Stress Estimation Using Biometric and Activity Indicators to Improve QoL of the Elderly †
Abstract
1. Introduction
- A new indicator, including biological and activity perspectives, was developed and used as a feature to estimate the health status of the elderly.
- The estimation performance was improved by 4–7% by introducing the developed indicator and some analytical methods (e.g., bagging, upsampler, downsampler, and SHAP) compared to baseline methods and our previous study [26].
2. Related Research
2.1. QoL Estimation
2.2. Stress Estimation
2.3. Recognition of Activities of Daily Living in the Home in a Smart Home
2.4. Health Management in Smart Home
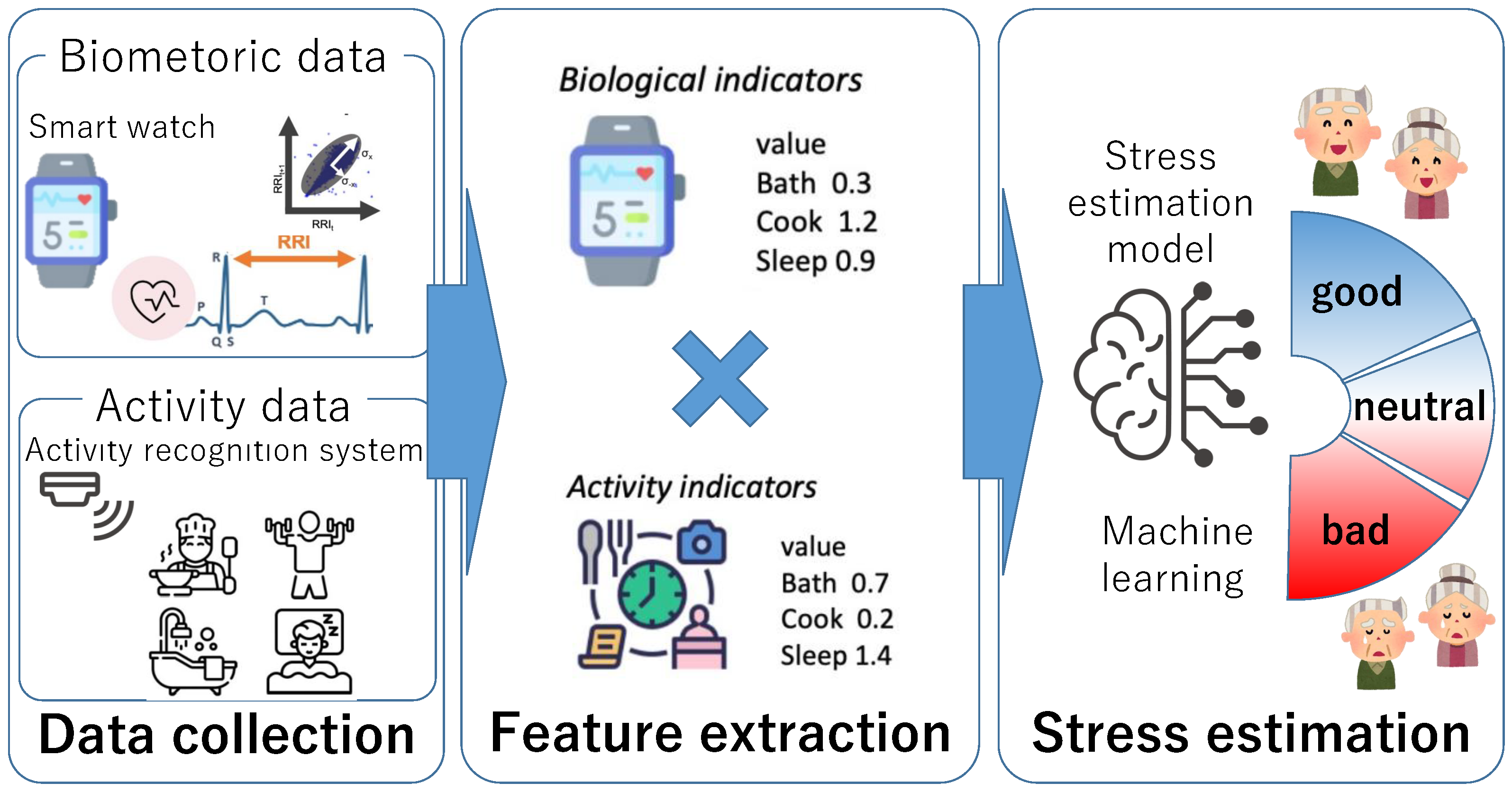
3. Stress Estimation Method Using Biometric and Activity Indicators
3.1. Overview of the Proposed Method
3.2. Data Collection
3.3. Feature Extraction
3.4. Stress Estimation
4. Evaluation Experiment
4.1. Data Collection Experiment
- MQ (morning question): Did you feel physically refreshed this morning?
- NQ (night question): Do you experience any physical stress due to physical pain or discomfort?
4.2. Construction of Stress Recognition Model
4.2.1. Overview of features used for stress recognition model
4.2.2. Detail of Stress Recognition Model
- Average RRI value in the last 24 h;
- Average RRI for 4 h after waking up;
- Lorenz plot area of the day;
- Lorenz plot area of the day for 4 h after waking up.
5. Results
5.1. Result of Introducing SMOTE-OverSampler, RandomUnderSampler, and Bagging
5.2. Effects of Different Features
5.3. Evaluation of Features by SHAP
6. Discussion and Limitations
6.1. Discussion
6.2. Limitation
6.3. Future Work
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomokazu, M.; Shinya, M.; Yuma, S.; Manato, F.; Hirohiko, S.; Keiichi, Y. Multi-Person Daily Activity Recognition with Non-Contact Sensors Based on Activity Co-Occurrence. In Proceedings of the 13th International Conference on Mobile Computing and Ubiquitous Networking (ICMU 2021), Tokyo, Japan, 17–19 November 2021; pp. 157–164. [Google Scholar]
- Payne, M.; Porter Starr, K.; Orenduff, M.; Mulder, H.; McDonald, S.; Spira, A.; Pieper, C.; Bales, C. Quality of life and mental health in older adults with obesity and frailty: Associations with a weight loss intervention. J. Nutr. Health Aging 2018, 22, 1259–1265. [Google Scholar] [CrossRef]
- Zurita-Cruz, J.N.; Manuel-Apolinar, L.; Arellano-Flores, M.L.; Gutierrez-Gonzalez, A.; Najera-Ahumada, A.G.; Cisneros-Gonzalez, N. Health and quality of life outcomes impairment of quality of life in type 2 diabetes mellitus: A cross-sectional study. Health Qual. Life Outcomes 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, X.; Wang, J.; Zhang, J.; Liu, J.; Wang, B.; Guan, W.; Zhang, H.; Xu, L.; Liu, G.; et al. Evaluation of quality of life and its influencing factors after transplantation of leukemia patients based on SF-36 score: A cohort study. Qual. Life Res. 2020, 29, 1809–1816. [Google Scholar] [CrossRef]
- Wunsch, K.; Nigg, C.; Niessner, C.; Schmidt, S.C.; Oriwol, D.; Hanssen-Doose, A.; Burchartz, A.; Eichsteller, A.; Kolb, S.; Worth, A.; et al. The impact of COVID-19 on the interrelation of physical activity, screen time and health-related quality of life in children and adolescents in Germany: Results of the Motorik-Modul Study. Children 2021, 8, 98. [Google Scholar] [CrossRef]
- Goes, M.; Lopes, M.; Marôco, J.; Oliveira, H.; Fonseca, C. Psychometric properties of the WHOQoL-BREF (PT) in a sample of elderly citizens. Health Qual. Life Outcomes 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Phyo, A.Z.Z.; Gonzalez-Chica, D.A.; Stocks, N.P.; Woods, R.L.; Fisher, J.; Tran, T.; Owen, A.J.; Ward, S.A.; Britt, C.J.; Ryan, J.; et al. Impact of economic factors, social health and stressful life events on physical health-related quality of life trajectories in older Australians. Qual. Life Res. 2022, 31, 1321–1333. [Google Scholar] [CrossRef]
- Sella, E.; Cellini, N.; Borella, E. How elderly people’s quality of life relates to their sleep quality and sleep-related beliefs. Behav. Sleep Med. 2022, 20, 112–124. [Google Scholar] [CrossRef]
- Group, T.W. The World Health Organization quality of life assessment (WHOQoL): Development and general psychometric properties. Soc. Sci. Med. 1998, 46, 1569–1585. [Google Scholar] [CrossRef]
- Amenomori, C.; Mizumoto, T.; Suwa, H.; Arakawa, Y.; Yasumoto, K. A method for simplified hrqol measurement by smart devices. In Proceedings of the International Conference on Wireless Mobile Communication and Healthcare, Vienna, Austria, 14–15 November 2017; pp. 91–98. [Google Scholar]
- Fukuda, S.; Matsuda, Y.; Tani, Y.; Arakawa, Y.; Yasumoto, K. Predicting Depression and Anxiety Mood by Wrist-Worn Sleep Sensor. In Proceedings of the 2020 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops), Austin, TX, USA, 23–27 March 2020; pp. 1–6. [Google Scholar]
- Jaques, N.; Taylor, S.; Nosakhare, E.; Sano, A.; Picard, R. Multi-Task Learning for Predicting Health, Stress, and Happiness; NIPS Workshop on Machine Learning for Healthcare: Cambridge, UK, 2016. [Google Scholar]
- Gjoreski, M.; Gjoreski, H.; Lutrek, M.; Gams, M. Automatic Detection of Perceived Stress in Campus Students Using Smartphones. In Proceedings of the 2015 International Conference on Intelligent Environments, Prague, Czech Republic, 15–17 July 2015; pp. 132–135. [Google Scholar]
- Toyofuku, F.; Yamaguchi, K.; Hagiwara, H. Simplified method for estimating parasympathetic nerves activity by Lorenz plot of ECG RR intervals. Jpn. J. Ergon. 2006, 42, 512–515. [Google Scholar] [CrossRef]
- Prasad, L.; Fredrick, J.; Aruna, R. The relationship between physical performance and quality of life and the level of physical activity among the elderly. J. Educ. Health Promot. 2021, 10, 68. [Google Scholar]
- Teixeira, E.; Fonseca, H.; Diniz-Sousa, F.; Veras, L.; Boppre, G.; Oliveira, J.; Pinto, D.; Alves, A.J.; Barbosa, A.; Mendes, R.; et al. Wearable devices for physical activity and healthcare monitoring in elderly people: A critical review. Geriatrics 2021, 6, 38. [Google Scholar] [CrossRef]
- Zapata-Lamana, R.; Poblete-Valderrama, F.; Ledezma-Dames, A.; Pavón-León, P.; Leiva, A.M.; Fuentes-Alvarez, M.T.; Cigarroa, I.; Parra-Rizo, M.A. Health, functional ability, and environmental quality as predictors of life satisfaction in physically active older adults. Soc. Sci. 2022, 11, 265. [Google Scholar] [CrossRef]
- Smets, E.; Rios Velazquez, E.; Schiavone, G.; Chakroun, I.; D’Hondt, E.; De Raedt, W.; Cornelis, J.; Janssens, O.; Van Hoecke, S.; Claes, S.; et al. Large-scale wearable data reveal digital phenotypes for daily-life stress detection. NPJ Digit. Med. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Kyriakou, K.; Resch, B.; Sagl, G.; Petutschnig, A.; Werner, C.; Niederseer, D.; Liedlgruber, M.; Wilhelm, F.; Osborne, T.; Pykett, J. Detecting moments of stress from measurements of wearable physiological sensors. Sensors 2019, 19, 3805. [Google Scholar] [CrossRef]
- Yao, W.; Kaminishi, K.; Yamamoto, N.; Hamatani, T.; Yamada, Y.; Kawada, T.; Hiyama, S.; Okimura, T.; Terasawa, Y.; Maeda, T.; et al. Passive Way of Measuring QoL/Well-Being Levels Using Smartphone Log. Front. Digit. Health 2022, 4, 780566. [Google Scholar] [CrossRef]
- Fukazawa, Y.; Yamamoto, N.; Hamatani, T.; Ochiai, K.; Uchiyama, A.; Ohta, K. Smartphone-based mental state estimation: A survey from a machine learning perspective. J. Inf. Process. 2020, 28, 16–30. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Murugappan, M.; Yaacob, S.B. DETECTION OF HUMAN STRESS USING SHORT-TERM ECG AND HRV SIGNALS. J. Mech. Med. Biol. 2013, 13, 1350038. [Google Scholar] [CrossRef]
- Setz, C.; Arnrich, B.; Schumm, J.; La Marca, R.; Tröster, G.; Ehlert, U. Discriminating Stress From Cognitive Load Using a Wearable EDA Device. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 410–417. [Google Scholar] [CrossRef]
- Matsui, T.; Onishi, K.; Misaki, S.; Suwa, H.; Fujimoto, M.; Mizumoto, T.; Sasaki, W.; Kimura, A.; Maruyama, K.; Yasumoto, K. Analysis of Visualized Bioindicators Related to Activities of Daily Living. In Proceedings of the International Conference on Advanced Information Networking and Applications, Toronto, ON, Canada, 12–14 May 2021; pp. 731–744. [Google Scholar]
- Cacioppo, J.; Tassinary, L. Inferring Psychological Significance From Physiological Signals. Am. Psychol. 1990, 45, 16–28. [Google Scholar] [CrossRef]
- Matsumoto, K.; Matsui, T.; Suwa, H.; Yasumoto, K. Stress Prediction Using Per-Activity Biometric Data to Improve QoL in the Elderly. In Proceedings of the 19th International Conference, ICOST 2022, Paris, France, 27–30 June 2022; pp. 196–208. [Google Scholar]
- Brennan, M.; Palaniswami, M.; Kamen, P. Poincare plot interpretation using a physiological model of HRV based on a network of oscillators. Am. J. -Physiol.-Heart Circ. Physiol. 2002, 283, H1873–H1886. [Google Scholar] [CrossRef]
- Group, W. The development of the World Health Organization quality of life assessment instrument (the WHOQoL). In Quality of Life Assessment: International Perspectives; Springer: Berlin/Heidelberg, Germany, 1994; pp. 41–57. [Google Scholar]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Whoqol Group Development of the World Health Organization WHOQoL-BREF quality of life assessment. Psychol. Med. 1998, 28, 551–558. [CrossRef]
- McDuff, D.; Gontarek, S.; Picard, R. Remote measurement of cognitive stress via heart rate variability. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2957–2960. [Google Scholar]
- Wijsman, J.; Grundlehner, B.; Liu, H.; Penders, J.; Hermens, H. Wearable physiological sensors reflect mental stress state in office-like situations. In Proceedings of the 2013 Humaine Association Conference on Affective Computing and Intelligent Interaction, Washington, DC, USA, 2–5 September 2013; pp. 600–605. [Google Scholar]
- Dedovic, K.; Renwick, R.; Mahani, N.K.; Engert, V.; Lupien, S.J.; Pruessner, J.C. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005, 30, 319. [Google Scholar]
- Bauer, G.; Lukowicz, P. Can smartphones detect stress-related changes in the behaviour of individuals? In Proceedings of the 2012 IEEE International Conference on Pervasive Computing and Communications Workshops, Lugano, Switzerland, 19–23 March 2012; pp. 423–426. [Google Scholar]
- Sano, A.; Picard, R.W. Stress recognition using wearable sensors and mobile phones. In Proceedings of the 2013 Humaine Association Conference on Affective Computing and Intelligent Interaction, Washington, DC, USA, 2–5 September 2013; pp. 671–676. [Google Scholar]
- Hernandez, J.; Morris, R.R.; Picard, R.W. Call center stress recognition with person-specific models. In Proceedings of the International Conference on Affective Computing and Intelligent Interaction, Memphis, TN, USA, 9–12 October 2011; pp. 125–134. [Google Scholar]
- Ghandeharioun, A.; Fedor, S.; Sangermano, L.; Ionescu, D.; Alpert, J.; Dale, C.; Sontag, D.; Picard, R. Objective assessment of depressive symptoms with machine learning and wearable sensors data. In Proceedings of the 2017 Seventh International Conference on Affective Computing and Intelligent Interaction (ACII), San Antonio, TX, USA, 23–26 October 2017; pp. 325–332. [Google Scholar]
- Garcia-Ceja, E.; Osmani, V.; Mayora, O. Automatic stress detection in working environments from smartphones’ accelerometer data: A first step. IEEE J. Biomed. Health Inform. 2015, 20, 1053–1060. [Google Scholar] [CrossRef]
- de Santos Sierra, A.; Ávila, C.S.; Casanova, J.G.; Bailador, G. A Stress-Detection System Based on Physiological Signals and Fuzzy Logic. IEEE Trans. Ind. Electron. 2011, 58, 4857–4865. [Google Scholar] [CrossRef]
- Wang, J.; Lin, C.; Yang, Y. A k-nearest-neighbor classifier with heart rate variability feature-based transformation algorithm for driving stress recognition. Neurocomputing 2013, 116, 136–143. [Google Scholar] [CrossRef]
- Union Tool Co.Sensor Products. Available online: https://www.uniontool.co.jp/en/product/sensor/ (accessed on 4 May 2022).
- Miyaji, A.; Matsui, T.; Zhang, Z.; Choi, H.; Fujimoto, M.; Yasumoto, K. Analysis on Nursing Care Activity Related Stress Level for Reduction of Caregiving Workload. In Proceedings of the 50th International Conference on Parallel Processing Workshop, Chicago, IL, USA, 9–12 August 2021; pp. 1–8. [Google Scholar]
- Healey, J.A.; Picard, R.W. Detecting stress during real-world driving tasks using physiological sensors. IEEE Trans. Intell. Transp. Syst. 2005, 6, 156–166. [Google Scholar] [CrossRef]
- Mynatt, E.D.; Rowan, J.; Craighill, S.; Jacobs, A. Digital family portraits: Supporting peace of mind for extended family members. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Seattle, WA, USA, March 31–April 5 2001; pp. 333–340. [Google Scholar]
- Mozer, M.C. 12 lessons from an adaptive home. In Smart Environments: Technologies, Protocols, and Applications; Boulder, CO, USA, 2005. [Google Scholar]
- Brumitt, B.; Cadiz, J. Let There Be Light! Comparing Interfaces for Homes of the Future; Technical report, MSR-TR-2000-92; Microsoft Research: Redmond, WA, USA, 2000. [Google Scholar]
- Jain, G.; Cook, D.; Jakkula, V. Monitoring Health by Detecting Drifts and Outliers for a Smart Environment Inhabitant; IOS Press: Belfast, UK, 2006. [Google Scholar]
- Consolvo, S.; Roessler, P.; Shelton, B.E.; LaMarca, A.; Schilit, B.; Bly, S. Technology for care networks of elders. IEEE Pervasive Comput. 2004, 3, 22–29. [Google Scholar] [CrossRef]
- Jakkula, V.R.; Cook, D.J.; Jain, G. Prediction models for a smart home based health care system. In Proceedings of the 21st International Conference on Advanced Information Networking and Applications Workshops (AINAW’07), Niagara Falls, ON, Canada, 21–23 May 2007; Volume 2, pp. 761–765. [Google Scholar]
- Baek, H.J.; Lee, H.B.; Kim, J.S.; Choi, J.M.; Kim, K.K.; Park, K.S. Nonintrusive biological signal monitoring in a car to evaluate a driver’s stress and health state. Telemed. e-Health 2009, 15, 182–189. [Google Scholar] [CrossRef]
- Yamamoto, K.; Toyoda, K.; Ohtsuki, T. Spectrogram-based non-contact RRI estimation by accurate peak detection algorithm. IEEE Access 2018, 6, 60369–60379. [Google Scholar] [CrossRef]
- Kamen, P.W.; Krum, H.; Tonkin, A.M. Poincare plot of heart rate variability allows quantitative display of parasympathetic nervous activity in humans. Clin. Sci. 1996, 91, 201–208. [Google Scholar] [CrossRef]
- Mourot, L.; Bouhaddi, M.; Perrey, S.; Rouillon, J.D.; Regnard, J. Quantitative Poincare plot analysis of heart rate variability: Effect of endurance training. Eur. J. Appl. Physiol. 2004, 91, 79–87. [Google Scholar] [CrossRef]
- Matsui, T.; Onishi, K.; Misaki, S.; Fujimoto, M.; Suwa, H.; Yasumoto, K. Salon: Simplified sensing system for activity of daily living in ordinary home. Sensors 2020, 20, 4895. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Luo, H.; Lee, P.A.; Clay, I.; Jaggi, M.; De Luca, V. Assessment of fatigue using wearable sensors: A pilot study. Digit. Biomarkers 2020, 4, 59–72. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.; Lee, H.Y.; Goh, H.; Abdigapporov, S.; Jeong, M.; Cho, H.; Han, K.; Noh, Y.; Lee, S.J.; et al. Prediction for Retrospection: Integrating Algorithmic Stress Prediction into Personal Informatics Systems for College Students’ Mental Health. In Proceedings of the 2022 CHI Conference on Human Factors in Computing Systems, New Orleans, LA, USA, 29 April–5 May 2022; Association for Computing Machinery: New York, NY, USA, 2022. [Google Scholar] [CrossRef]









| Time | Activity |
|---|---|
| –7:00 | Sleeping |
| 7:30–8:00 | Cooking |
| 8:00–8:30 | Eating Meals |
| 8:30–9:00 | Resting |
| 9:00–18:00 | Going Out |
| 18:00–18:30 | Cooking |
| 18:30–19:30 | Eating Meals |
| 19:30–21:00 | Resting |
| 21:00–21:30 | Bathing |
| 21:30–23:00 | Resting |
| 23:00– | Sleeping |
| Activity | RRI Variance | LP Area |
|---|---|---|
| Sleeping | 0.8 | 1.0 |
| Cooking | 0.3 | 0.5 |
| Eating Meals | 0.2 | 0.4 |
| Resting | 0.3 | 0.3 |
| Going out | 0.1 | 0.3 |
| Bathing | 0.5 | 0.7 |
| Sleep | 0.9 | 0.9 |
| Activity | Value |
|---|---|
| Bathing | 1.13 |
| Cooking | 1.21 |
| Eating | 0.67 |
| Going out | 0.80 |
| Sleeping | 0.95 |
| Other | 1.10 |
| Activity | Value |
|---|---|
| Bathing | 0.40 |
| Cooking | 1.84 |
| Eating | 0.66 |
| Going out | 0.71 |
| Sleeping | 1.11 |
| Other | 1.14 |
| Baseline 1 | Baseline 2 | Previous Method | Proposed Method 1 | Proposed Method 2 | |
|---|---|---|---|---|---|
| Basic features | X | X | X | X | X |
| Sleep duration | X | X | X | X | |
| Lorenz plot area per activity | X | X | X | ||
| Lorenz plot area per activity for 4 h after waking up | X | X | X | ||
| Time per activity | X | X | |||
| Time per activity for 4 h after waking up | X | X | |||
| Mixed indicator multiplied by Lorenz plot area per activity | X | ||||
| Mixed indicator multiplied by time per activity | X |
| Not Manipulated | OverSampler | UnderSampler | UnderSampler and Bagging | Over, UnderSampler, and Bagging | |
|---|---|---|---|---|---|
| Accuracy | 0.65 | 0.65 | 0.46 | 0.44 | 0.58 |
| F1-measure | |||||
| Bad | 0.27 | 0.37 | 0.41 | 0.43 | 0.47 |
| Neutral | 0.24 | 0.37 | 0.38 | 0.35 | 0.38 |
| Good | 0.76 | 0.75 | 0.54 | 0.49 | 0.66 |
| Baseline 1 | Baseline 2 | Previous Method | Proposed Method 1 | Proposed Method 2 | |
|---|---|---|---|---|---|
| MQ: Morning physical stress | 0.49 | 0.49 | 0.54 | 0.55 | 0.56 |
| NQ: Nighttime physical stress | 0.55 | 0.57 | 0.61 | 0.64 | 0.62 |
| Mean | 0.52 | 0.55 | 0.57 | 0.60 | 0.59 |
| Baseline 1 | Baseline 2 | Previous Method | Proposed Method 1 | Proposed Method 2 | Support | |
|---|---|---|---|---|---|---|
| Accuracy | 0.49 | 0.53 | 0.52 | 0.55 | 0.56 | 143 |
| ine Recall | ||||||
| Bad | 0.35 | 0.05 | 0.15 | 0.14 | 0.23 | 21 |
| Neutral | 0.38 | 0.42 | 0.39 | 0.33 | 0.42 | 36 |
| Good | 0.66 | 0.61 | 0.67 | 0.74 | 0.70 | 83 |
| ine F1-measure | ||||||
| Bad | 0.36 | 0.04 | 0.16 | 0.23 | 0.45 | 21 |
| Neutral | 0.34 | 0.38 | 0.38 | 0.36 | 0.44 | 36 |
| Good | 0.66 | 0.64 | 0.67 | 0.71 | 0.69 | 83 |
| Baseline 1 | Baseline 2 | Previous Method | Proposed Method 1 | Proposed Method 2 | Support | |
|---|---|---|---|---|---|---|
| Accuracy | 0.55 | 0.57 | 0.61 | 0.64 | 0.62 | 157 |
| ine Recall | ||||||
| Bad | 0.56 | 0.54 | 0.53 | 0.60 | 0.64 | 55 |
| Neutral | 0.42 | 0.42 | 0.58 | 0.32 | 0.32 | 19 |
| Good | 0.57 | 0.61 | 0.67 | 0.73 | 0.69 | 83 |
| ine F1-measure | ||||||
| Bad | 0.56 | 0.60 | 0.38 | 0.66 | 0.67 | 55 |
| Neutral | 0.32 | 0.31 | 0.42 | 0.26 | 0.28 | 19 |
| Good | 0.61 | 0.65 | 0.68 | 0.73 | 0.68 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, K.; Matsui, T.; Suwa, H.; Yasumoto, K. Stress Estimation Using Biometric and Activity Indicators to Improve QoL of the Elderly. Sensors 2023, 23, 535. https://doi.org/10.3390/s23010535
Matsumoto K, Matsui T, Suwa H, Yasumoto K. Stress Estimation Using Biometric and Activity Indicators to Improve QoL of the Elderly. Sensors. 2023; 23(1):535. https://doi.org/10.3390/s23010535
Chicago/Turabian StyleMatsumoto, Kanta, Tomokazu Matsui, Hirohiko Suwa, and Keiichi Yasumoto. 2023. "Stress Estimation Using Biometric and Activity Indicators to Improve QoL of the Elderly" Sensors 23, no. 1: 535. https://doi.org/10.3390/s23010535
APA StyleMatsumoto, K., Matsui, T., Suwa, H., & Yasumoto, K. (2023). Stress Estimation Using Biometric and Activity Indicators to Improve QoL of the Elderly. Sensors, 23(1), 535. https://doi.org/10.3390/s23010535









