Recent Progress and Applications of Thermal Lens Spectrometry and Photothermal Beam Deflection Techniques in Environmental Sensing
Abstract
:1. Introduction
2. Instrumental
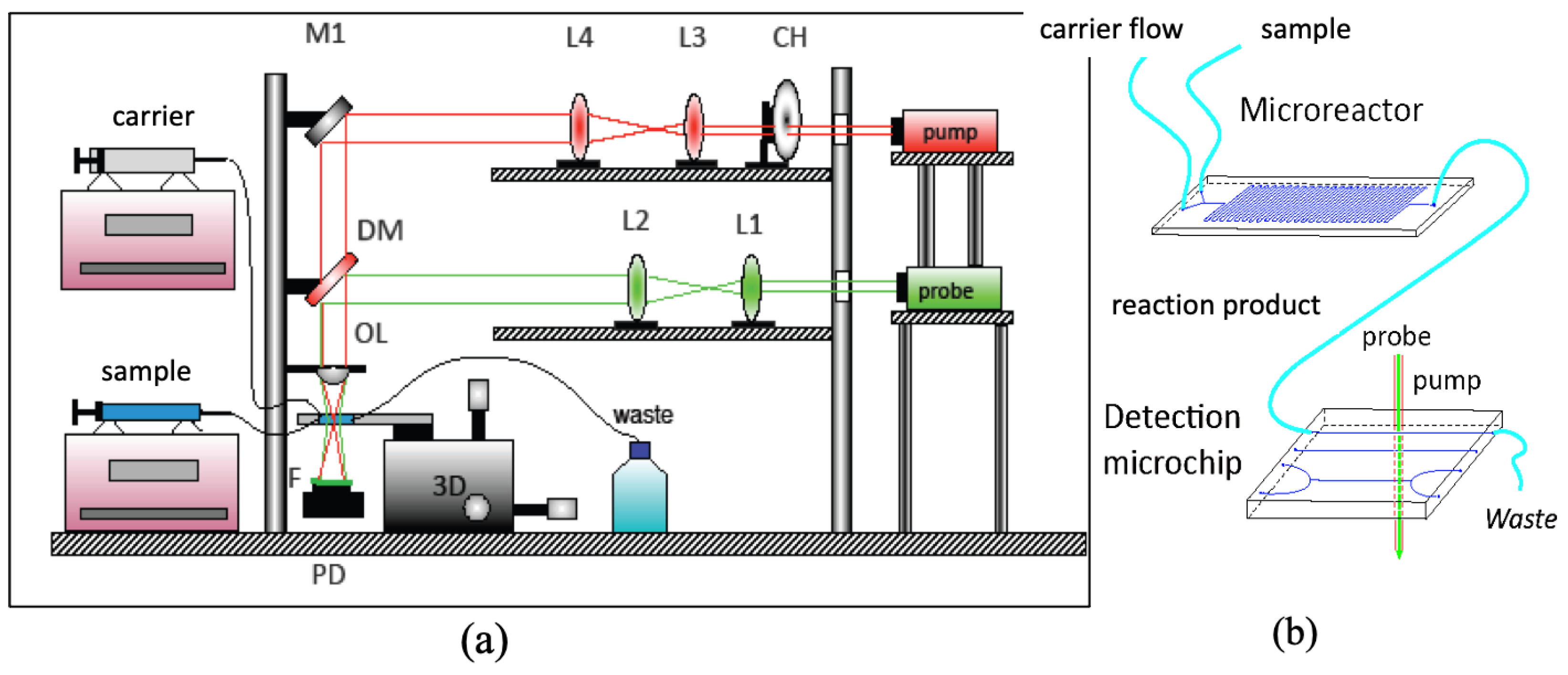
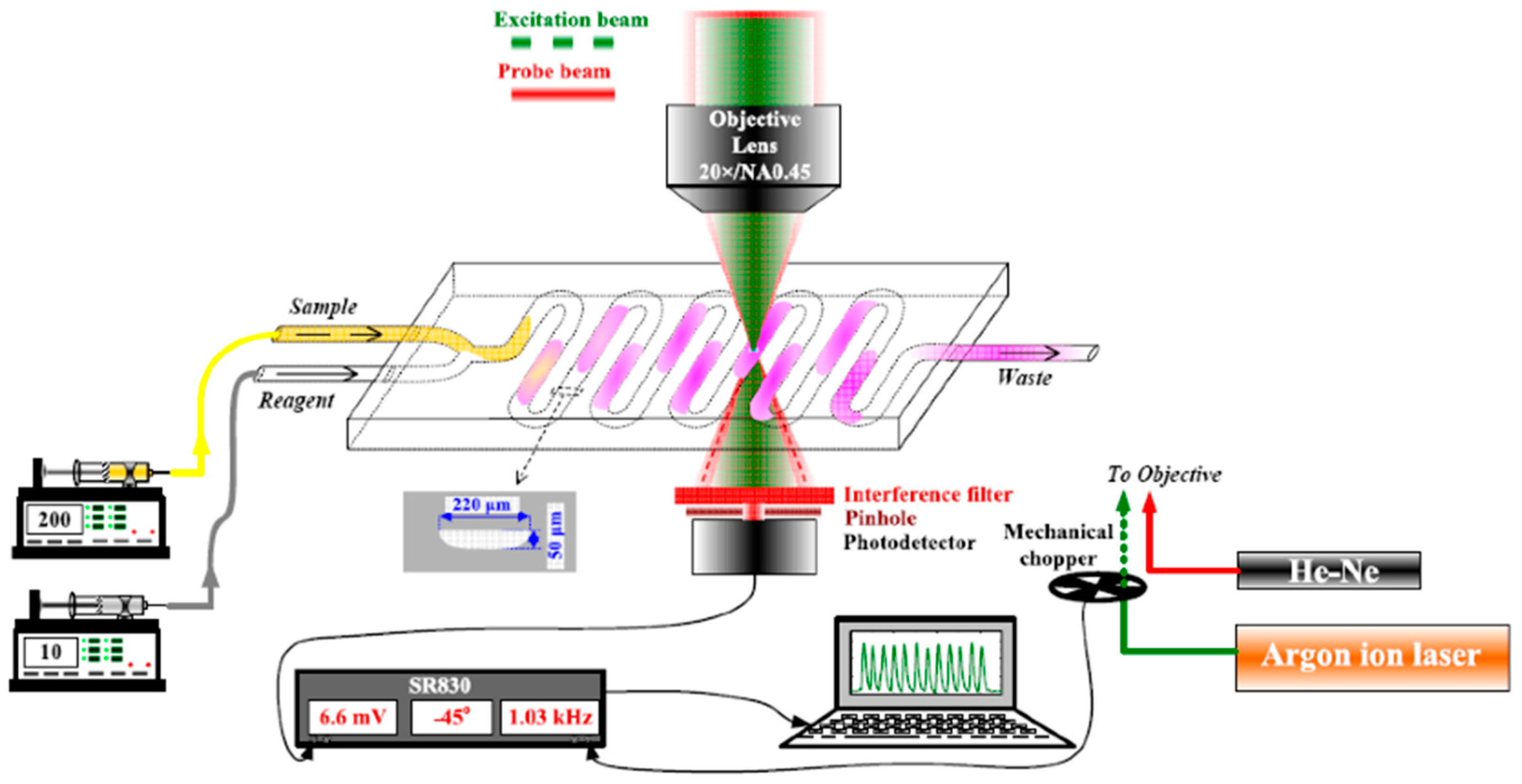
2.1. Thermal Lens Microscope and Microfluidic System
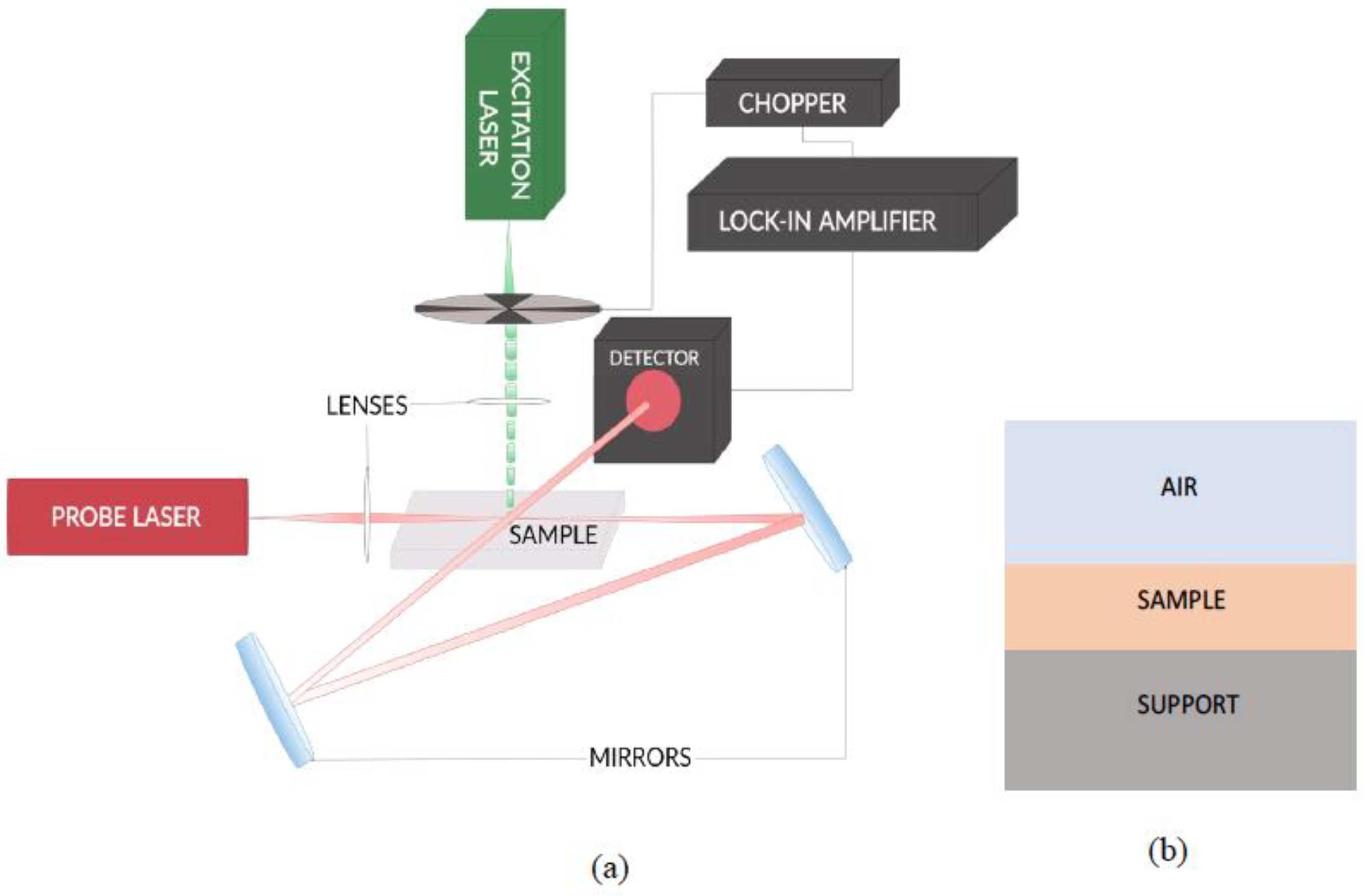
2.2. Beam Deflection Spectrometer
3. Results and Discussion
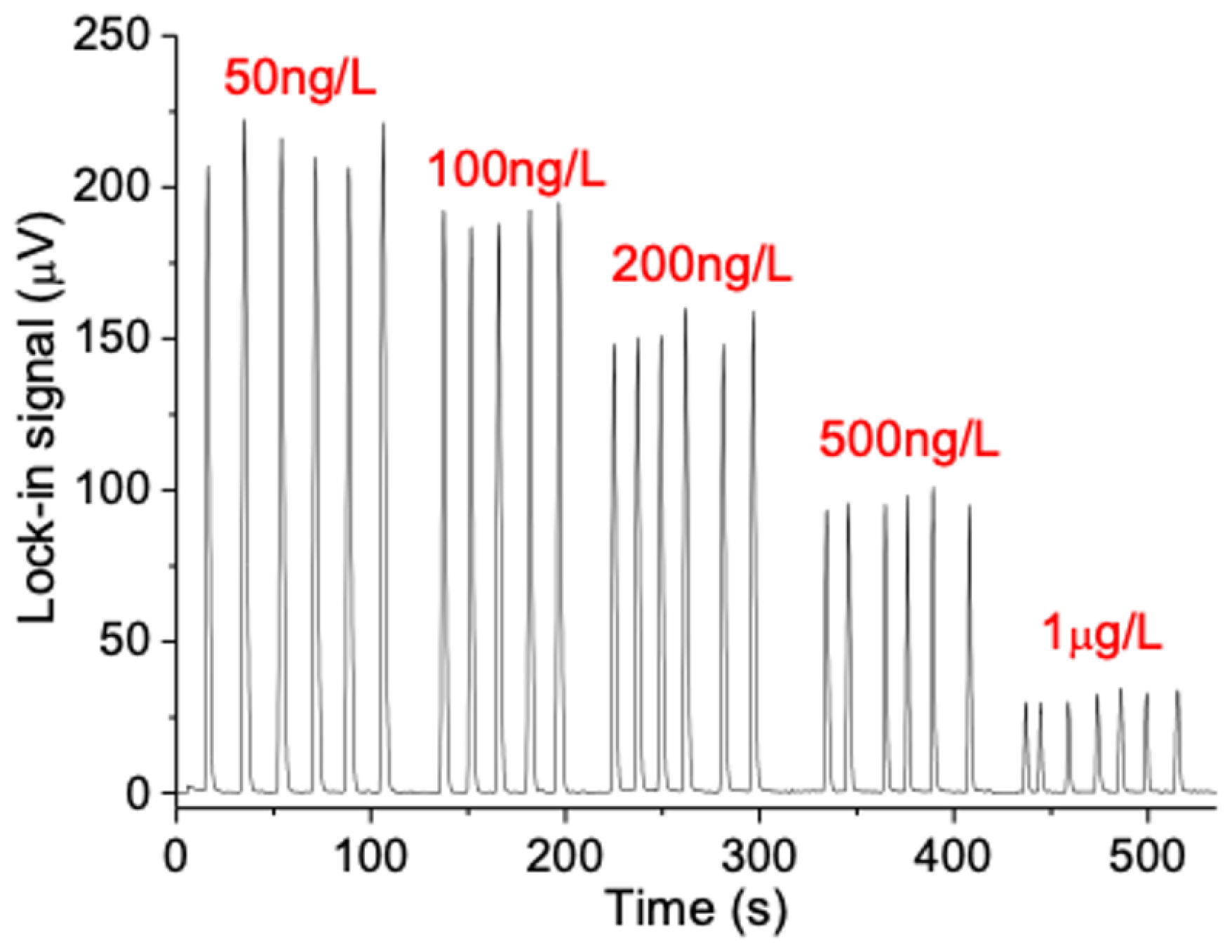
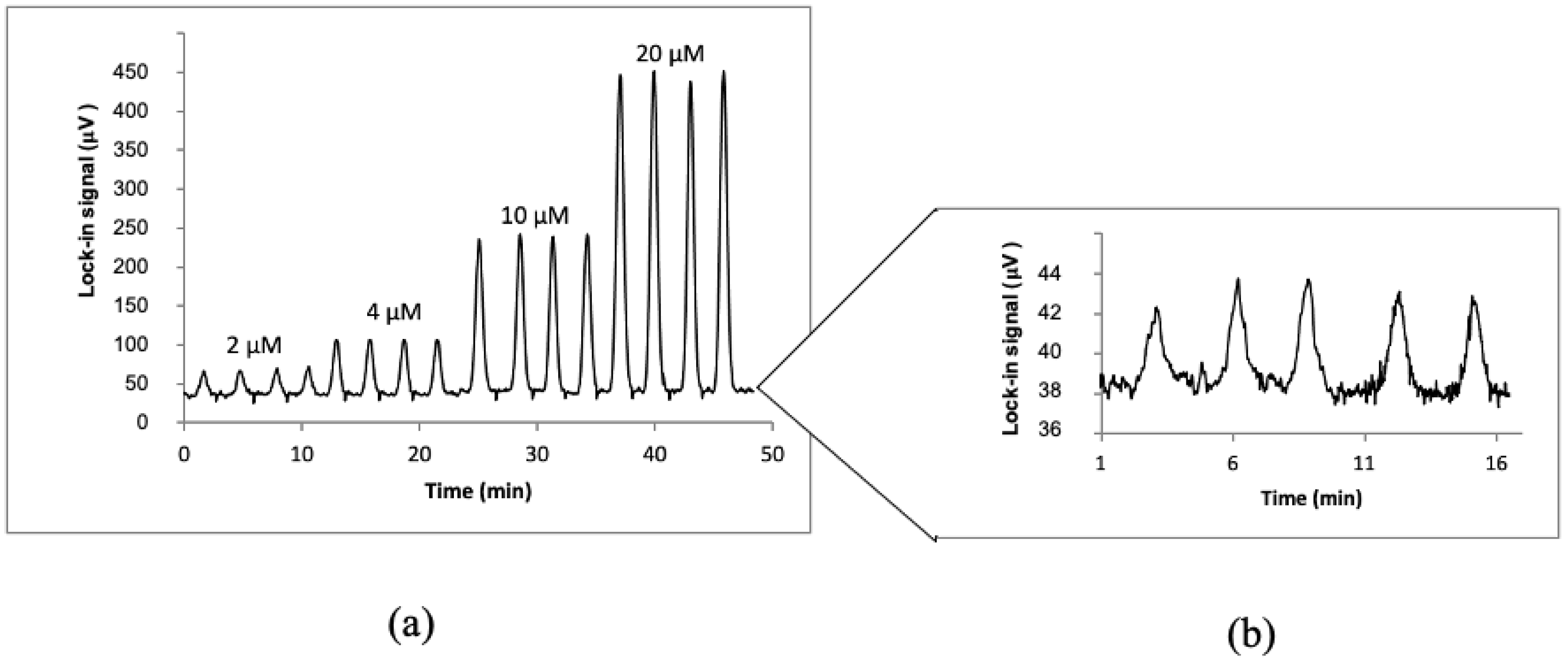
3.1. Determination of Microcystin−LR in Water by µFIA-TLM
3.2. Determination of Total Fe and Fe(II) in Synthetic Cloudwater by µFIA-TLM
3.3. Determination of Ammonium in Water by µFIA-TLM
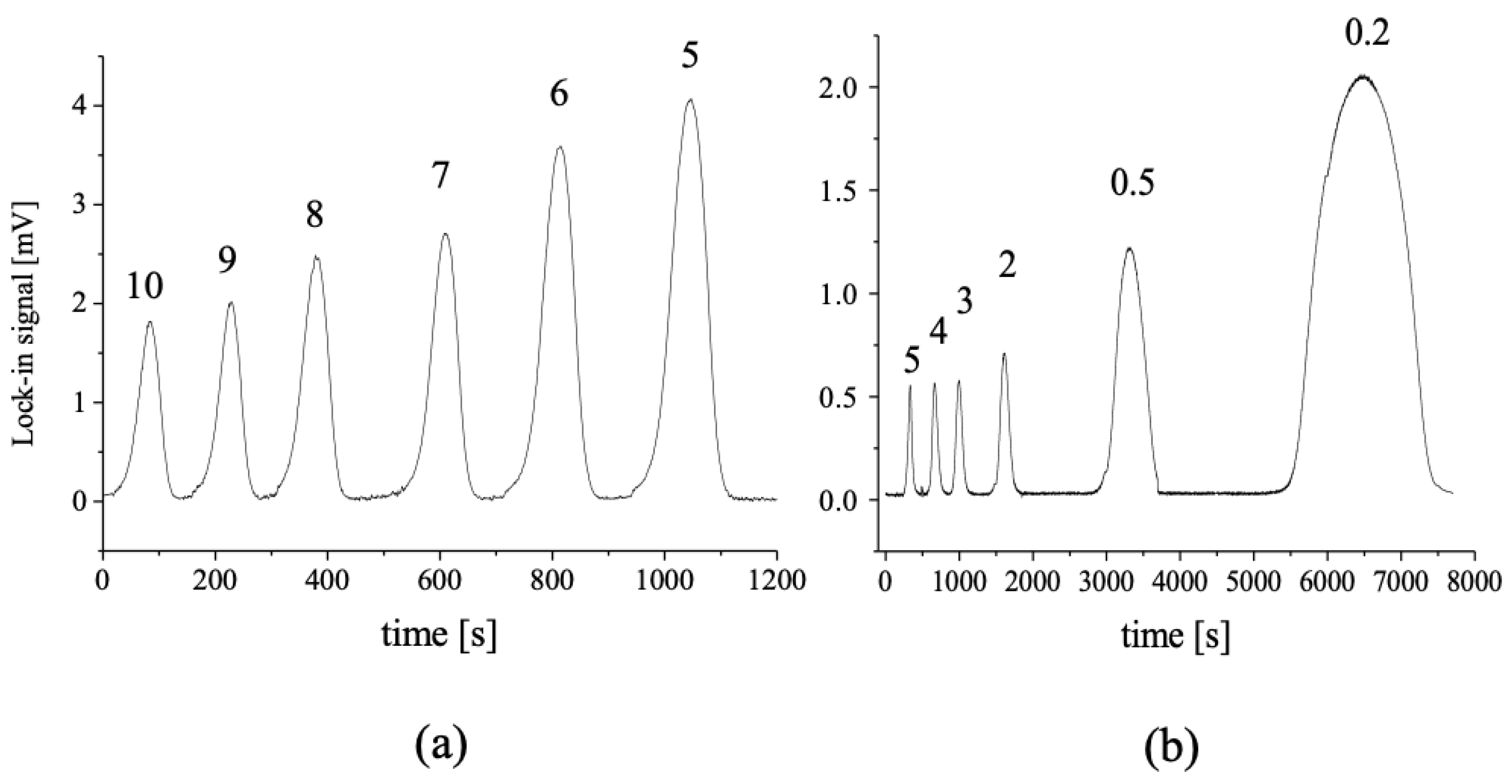
3.4. Determination of Iron Species in Water and Polar Sea Ice by DGT-BDS
3.4.1. Performance Characteristics
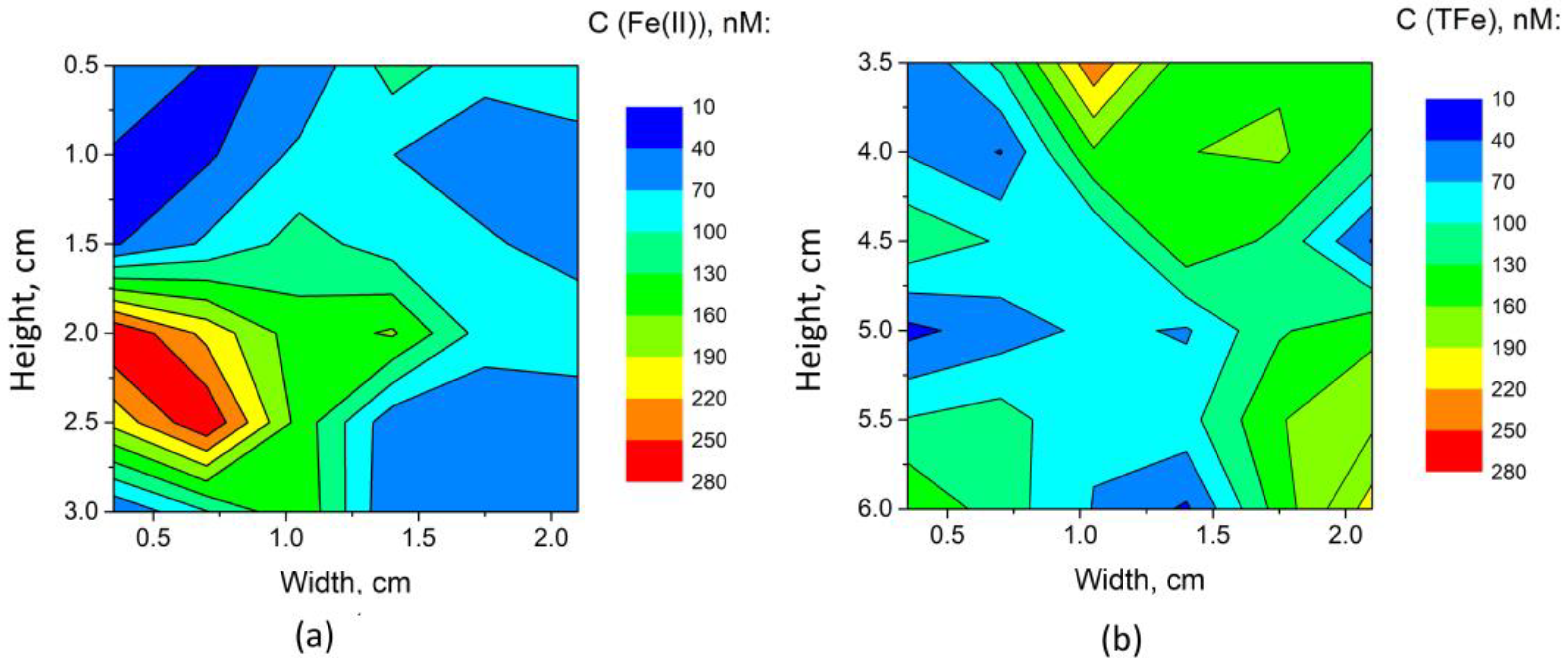
3.4.2. Determination of Fe(II) and Total Fe Distribution in Polar Sea Ice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, K.R. Recent advances in biosensor techniques for environmental monitoring. Anal. Chim. Acta 2006, 568, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent progress in biosensors for environmental monitoring: A review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampreti, M.; Gotti, R. (Eds.) Recent Developments in Novel Solid State Lasers; Optics, Special Issue; MDPI: Basel, Switzerland, 2022. [Google Scholar]
- Fiddler, M.N.; Begashaw, I.; Mickens, M.A.; Collingwood, M.S.; Assefa, Z.; Bililign, S. Laser spectroscopy for atmospheric and environmental sensing. Sensors 2009, 9, 10447–10512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.P. Molecular and Laser Spectroscopy: Advances and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Svanberg, S.; Zhao, G.; Zhang, H.; Huang, J.; Lian, M.; Li, T.; Zhu, S.; Li, Y.Z.; Lin, H.; Svanberg, K. Laser spectroscopy applied to environmental, ecological, food safety, and biomedical research. Opt. Express 2016, 24, A515–A527. [Google Scholar] [CrossRef]
- He, S.; Jun, H.; Shihao, S.; Siyu, L.; Baodui, W. Palladium coordination polymers nanosheets: New strategy for sensitive photothermal detection of H2S. Anal. Chem. 2019, 91, 10823–10829. [Google Scholar] [CrossRef]
- Xu, S.; Xilin, B.; Leyu, W. Exploration of photothermal sensors based on photothermally responsive materials: A brief review. Inorg. Chem. Front 2018, 5, 751–759. [Google Scholar] [CrossRef]
- Sigrist, M.W. Photoacoustic spectroscopy, Applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Schmid, T.; Helmbrecht, C.; Haisch, C.; Panne, U.; Niessner, R. On-line monitoring of opaque liquids by photoacoustic spectroscopy. Anal. Bioanal. Chem. 2003, 375, 1130–1135. [Google Scholar] [CrossRef]
- Hanyecz, V.; Mohacsi, A.; Puskas, S.; Vago, A.; Szabo, G. Photoacoustic spectroscopy-based detector for measuring benzene and toluene concentration in gas and liquid samples. Meas. Sci. Technol. 2011, 22, 125602. [Google Scholar] [CrossRef]
- Franko, M.; Liu, M.; Boškin, A.; Delneri, A.; Proskurnin, M.A. Fast screening techniques for neurotoxigenic substances and other toxicants and pollutants based on thermal lensing and microfluidic chips. Anal. Sci. 2016, 32, 23–30. [Google Scholar] [CrossRef]
- Liu, M.; Malovrh, S.; Franko, M. Microfluidic flow-injection thermal-lens microscopy for high-throughput and sensitive analysis of sub-μL samples. Anal. Methods 2016, 8, 5053–5060. [Google Scholar] [CrossRef] [Green Version]
- Rivoira, L.; Žorž, M.; Martelanc, M.; Budal, S.; Carena, D.; Franko, M.; Bruzzoniti, M.C. Novel approaches for the determination of biogenic amines in food samples. Studia UBB Chemia 2017, 62, 103–122. [Google Scholar] [CrossRef]
- Berden Zrimec, M.; Kožar Logar, J.; Zrimec, A.; Drinovec, L.; Franko, M.; Malej, A. New approach in studies of microalgal celllysis. Cent. Eur. J. Biol. 2009, 4, 313–320. [Google Scholar] [CrossRef]
- Korte, D.; Bruzzoniti, M.C.; Sarzanini, C.; Franko, M. Thermal lens spectrometric determination of colloidal and ionic silver in water. Int. J. Thermophys. 2011, 32, 818–827. [Google Scholar] [CrossRef]
- Budal, S.; Martelanc, M.; Radovanović, T.; Franko, M. Thermal lens spectrometric determination of ammonium in water samples based on indophenol formation with sodium salicylate. Int. J. Environ. Health 2014, 7, 101–113. [Google Scholar] [CrossRef]
- Pogačnik, L.; Franko, M. Detection of organophosphate and carbamate pesticides in vegetable samples by a photothermal biosensor. Biosens. Bioelectron. 2003, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Franko, M. Thermal lens spectrometry: Still a technique on the horizon? Int. J. Thermophys. 2016, 37, 37–67. [Google Scholar] [CrossRef]
- Franko, M. Thermal lens spectrometric detection in flow injection analysis and separation techniques. Appl. Spectrosc. Rev. 2008, 43, 358–388. [Google Scholar] [CrossRef]
- Franko, M. Recent applications of thermal lens spectrometry in food analysis and environmental research. Talanta 2001, 54, 1–13. [Google Scholar] [CrossRef]
- Franko, M.; Tran, C.D. Thermal lens spectroscopy. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Trebše, P.; Franko, M.; Bavcon Kralj, M.; Černigoj, U.; Boškin, A. Recent progress in methods for determination and AOM removal of organophosphorus and neonicotinoid insecticides. In Progress in Pesticides Research; Kanzantzakis, C.M., Ed.; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Franko, M. Bioanalytical applications of thermal lens spectrometry. In Thermal Wave Physics and Related Photothermal Techniques: Basic Principles and Recent Developments; Marín Moares, E., Ed.; Transworld Research Network: Trivandrum, India, 2009; pp. 309–327. [Google Scholar]
- Budasheva, H.; Kravos, A.; Korte, D.; Bratkič, A.; Gao, Y.; Franko, M. Determination of dissolved iron redox species in freshwater sediment using DGT technique coupled to BDS. Acta Chim. Slov. 2019, 66, 239–246. [Google Scholar] [CrossRef]
- Afjeh-Dana, E.; Asadian, E.; Razzaghi, M.R.; Rafii-Tabar, H.; Sasanpour, P. Deflection-based laser sensing platform for selective and sensitive detection of H2S using plasmonic nanostructures. Sci. Rep. 2022, 12, 15789–15798. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.; Thundat, T. Photothermal cantilever deflection spectroscopy. EPJ Technol. Instrum. 2014, 1, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Kožar Logar, J.; Malej, A.; Franko, M. Hyphenated high performance liquid chromatography-thermal lens spectrometry technique as a tool for investigations of xanthophyll cycle pigments in different taxonomic groups of marine phytoplankton. Rev. Sci. Instrum. 2003, 74, 776–778. [Google Scholar] [CrossRef]
- Guzsvany, V.; Madžgalj, A.; Trebše, P.; Gaál, F.; Franko, M. Determination of selected neonicotinoid insecticides by liquid chromatography with thermal lens spectrometric detection. Environ. Chem. Lett. 2007, 5, 203–208. [Google Scholar] [CrossRef]
- Madžgalj, A.; Baesso, M.L.; Franko, M. Flow injection thermal lens spectrometric detection of hexavalent chromium. Eur. Phys. J. Spec. Top. 2008, 153, 503–506. [Google Scholar] [CrossRef]
- Bavcon Kralj, M.; Trebše, P.; Franko, M. Applications of bioanalytical techniques in evaluating advanced oxidation processes in pesticide degradation. TrAC Trends Anal. Chem. 2007, 26, 1020–1031. [Google Scholar] [CrossRef]
- Franko, M.; Tran, C.D. Analytical thermal lens instrumentation. Rev. Sci. Instrum. 1996, 67, 1–18. [Google Scholar] [CrossRef]
- Kitamori, T.; Tokeshi, M.; Hibara, A.; Sato, K. Thermal lens microscopy and microchip chemistry. Anal. Chem. 2004, 76, 52A–60A. [Google Scholar] [CrossRef] [Green Version]
- Bialkowski, S.E.; Astrath, N.G.C.; Proskurnin, M.A. Photothermal Spectroscopy Methods, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Liu, M.; Franko, M. Progress in thermal lens spectrometry and its applications in microscale analytical devices. CRAC Crit. Rev. Anal. Chem. 2014, 44, 328–353. [Google Scholar] [CrossRef]
- Mawatari, K.; Ohashi, T.; Ebata, T.; Tokeshi, M.; Kitamori, T. Thermal lens detection device. Lab Chip 2011, 11, 2990–2993. [Google Scholar] [CrossRef]
- Budasheva, H.; Korte, D.; Bratkič, A. Optimization of photothermal beam deflection spectrometry for determination of iron redox species bound in Chelex-based resin hydrogel. Sci. Total Environ. 2022; submitted. [Google Scholar]
- Carmichael, W.W.; Azevedo, S.M.F.O.; An, J.S.; Molica, R.J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Microcystins ADDA-ELISA Kit (Eurofins Abraxis). Available online: https://tecna.eurofins-technologies.com/media/9347/microcystins-adda-plate-520011.pdf (accessed on 20 December 2022).
- Microcystins/Nodularins PP2A Microtiter Plate Kit (Eurofins Abraxis). Available online: https://abraxis.eurofins-technologies.com/media/7737/microcystins-pp2a-plate-r06042020.pdf (accessed on 20 December 2022).
- Lebogang, L.; Mattiasson, B.; Hedström, M. Capacitive sensing of microcystin variants of Microcystis aeruginosa using a gold immunoelectrode modified with antibodies, gold nanoparticles and polytyramine. Microchim. Acta 2014, 18, 1009–1017. [Google Scholar] [CrossRef]
- Parazols, M.; Marinoni, A.; Amato, P.; Abida, O.; Laj, P.; Mailhot, G. Speciation and role of iron in cloud droplets at the puy de Dôme station. J. Atmos. Chem. 2006, 54, 267–281. [Google Scholar] [CrossRef]
- Deguillaume, L.; Charbouillot, T.; Joly, M.; Vaïtilingom, M.; Parazols, M.; Marinoni, A.; Amato, P.; Delort, A.M.; Vinatier, V.; Flossmann, A.; et al. Classification of clouds sampled at the puy de Dôme (France) based on 10 yr of monitoring of their physicochemical properties. Atmos. Chem. Phys. 2014, 14, 1485–1506. [Google Scholar] [CrossRef] [Green Version]
- Vaïtilingom, M.; Parazols, M.; Sancelme, M.; Charbouillot, T.; Deguillaume, L.; Mailhot, G.; Delort, A.M. Is microbiolgy an alternative route to photochemistry in atmospheric chemistry. In Proceedings of the 10th European Meeting on Environmental Chemistry (EMEC 10), 2–5 December 2009, Limoges, France.
- Sato, K.; Tokeshi, M.; Kitamori, T.; Sawada, T. Integration of flow injection analysis and zeptomole-level detection of the Fe(II)-o−phenanthroline complex. Anal. Sci. 1999, 15, 641–645. [Google Scholar] [CrossRef] [Green Version]
- USA Environmental Protection Agency, USA EPA. Guidance for Methods Development and Methods Validation for the Resource Conservation and Recovery Act (RCRA) Program. 2012. Available online: https://www.epa.gov/hw-sw846/guidance-methods-development-and-methods-validation-resource-conservation-and-recovery-act (accessed on 20 December 2022).
- Neal, C.; Reynolds, B.; Neal, M.; Hill, L.; Wickham, H.; Pugh, B. Nitrogen in rainfall, cloud water, throughfall, stemflow, stream water and groundwater for the Plynlimon catchments of mid-Wales. Sci. Total Environ. 2003, 314–316, 121–151. [Google Scholar] [CrossRef]
- Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption (Amended by Regulations 1882/2003/EC and 596/2009/EC); Council of the European Union. 1998. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01998L0083-19981225&from=EN (accessed on 20 December 2022).
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A guide for selecting the most appropriate method for ammonium determination in water analysis. TrAC Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Davison, W.; Zhang, H. Progress in understanding the use of diffusive gradients in thin films (DGT)—Back to basics. Environ. Chem. 2012, 9, 1–13. [Google Scholar] [CrossRef]
- Moore, J.K.; Braucher, O. Observations of dissolved iron concentrations in the World Ocean: Implications and constraints for ocean biogeochemical models. Biogeosci. Discuss 2007, 4, 1241–1277. [Google Scholar]
- Birchill, A.J.; Hartner, N.T.; Kunde, K.; Siemering, B.; Daniels, C.; González-Santana, D.; Milne, A.; Ussher, S.J.; Worsfold, P.J.; Leopold, K.; et al. The eastern extent of seasonal iron limmitation in the high latitude North Atlantic Ocean. Sci. Rep. 2019, 9, 1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leermakers, M.; Gao, Y.; Gabelle, C.; Lojen, S.; Ouddane, B.; Wartel, M.; Baeyens, W. Determination of high resolution pore water profiles of trace metals in sediments of the Rupel River (Belgium) using DET (Diffusive equilibrium in thin films) and DGT (Diffusive gradients in thin films) techniques. Water Air Soil Poll 2005, 166, 265–286. [Google Scholar] [CrossRef]
- Radovanivić Vukajlović, T. Novel Methods for Detection of Bioactive Substances and Their Effects in Organisms and in the Environment, Dissertation, University of Nova Gorica, Nova Gorica. 2017. Available online: http://repozitorij.ung.si/IzpisGradiva.php?id=2916 (accessed on 20 December 2022).
- Liu, M.; Franko, M. An incoherent light source excited thermal lens microscope. Appl Phys Lett. 2012, 100, 121110-1–121110-4. [Google Scholar] [CrossRef]
- Cabrera, H.; Akbara, J.; Korte, D.; Ramírez-Miquet, E.E.; Marín, E.; Niemela, J.; Ebrahimpour, Z.; Mannatunga, K.; Franko, M. Trace detection and photothermal spectral characterization by a tuneable thermal lens spectrometer with white-light excitation. Talanta 2018, 183, 158–163. [Google Scholar] [CrossRef]
- Budasheva, H. Nondestructive Thermal, Optical, Chemical and Structural Characterization of Advanced Materials by Optothermal Techniques, Dissertation, University of Nova Gorica, Nova Gorica. 2022. Available online: https://repozitorij.ung.si/IzpisGradiva.php?id=7503&lang=eng (accessed on 20 December 2022).
- Liu, M.; Korte, D.; Franko, M. Theoretical description of thermal lens spectrometry in micro space. J. Appl. Phys. 2012, 111, 033109. [Google Scholar] [CrossRef]

| Detection Method | LOD | Linear Range | Sample Throughput/ Analysis Time | Ref. |
|---|---|---|---|---|
| μFIA-TLM PP2A | 80 ng/L | 0.08–1 μg/L | 4 samples/min (triplicate injection) | This work |
| ELISA | 0.1 μg/L | 0.15–5 μg/L | 150 min/96 samples | [40] |
| PP2A microtiter plate | 0.25 μg/L | 0.25–2.5 μg/L | 30 min/96 samples | [41] |
| Immuno−electrode | 0.01 ng/L | 0.0001–0.1 μg/L | 37 min/sample | [42] |
| Added Fe(II) + Fe(III), μmol/L | Determined Fe(II), μmol/L | Recovery Fe(II), | Determined Total Fe, μmol/L | Recovery Total Fe, |
|---|---|---|---|---|
| 0.5 + 0.5 | 0.65 ± 0.01 | 130 ± 1 | 1.05 ± 0.01 | 105 ± 1 |
| 2 + 2 | 2.8 ± 0.2 | 140 ± 8 | 4.09 ± 0.09 | 102 ± 2 |
| 10 + 10 | 10.8 ± 0.3 | 108 ± 3 | 20.5 ± 0.7 | 102 ± 3 |
| Added Concentration, nM | Measured Concentration, nM | Yield, % |
|---|---|---|
| 200 | 187 ± 30 | 93 ± 15 |
| 600 | 577 ± 45 | 96 ± 8 |
| 1000 | 992 ± 60 | 99 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franko, M.; Goljat, L.; Liu, M.; Budasheva, H.; Žorž Furlan, M.; Korte, D. Recent Progress and Applications of Thermal Lens Spectrometry and Photothermal Beam Deflection Techniques in Environmental Sensing. Sensors 2023, 23, 472. https://doi.org/10.3390/s23010472
Franko M, Goljat L, Liu M, Budasheva H, Žorž Furlan M, Korte D. Recent Progress and Applications of Thermal Lens Spectrometry and Photothermal Beam Deflection Techniques in Environmental Sensing. Sensors. 2023; 23(1):472. https://doi.org/10.3390/s23010472
Chicago/Turabian StyleFranko, Mladen, Leja Goljat, Mingqiang Liu, Hanna Budasheva, Mojca Žorž Furlan, and Dorota Korte. 2023. "Recent Progress and Applications of Thermal Lens Spectrometry and Photothermal Beam Deflection Techniques in Environmental Sensing" Sensors 23, no. 1: 472. https://doi.org/10.3390/s23010472





