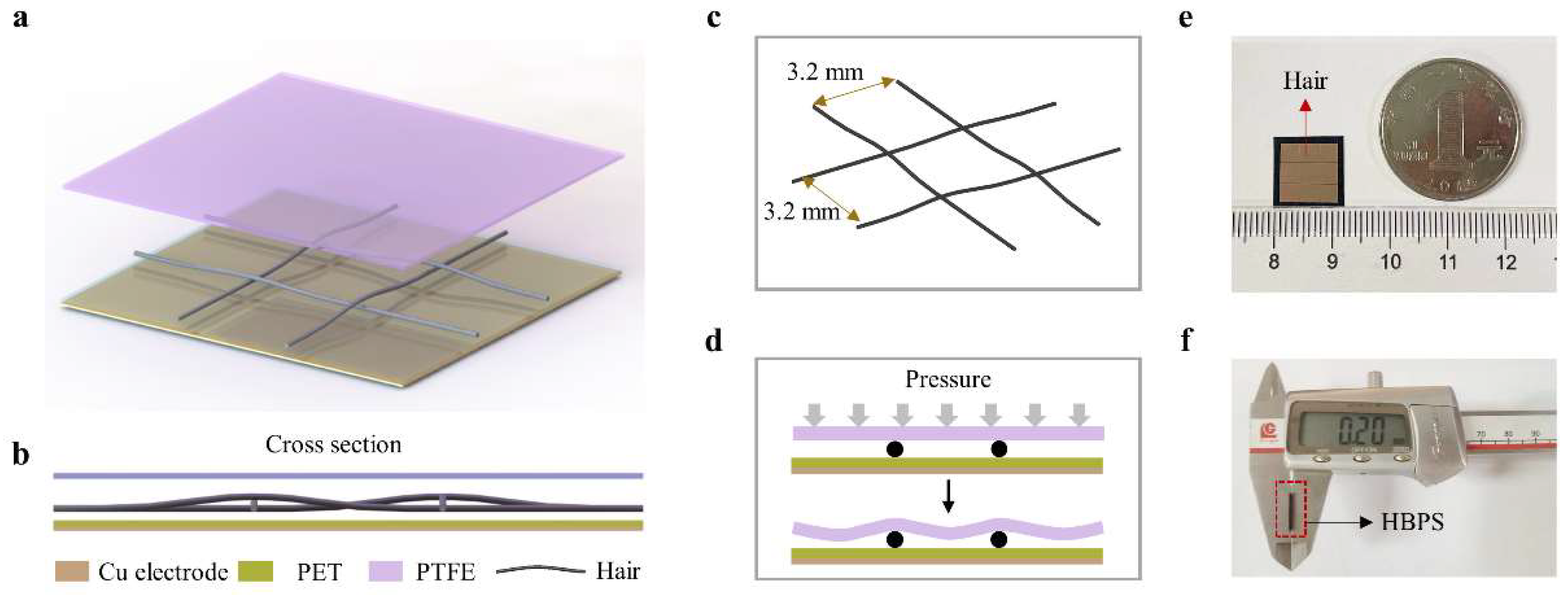
2.1. Structure and Fabrication of the Proposed HBPS
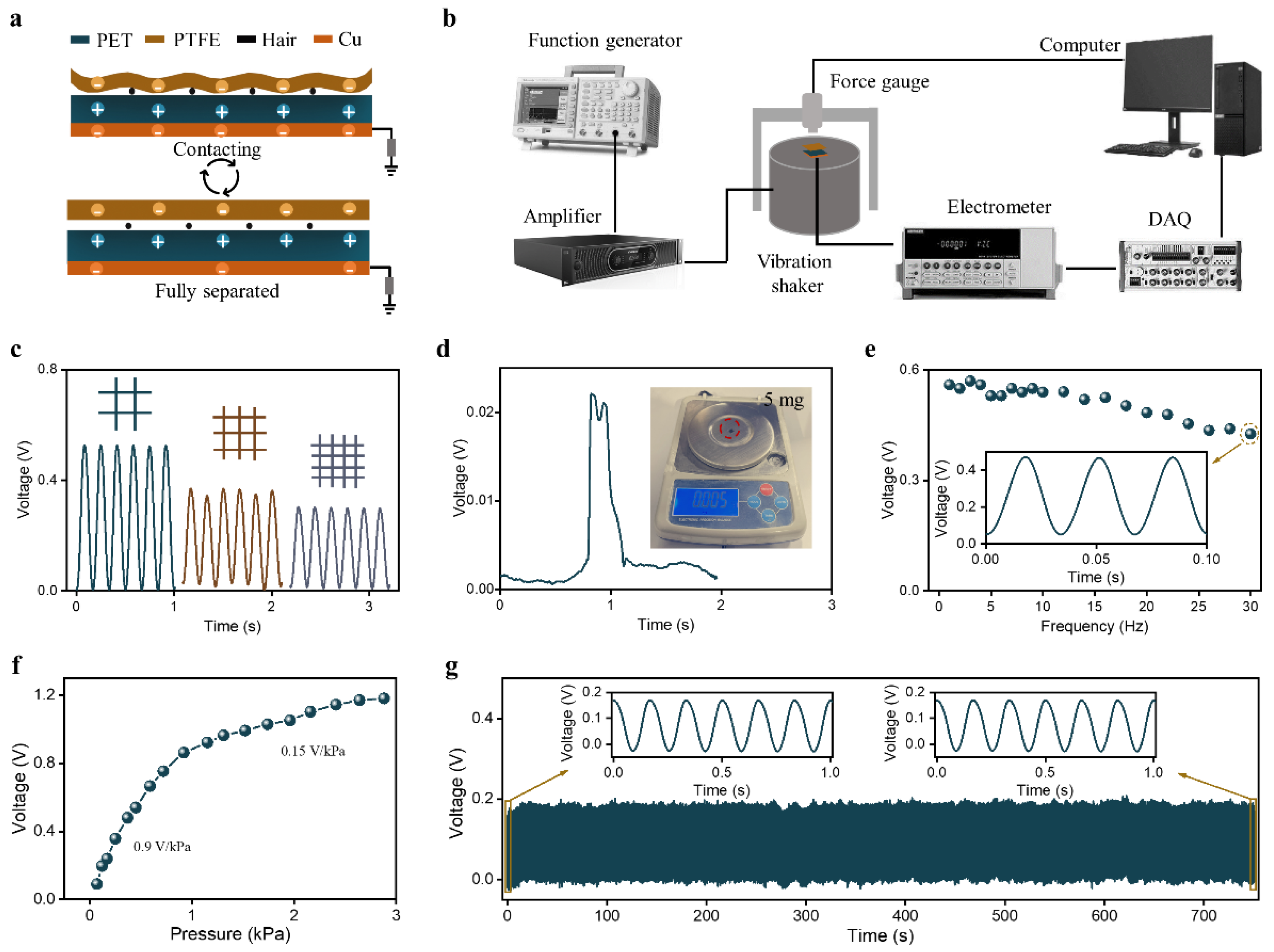
2.2. HBPS Output Performance Characterization
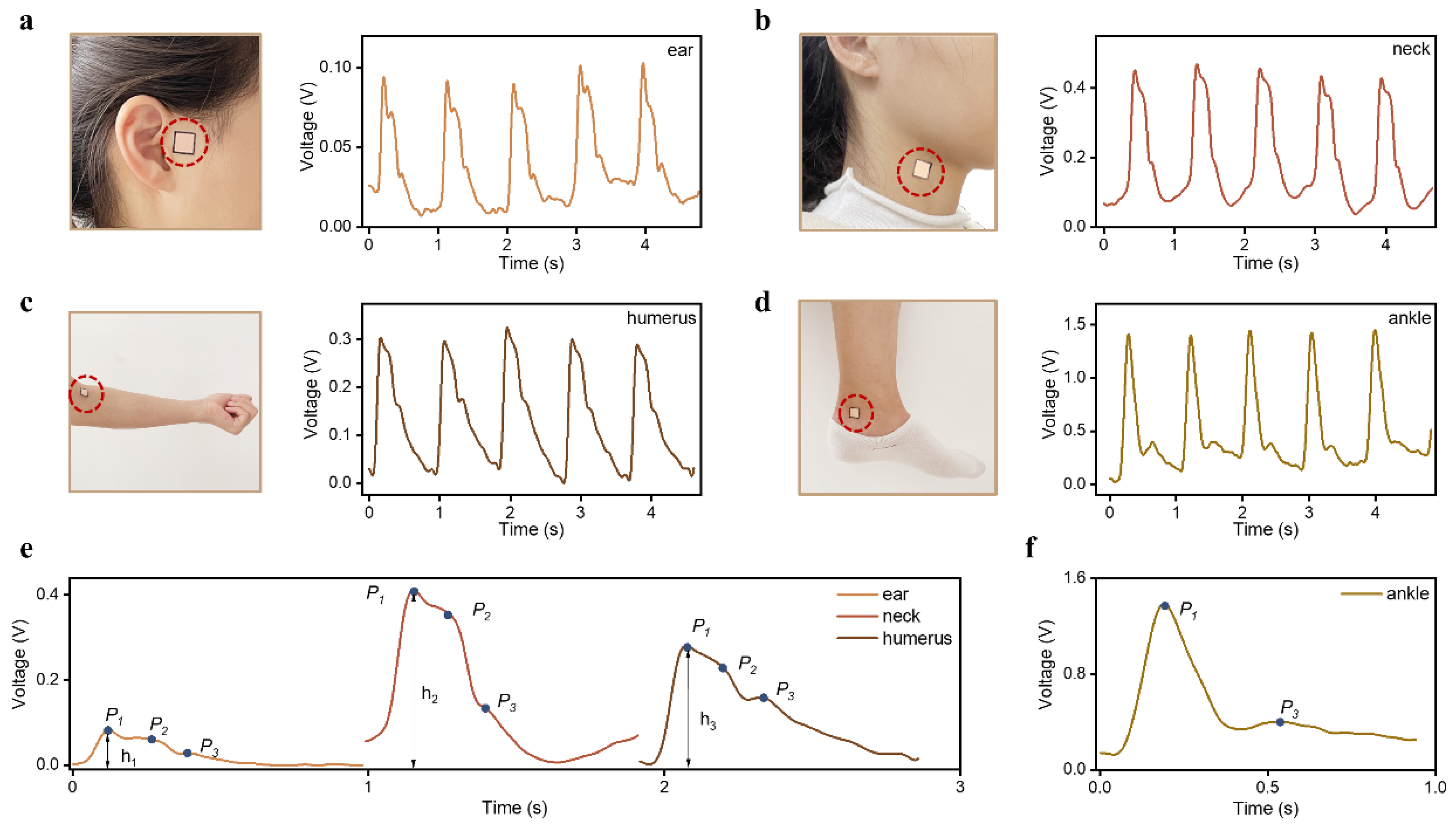
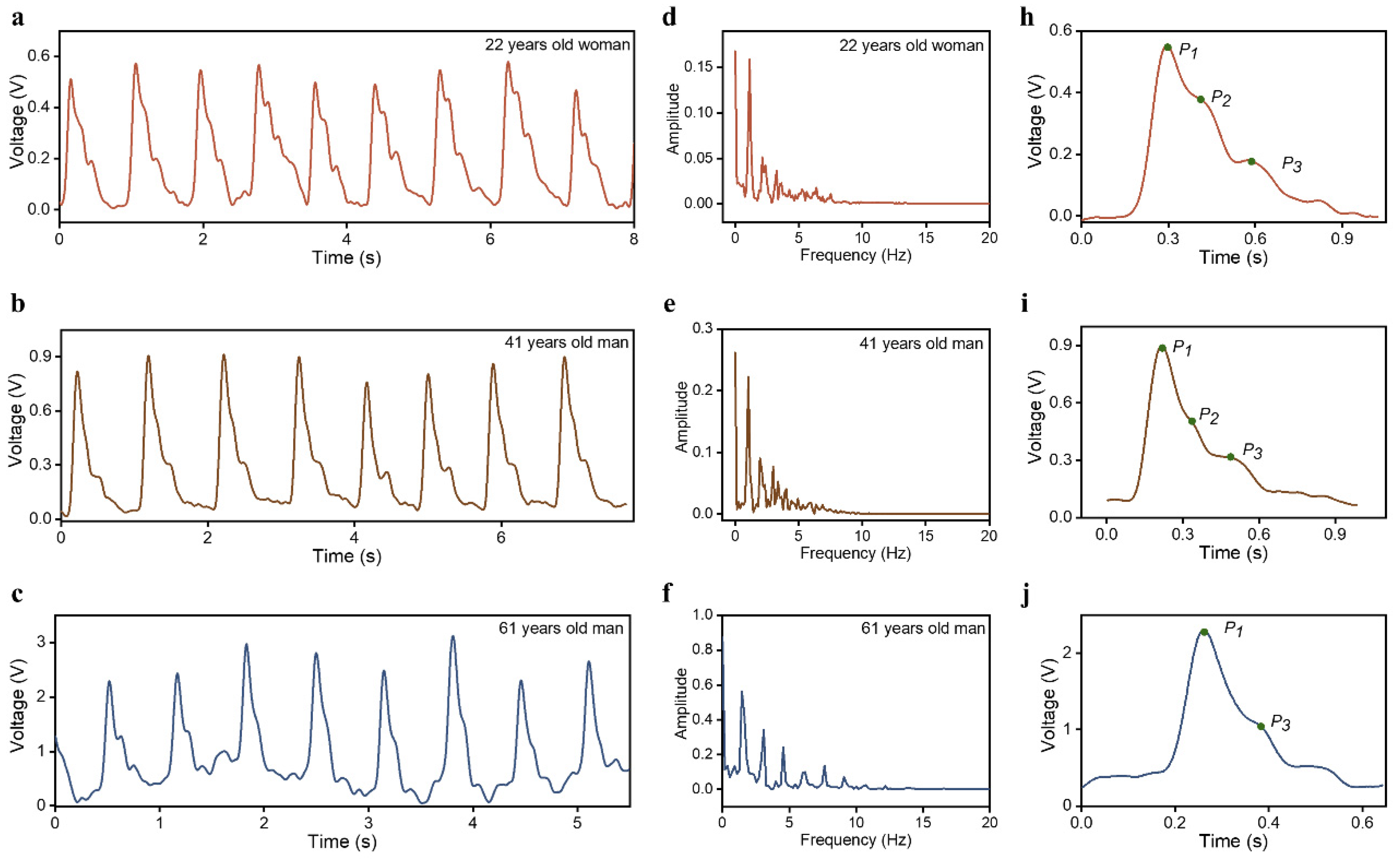
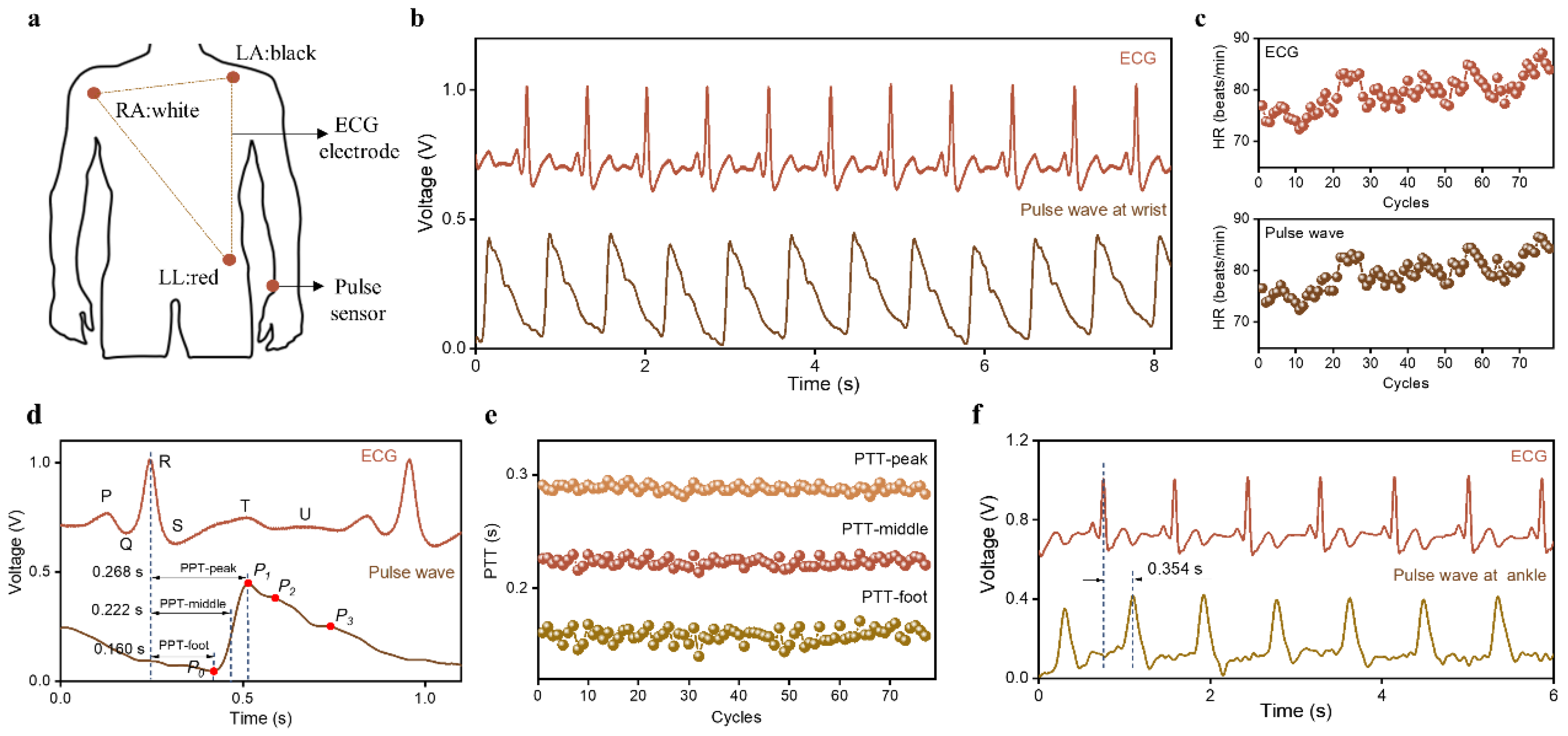
2.3. Human Epidermal Pulse Wave Monitoring
2.4. PTT Measurement
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.H.; Jang, D.G.; Park, J.W.; Youm, S.K. Wearable Sensing of In-Ear Pressure for Heart Rate Monitoring with a Piezoelectric Sensor. Sensors 2015, 15, 23402–23417. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Chirinos, J.A.; Townsend, R.R.; Weber, M.A.; Scuteri, A.; Avolio, A.; Hoshide, S.; Kabutoya, T.; Tomiyama, H.; Node, K.; et al. Systemic hemodynamic atherothrombotic syndrome (SHATS)—Coupling vascular disease and blood pressure variability: Proposed concept from pulse of Asia. Prog. Cardiovasc. Dis. 2020, 63, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, K.; Panchatcharam, P.; Chandrasekhar, A.; Shanmugasundaram, V. Comprehensive Review on Triboelectric Nanogenerator Based Wrist Pulse Measurement: Sensor Fabrication and Diagnosis of Arterial Pressure. ACS Sens. 2021, 6, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Izumi, S.; Katsuura, T.; Kawaguchi, H.; Yoshimoto, M. Multimodal Cardiovascular Information Monitor Using Piezoelectric Transducers for Wearable Healthcare. J. Signal Process. Syst. 2018, 91, 1053–1062. [Google Scholar] [CrossRef]
- Roh, D.; Han, S.; Park, J.; Shin, H. Development of a Multi-Array Pressure Sensor Module for Radial Artery Pulse Wave Measurement. Sensors 2019, 20, 33. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Meng, K.; He, Q.; Zhang, G.; Zhou, Z.; Tan, X.; Feng, Z.; Sun, C.; Yang, J.; et al. Enabling the Unconstrained Epidermal Pulse Wave Monitoring via Finger-Touching. Adv. Funct. Mater. 2021, 31, 2102378. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De la Paz, E.; Pei, K.; Sonsa-Ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef]
- Kim, J.; Gutruf, P.; Chiarelli, A.M.; Heo, S.Y.; Cho, K.; Xie, Z.; Banks, A.; Han, S.; Jang, K.I.; Lee, J.W.; et al. Miniaturized Battery-Free Wireless Systems for Wearable Pulse Oximetry. Adv. Funct. Mater. 2017, 27, 1604373. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, C.S.; Kim, S.; Hur, J.; Lee, S.; Shin, K.W.; Yoon, Y.Z.; Choi, M.K.; Yang, J.; Kim, D.H.; et al. Fully Stretchable Optoelectronic Sensors Based on Colloidal Quantum Dots for Sensing Photoplethysmographic Signals. ACS Nano 2017, 11, 5992–6003. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.S.; Huang, Y.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Lee, Y.; Lee, H.S.; Ko, H. Fingertip Skin-inspired Microstructured Ferroelectric Skins Discriminate Static/dynamic Pressure and Temperature Stimuli. Sci. Adv. 2015, 1, e1500661. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Tang, X.; Chen, Y.-L.; Liu, J.-H.; Shen, Q.-D. Flexible Polymer Transducers for Dynamic Recognizing Physiological Signals. Adv. Funct. Mater. 2016, 26, 3640–3648. [Google Scholar] [CrossRef]
- Su, Y.; Chen, C.; Pan, H.; Yang, Y.; Chen, G.; Zhao, X.; Li, W.; Gong, Q.; Xie, G.; Zhou, Y.; et al. Muscle Fibers Inspired High-Performance Piezoelectric Textiles for Wearable Physiological Monitoring. Adv. Funct. Mater. 2021, 31, 2010962. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Ichiki, M. MEMS-Based Sensor for Simultaneous Measurement of Pulse Wave and Respiration Rate. Sensors 2019, 19, 4942. [Google Scholar] [CrossRef]
- Choong, C.L.; Shim, M.B.; Lee, B.S.; Jeon, S.; Ko, D.S.; Kang, T.H.; Bae, J.; Lee, S.H.; Byun, K.E.; Im, J.; et al. Highly stretchable resistive pressure sensors using a conductive elastomeric composite on a micropyramid array. Adv. Mater. 2014, 26, 3451–3458. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Pang, C.; Koo, J.H.; Nguyen, A.; Caves, J.M.; Kim, M.G.; Chortos, A.; Kim, K.; Wang, P.J.; Tok, J.B.; Bao, Z. Highly skin-conformal microhairy sensor for pulse signal amplification. Adv. Mater. 2015, 27, 634–640. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, F.; Huang, D.; Gao, X.; Di, C.A.; Zhu, D. Flexible suspended gate organic thin-film transistors for ultra-sensitive pressure detection. Nat. Commun. 2015, 6, 6269. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Feng, Z.; Zhang, G.; Qiu, J.; Wu, Y.; Yang, J. Graded Microstructured Flexible Pressure Sensors with High Sensitivity and an Ultrabroad Pressure Range for Epidermal Pulse Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 55747–55755. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Xia, Y.; Zhang, G.; Wang, L.; Chen, L.; Wu, Y.; Yang, J.; Wang, Z.L. Flexible pressure sensor for high-precision measurement of epidermal arterial pulse. Nano Energy 2022, 102, 107710. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Su, Y.; Jing, Q.; Li, Z.; Yi, F.; Wen, X.; Wang, Z.; Wang, Z.L. Eardrum-inspired active sensors for self-powered cardiovascular system characterization and throat-attached anti-interference voice recognition. Adv. Mater. 2015, 27, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Yi, C.; Liu, S.; Wang, Y.; Liu, L.; Liu, X.; Xu, X.; Wang, L. Ultrasensitive, passive and wearable sensors for monitoring human muscle motion and physiological signals. Biosens. Bioelectron. 2016, 77, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, N.; Lin, S.; Duan, J.; Xu, Z.; Pan, Y.; Zhang, H.; Xu, Z.; Huang, L.; Hu, B.; et al. Hierarchical elastomer tuned self-powered pressure sensor for wearable multifunctional cardiovascular electronics. Nano Energy 2020, 70, 104460. [Google Scholar] [CrossRef]
- Dong, K.; Wu, Z.; Deng, J.; Wang, A.C.; Zou, H.; Chen, C.; Hu, D.; Gu, B.; Sun, B.; Wang, Z.L. A Stretchable Yarn Embedded Triboelectric Nanogenerator as Electronic Skin for Biomechanical Energy Harvesting and Multifunctional Pressure Sensing. Adv. Mater. 2018, 30, e1804944. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Zeng, X.; Fu, X.; Hu, Y. Expandable microsphere-based triboelectric nanogenerators as ultrasensitive pressure sensors for respiratory and pulse monitoring. Nano Energy 2019, 59, 295–301. [Google Scholar] [CrossRef]
- Ouyang, H.; Tian, J.; Sun, G.; Zou, Y.; Liu, Z.; Li, H.; Zhao, L.; Shi, B.; Fan, Y.; Fan, Y.; et al. Self-Powered Pulse Sensor for Antidiastole of Cardiovascular Disease. Adv. Mater. 2017, 29, 1703456. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Gao, F.; Zhao, X.; Xun, X.; Kang, Z.; Liao, Q.; Zhang, Y. Self-powered ultrasensitive pulse sensors for noninvasive multi-indicators cardiovascular monitoring. Nano Energy 2021, 81, 105614. [Google Scholar] [CrossRef]
- Neema, S.; Vashisht, D.; Yadav, A.K.; Sinha, A.; Radhakrishnan, S. Trichoscopy of Pressure-Induced Alopecia and Alopecia Areata: A Comparative Study. Int. J. Trichology 2022, 14, 17–20. [Google Scholar]
- Wu, S. Hair and Nail Conditions: Alopecia Evaluation. FP Essent. 2022, 517, 11–16. [Google Scholar]
- Ghasemzadeh, N.; Zafari, A.M. A brief journey into the history of the arterial pulse. Cardiol. Res. Pract. 2011, 2011, 164832. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, Y.J.; Lee, H.J.; Kim, J.Y. Variations in Non-invasive Radial Artery Pulse Waveform at Different Measuring Positions. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 1415–1418. [Google Scholar]
- Wang, T.W.; Lin, S.F. Wearable Piezoelectric-Based System for Continuous Beat-to-Beat Blood Pressure Measurement. Sensors 2020, 20, 851. [Google Scholar] [CrossRef]
- Rastegar, S.; GholamHosseini, H.; Lowe, A. Non-invasive Continuous Blood Pressure Monitoring Systems: Current and Proposed Technology Issues and Challenges. Phys. Eng. Sci. Med. 2019, 43, 11–28. [Google Scholar] [CrossRef]
- Zambrana-Vinaroz, D.; Vicente-Samper, J.M.; Juan, C.G.; Esteve-Sala, V.; Sabater-Navarro, J.M. Non-Invasive Device for Blood Pressure Wave Acquisition by Means of Mechanical Transducer. Sensors 2019, 19, 4311. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Wu, C.-D.; Lu, S.-S.; Tsao, Y. A Linear Regression Model with Dynamic Pulse Transit Time Features for Noninvasive Blood Pressure Prediction. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BIOCAS), Shanghai, China, 17–19 October 2017; pp. 604–607. [Google Scholar]
- Ding, X.R.; Zhang, Y.T. Pulse Transit Time Technique for Cuffless Unobtrusive Blood Pressure Measurement: From Theory to Algorithm. Biomed. Eng. Lett. 2019, 9, 37–52. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Feng, Z.; Li, P.; Wang, L.; Chen, L.; Wu, Y.; Yang, J. A Flexible Pressure Sensor with a Mesh Structure Formed by Lost Hair for Human Epidermal Pulse Wave Monitoring. Sensors 2023, 23, 45. https://doi.org/10.3390/s23010045
Wang X, Feng Z, Li P, Wang L, Chen L, Wu Y, Yang J. A Flexible Pressure Sensor with a Mesh Structure Formed by Lost Hair for Human Epidermal Pulse Wave Monitoring. Sensors. 2023; 23(1):45. https://doi.org/10.3390/s23010045
Chicago/Turabian StyleWang, Xue, Zhiping Feng, Peng Li, Luna Wang, Liang Chen, Yufen Wu, and Jin Yang. 2023. "A Flexible Pressure Sensor with a Mesh Structure Formed by Lost Hair for Human Epidermal Pulse Wave Monitoring" Sensors 23, no. 1: 45. https://doi.org/10.3390/s23010045
APA StyleWang, X., Feng, Z., Li, P., Wang, L., Chen, L., Wu, Y., & Yang, J. (2023). A Flexible Pressure Sensor with a Mesh Structure Formed by Lost Hair for Human Epidermal Pulse Wave Monitoring. Sensors, 23(1), 45. https://doi.org/10.3390/s23010045





