Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex Increases Posterior Theta Rhythm and Reduces Latency of Motor Imagery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
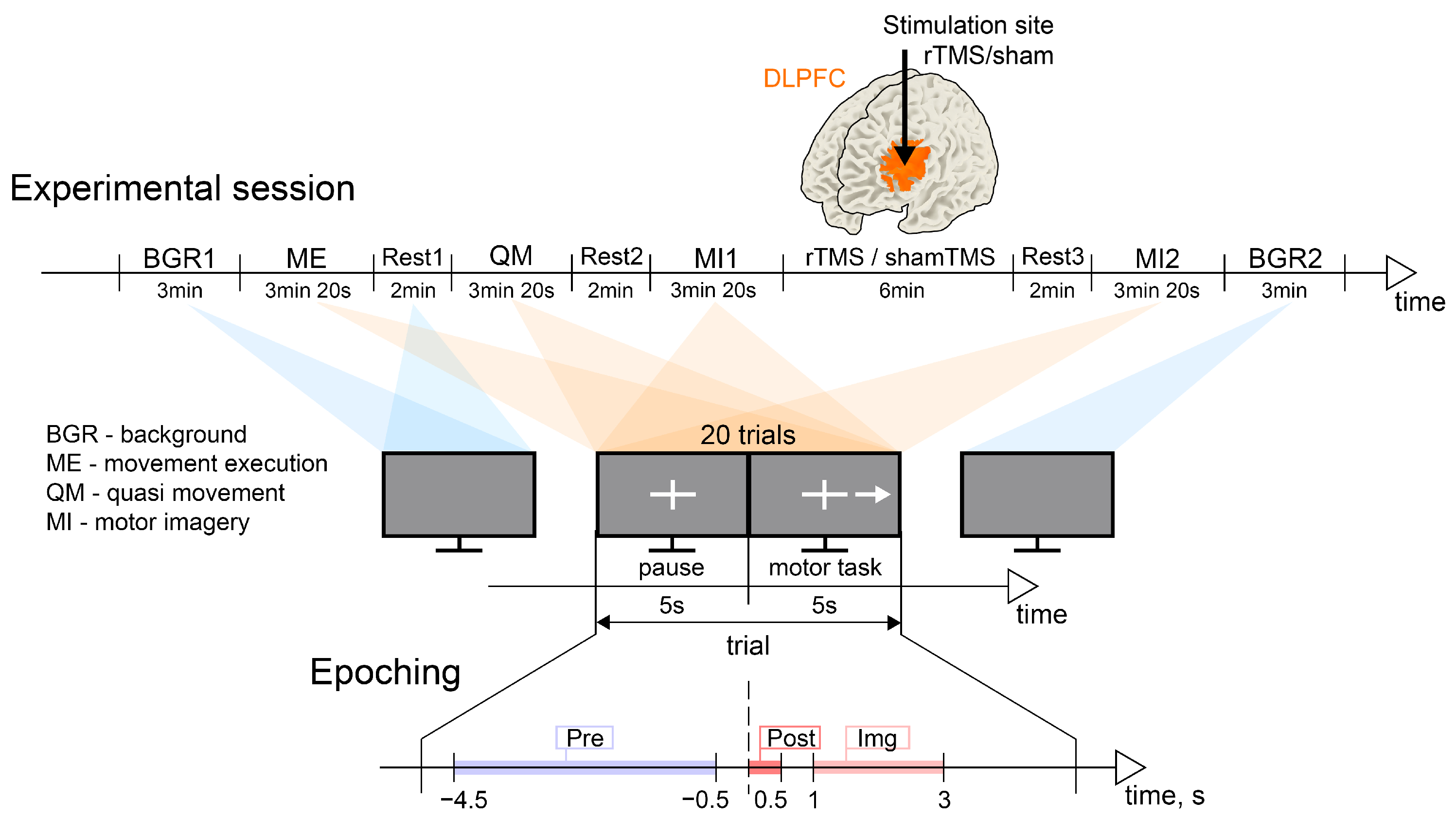
2.2. Experimental Design
2.3. Experimental Equipment
2.3.1. Electroencephalography Recording
2.3.2. Repetitive Transcranial Magnetic Stimulation
2.3.3. Electromyography Recordings
2.4. Data Analysis
2.4.1. EEG Preprocessing
2.4.2. EEG Data Epoching
2.4.3. Experimental Conditions
2.4.4. Sensor-Level Analysis
2.4.5. Estimation of the MI Brain Response Time
2.4.6. Source Reconstruction
2.4.7. Definition of ROIs
2.4.8. Connectivity Analysis
2.5. Statistical Analysis
3. Results
3.1. Neural Substrates of MI
3.2. Neural Substrates Induced by TMS before MI
3.3. Similarity between Brain Patterns of Sensorimotor Integration and Preactivation with TMS
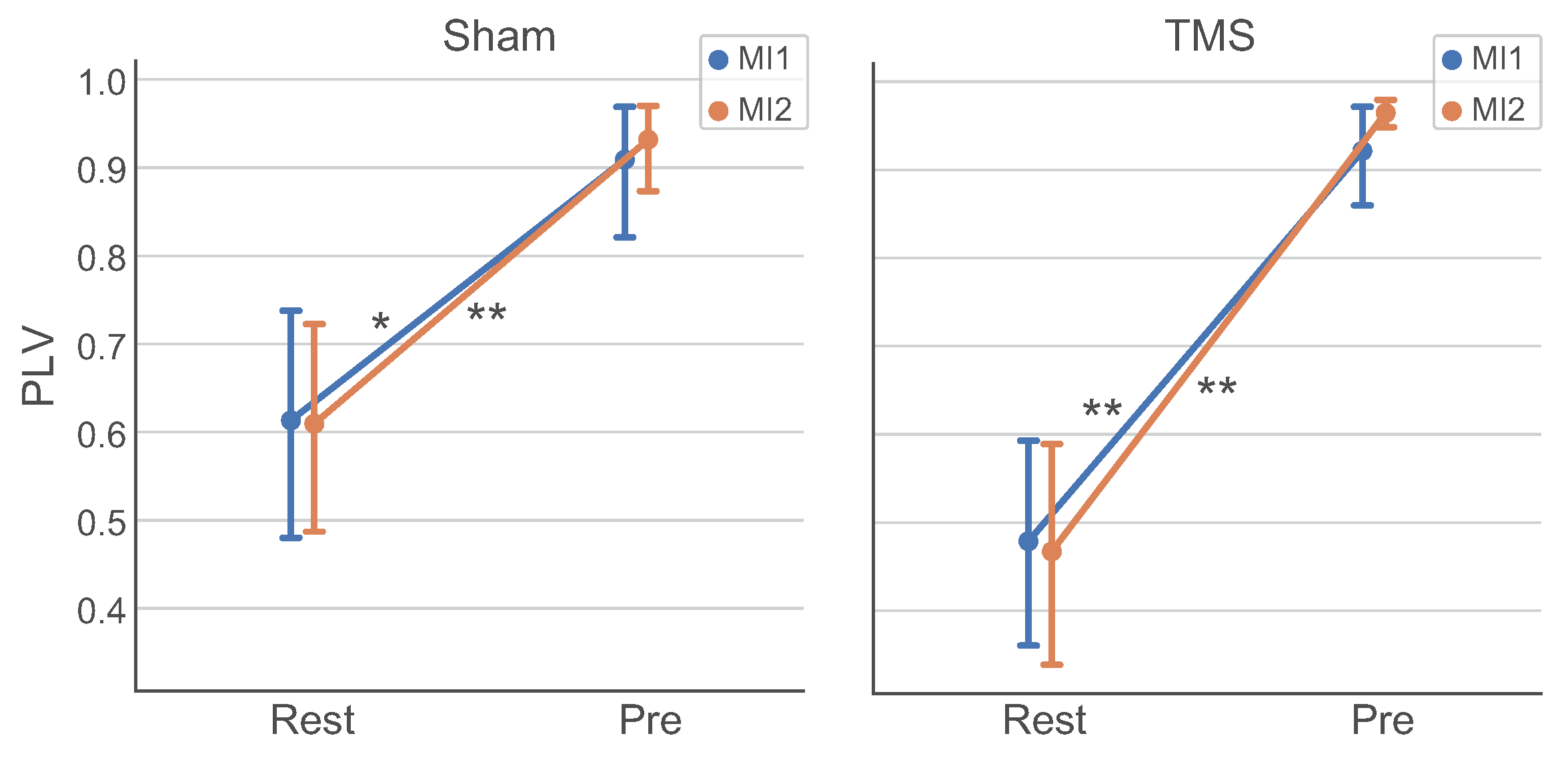
3.4. Analysis of Connectivity between ROIs
3.5. Effect of TMS on MI Brain Response Time
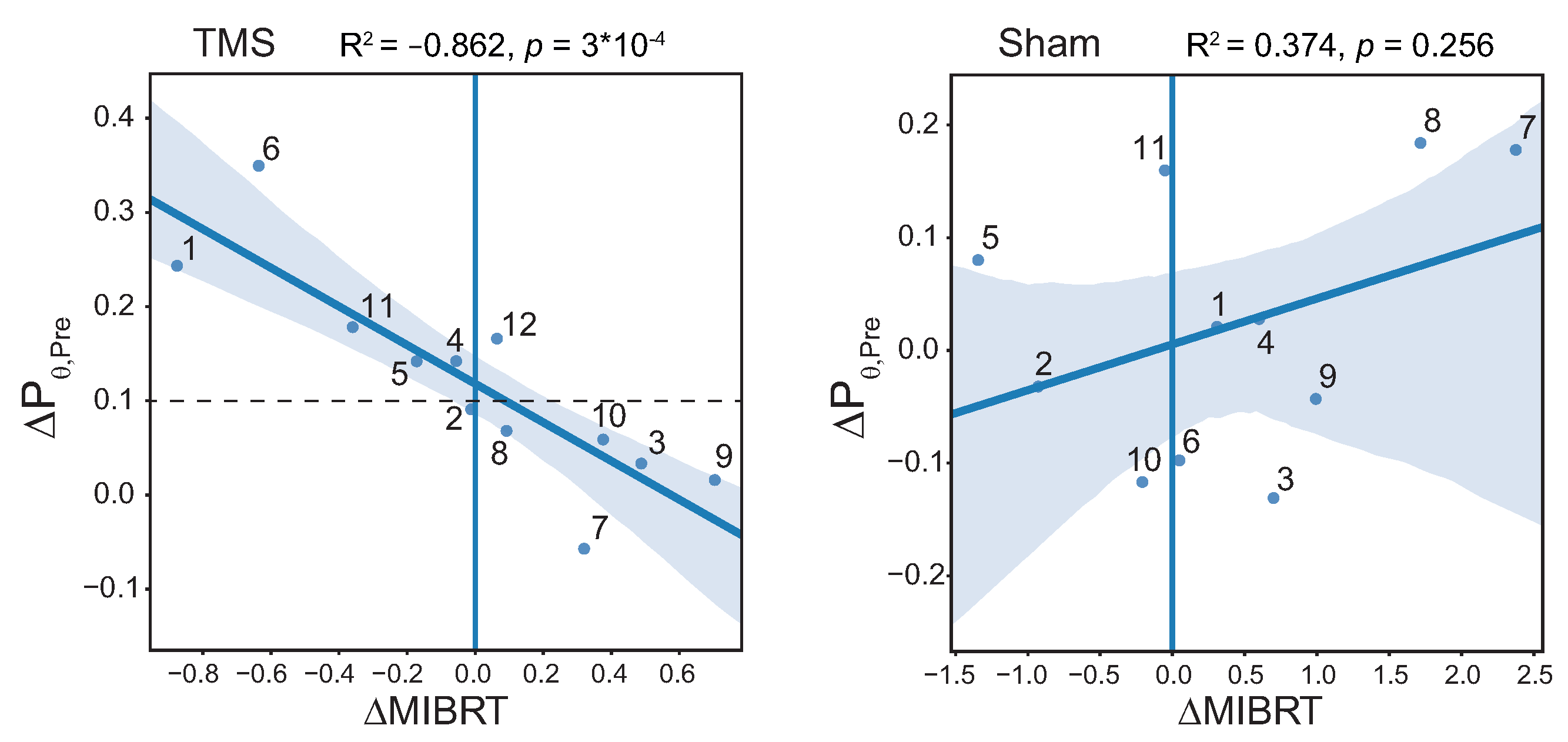
3.6. Correlation between MIBRT and Level of Brain Preactivation with TMS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EEG | Electroencephalogram |
| DLPFC | Dorsolateral prefrontal cortex |
| MI | Motor imagery |
| rTMS | repetitive Transcranial magnetic stimulation |
| M1 | Primary motor cortex |
| SMA | Supplementary motor area |
| PMd | Dorsal premotor cortex |
| CEN | Central executive network |
| DMN | Default mode network |
| ERD | Event-related desynchronization |
| SD | Standard deviation |
| ME | Motor execution |
| QM | Quasi-movement |
| EMG | Electromyography |
| TOI | Time interval of interest |
| ROI | Region of interest |
| RMT | Resting motor threshold |
| MIBRT | MI brain response time |
| ICA | Independent component analysis |
| PLV | Phase-locking value |
| BCI | Brain-computer interface |
References
- Decety, J. The neurophysiological basis of motor imagery. Behav. Brain Res. 1996, 77, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Lafleur, M.; Malouin, F.; Richards, C.; Doyon, J. Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch. Phys. Med. Rehabil. 2001, 82, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Pomeroy, V.M.; Baron, J.C. Motor imagery: A backdoor to the motor system after stroke? Stroke 2006, 37, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Mulder, T. Motor imagery and action observation: Cognitive tools for rehabilitation. J. Neural Transm. 2007, 114, 1265–1278. [Google Scholar] [CrossRef]
- Eaves, D.L.; Riach, M.; Holmes, P.S.; Wright, D.J. Motor Imagery during Action Observation: A Brief Review of Evidence, Theory and Future Research Opportunities. Front. Neurosci. 2016, 10, 514. [Google Scholar] [CrossRef]
- Bello, U.M.; Winser, S.J.; Chan, C.C. Role of kinaesthetic motor imagery in mirror-induced visual illusion as intervention in post-stroke rehabilitation. Rev. Neurosci. 2020, 31, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Grigorev, N.A.; Savosenkov, A.O.; Lukoyanov, M.V.; Udoratina, A.; Shusharina, N.N.; Kaplan, A.Y.; Hramov, A.E.; Kazantsev, V.B.; Gordleeva, S. A bci-based vibrotactile neurofeedback training improves motor cortical excitability during motor imagery. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1583–1592. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C. Construction of the Motor Imagery Integrative Model in Sport: A review and theoretical investigation of motor imagery use. Int. Rev. Sport Exerc. Psychol. 2008, 1, 31–44. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L. Mental practice for relearning locomotor skills. Phys. Ther. 2010, 90, 240–251. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Ruffino, C.; Lebon, F. Motor imagery and cortico-spinal excitability: A review. Eur. J. Sport Sci. 2015, 16, 317–324. [Google Scholar] [CrossRef]
- Ruffino, C.; Papaxanthis, C.; Lebon, F. Neural plasticity during motor learning with motor imagery practice: Review and perspectives. Neuroscience 2017, 341, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Ladda, A.M.; Lebon, F.; Lotze, M. Using motor imagery practice for improving motor performance—A review. Brain Cogn. 2021, 150, 105705. [Google Scholar] [CrossRef]
- Gerardin, E.; Sirigu, A.; Lehéricy, S.; Poline, J.B.; Gaymard, B.; Marsault, C.; Agid, Y.; Bihan, D.L. Partially overlapping neural networks for real and imagined hand movements. Cereb. Cortex 2000, 10, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.P.; Eugène, F.; Michon, P.E.; Jackson, P.L. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Chepurova, A.; Hramov, A.; Kurkin, S. Motor imagery: How to assess, improve its performance, and apply it for psychosis diagnostics. Diagnostics 2022, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.; Glover, S. TMS over dorsolateral prefrontal cortex affects the timing of motor imagery but not overt action: Further support for the motor-cognitive model. Behav. Brain Res. 2023, 437, 114125. [Google Scholar] [CrossRef] [PubMed]
- Zanto, T.P.; Rubens, M.T.; Thangavel, A.; Gazzaley, A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat. Neurosci. 2011, 14, 656–661. [Google Scholar] [CrossRef]
- Rottschy, C.; Langner, R.; Dogan, I.; Reetz, K.; Laird, A.R.; Schulz, J.B.; Fox, P.T.; Eickhoff, S.B. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage 2012, 60, 830–846. [Google Scholar] [CrossRef]
- Mars, R.B.; Grol, M.J. Dorsolateral prefrontal cortex, working memory, and prospective coding for action. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 1801–1802. [Google Scholar] [CrossRef]
- Blasi, G.; Goldberg, T.E.; Weickert, T.; Das, S.; Kohn, P.; Zoltick, B.; Bertolino, A.; Callicott, J.H.; Weinberger, D.R.; Mattay, V.S. Brain regions underlying response inhibition and interference monitoring and suppression. Eur. J. Neurosci. 2006, 23, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Coxon, J.P.; Goble, D.J.; Leunissen, I.; Impe, A.V.; Wenderoth, N.; Swinnen, S.P. Functional Brain Activation Associated with Inhibitory Control Deficits in Older Adults. Cereb. Cortex 2016, 26, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Nigel, R.; Daskalakis, Z.; Fitzgerald, P. The relationship between dorsolateral prefrontal cortical inhibition and working memory performance: A combined TMS-EEG study. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Stephan, K.M.; Fink, G.R.; Passingham, R.E.; Silbersweig, D.; Ceballos-Baumann, A.O.; Frith, C.D.; Frackowiak, R.S. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J. Neurophysiol. 1995, 73, 372–386. [Google Scholar] [CrossRef]
- Dodakian, L.; Stewart, J.C.; Cramer, S.C. Motor imagery during movement activates the brain more than movement alone after stroke: A pilot study. J. Rehabil. Med. 2014, 46, 843–848. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C.; Nguyen, V.A.; Malouin, F.; Richards, C.; Doyon, J. Functional neuroanatomical networks associated with expertise in motor imagery. Neuroimage 2008, 41, 1471–1483. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C.; Nguyen, V.A.; Malouin, F.; Richards, C.; Doyon, J. Brain activity during visual versus kinesthetic imagery: An fMRI study. Hum. Brain Mapp. 2009, 30, 2157–2172. [Google Scholar] [CrossRef]
- Lotze, M.; Scheler, G.; Tan, H.R.M.; Braun, C.; Birbaumer, N. The musician’s brain: Functional imaging of amateurs and professionals during performance and imagery. Neuroimage 2003, 20, 1817–1829. [Google Scholar] [CrossRef]
- Macuga, K.L.; Frey, S.H. Differential contributions of the superior and inferior parietal cortex to feedback versus feedforward control of tools. Neuroimage 2014, 92, 36–45. [Google Scholar] [CrossRef]
- Tacchino, A.; Saiote, C.; Brichetto, G.; Bommarito, G.; Roccatagliata, L.; Cordano, C.; Battaglia, M.A.; Mancardi, G.L.; Inglese, M. Motor Imagery as a Function of Disease Severity in Multiple Sclerosis: An fMRI Study. Front. Hum. Neurosci. 2018, 11, 628. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, E.; Lee, A.; Im, C.H.; Kim, Y.H. Changes in network connectivity during motor imagery and execution. PLoS ONE 2018, 13, e0190715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, T.; Li, F.; Li, M.; Liu, D.; Zhang, R.; He, H.; Li, P.; Gong, J.; Luo, C.; et al. Structural and functional correlates of motor imagery BCI performance: Insights from the patterns of fronto-parietal attention network. Neuroimage 2016, 134, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Yoon, J.G.; Lee, S.W. Predicting Motor Imagery Performance From Resting-State EEG Using Dynamic Causal Modeling. Front. Hum. Neurosci. 2020, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, N.; Nakata, H.; Hayashi, T.; Sakamoto, M.; Muraoka, T.; Uchida, Y.; Kanosue, K. Brain activity during motor imagery of an action with an object: A functional magnetic resonance imaging study. Neurosci. Res. 2013, 76, 150–155. [Google Scholar] [CrossRef]
- Silvanto, J.; Muggleton, N.G. New light through old windows: Moving beyond the “virtual lesion” approach to transcranial magnetic stimulation. Neuroimage 2008, 39, 549–552. [Google Scholar] [CrossRef]
- Walsh, V.; Cowey, A. Transcranial magnetic stimulation and cognitive neuroscience. Nat. Rev. Neurosci. 2000, 1, 73–80. [Google Scholar] [CrossRef]
- Fadiga, L.; Buccino, G.; Craighero, L.; Fogassi, L.; Gallese, V.; Pavesi, G. Corticospinal excitability is specifically modulated by motor imagery: A magnetic stimulation study. Neuropsychologia 1998, 37, 147–158. [Google Scholar] [CrossRef]
- Stinear, C.M.; Byblow, W.D.; Steyvers, M.; Levin, O.; Swinnen, S.P. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp. Brain Res. 2006, 168, 157–164. [Google Scholar] [CrossRef]
- Guillot, A.; Di Rienzo, F.; MacIntyre, T.; Moran, A.; Collet, C. Imagining is not doing but involves specific motor commands: A review of experimental data related to motor inhibition. Front. Hum. Neurosci. 2012, 6, 247. [Google Scholar] [CrossRef]
- Solomon, J.P.; Kraeutner, S.N.; O’Neil, K.; Boe, S.G. Examining the role of the supplementary motor area in motor imagery-based skill acquisition. Exp. Brain Res. 2021, 239, 3649–3659. [Google Scholar] [CrossRef]
- Cona, G.; Marino, G.; Semenza, C. TMS of supplementary motor area (SMA) facilitates mental rotation performance: Evidence for sequence processing in SMA. Neuroimage 2017, 144, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Cona, G.; Panozzo, G.; Semenza, C. The role of dorsal premotor cortex in mental rotation: A transcranial magnetic stimulation study. Brain Cogn. 2017, 116, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Oldrati, V.; Finisguerra, A.; Avenanti, A.; Aglioti, S.M.; Urgesi, C. Differential influence of the dorsal premotor and primary somatosensory cortex on corticospinal excitability during kinesthetic and visual motor imagery: A low-frequency repetitive transcranial magnetic stimulation study. Brain Sci. 2021, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.K.; Stinear, C.M.; Byblow, W.D. Bilateral parietal cortex function during motor imagery. Exp. Brain Res. 2010, 201, 499–508. [Google Scholar] [CrossRef]
- Kraeutner, S.N.; Keeler, L.T.; Boe, S.G. Motor imagery-based skill acquisition disrupted following rTMS of the inferior parietal lobule. Exp. Brain Res. 2016, 234, 397–407. [Google Scholar] [CrossRef]
- Kraeutner, S.N.; El-Serafi, M.; Lee, J.; Boe, S.G. Disruption of motor imagery performance following inhibition of the left inferior parietal lobe. Neuropsychologia 2019, 127, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C. Motor imagery activates primary sensorimotor area in humans. Neurosci. Lett. 1997, 239, 65–68. [Google Scholar] [CrossRef]
- Chholak, P.; Niso, G.; Maksimenko, V.A.; Kurkin, S.A.; Frolov, N.S.; Pitsik, E.N.; Hramov, A.E.; Pisarchik, A.N. Visual and kinesthetic modes affect motor imagery classification in untrained subjects. Sci. Rep. 2019, 9, 9838. [Google Scholar] [CrossRef]
- Oldfield, R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Rossini, P.; Burke, D.; Chen, R.; Cohen, L.; Daskalakis, Z.; Iorio, R.D.; Lazzaro, V.D.; Ferreri, F.; Fitzgerald, P.; George, M.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.J.; Hoge, R.; Collins, L.; Woods, R.; Toga, A.W.; Evans, A.C. Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 1998, 22, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Mylius, V.; Ayache, S.; Ahdab, R.; Farhat, W.; Zouari, H.; Belke, M.; Brugières, P.; Wehrmann, E.; Krakow, K.; Timmesfeld, N.; et al. Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: Inter-rater reliability, accuracy, and influence of gender and age. Neuroimage 2013, 78, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Smittenaar, P.; FitzGerald, T.H.; Romei, V.; Wright, N.D.; Dolan, R.J. Disruption of dorsolateral prefrontal cortex decreases model-based in favor of model-free control in humans. Neuron 2013, 80, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Halko, M.A.; Eldaief, M.C.; Pascual-Leone, A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 2012, 62, 2232–2243. [Google Scholar] [CrossRef]
- Rossini, P.; Barker, A.; Berardelli, A.; Caramia, M.; Caruso, G.; Cracco, R.; Dimitrijević, M.; Hallett, M.; Katayama, Y.; Lücking, C.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Rossi, S.; Cappa, S.F.; Babiloni, C.; Pasqualetti, P.; Miniussi, C.; Carducci, F.; Babiloni, F.; Rossini, P.M. Prefontal cortex in long-term memory: An “interference” approach using magnetic stimulation. Nat. Neurosci. 2001, 4, 948–952. [Google Scholar] [CrossRef]
- Esser, S.; Huber, R.; Massimini, M.; Peterson, M.; Ferrarelli, F.; Tononi, G. A direct demonstration of cortical LTP in humans: A combined TMS/EEG study. Brain Res. Bull. 2006, 69, 86–94. [Google Scholar] [CrossRef]
- Matsunaga, K.; Maruyama, A.; Fujiwara, T.; Nakanishi, R.; Tsuji, S.; Rothwell, J.C. Increased corticospinal excitability after 5 Hz rTMS over the human supplementary motor area. J. Physiol. 2004, 562, 295–306. [Google Scholar] [CrossRef]
- Rizzo, V.; Siebner, H.R.; Modugno, N.; Pesenti, A.; Münchau, A.; Gerschlager, W.; Webb, R.M.; Rothwell, J.C. Shaping the excitability of human motor cortex with premotor rTMS. J. Physiol. 2004, 554, 483–495. [Google Scholar] [CrossRef]
- Berardelli, A.; Inghilleri, M.; Rothwell, J.C.; Romeo, S.; Currà, A.; Gilio, F.; Modugno, N.; Manfredi, M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp. Brain Res. 1998, 122, 79–84. [Google Scholar] [CrossRef]
- Thut, G.; Pascual-Leone, A. A Review of Combined TMS-EEG Studies to Characterize Lasting Effects of Repetitive TMS and Assess Their Usefulness in Cognitive and Clinical Neuroscience. Brain Topogr. 2009, 22, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, E.M. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1998, 108, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Mishory, A.; Nahas, Z.; Borckardt, J.J.; Yamanaka, K.; Rastogi, K.; George, M.S. Tolerability and Safety of High Daily Doses of Repetitive Transcranial Magnetic Stimulation in Healthy Young Men. J. ECT 2006, 22, 49–53. [Google Scholar] [CrossRef]
- Machii, K.; Cohen, D.; Ramos-Estebanez, C.; Pascual-Leone, A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin. Neurophysiol. 2006, 117, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Chang, D.; Zhang, J.; Peng, W.; Song, D.; Gao, X.; Wang, Z. Theta-burst transcranial magnetic stimulation induced functional connectivity changes between dorsolateral prefrontal cortex and default-mode-network. Brain Imaging Behav. 2020, 14, 1955–1963. [Google Scholar] [CrossRef]
- Izuma, K.; Akula, S.; Murayama, K.; Wu, D.A.; Iacoboni, M.; Adolphs, R. A causal role for posterior medial frontal cortex in choice-induced preference change. J. Neurosci. 2015, 35, 3598–3606. [Google Scholar] [CrossRef]
- Subasi, A. Practical Guide for Biomedical Signals Analysis Using Machine Learning Techniques: A MATLAB Based Approach; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef]
- Frolov, N.S.; Pitsik, E.N.; Maksimenko, V.A.; Grubov, V.V.; Kiselev, A.R.; Wang, Z.; Hramov, A.E. Age-related slowing down in the motor initiation in elderly adults. PLoS ONE 2020, 15, e0233942. [Google Scholar] [CrossRef]
- Pernet, C.; Garrido, M.I.; Gramfort, A.; Maurits, N.; Michel, C.M.; Pang, E.; Salmelin, R.; Schoffelen, J.M.; Valdes-Sosa, P.A.; Puce, A. Issues and recommendations from the OHBM COBIDAS MEEG committee for reproducible EEG and MEG research. Nat. Neurosci. 2020, 23, 1473–1483. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Brunner, C.; Schlögl, A.; Da Silva, F.L. Mu rhythm (de) synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage 2006, 31, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG-and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Zabielska-Mendyk, E.; Francuz, P.; Jaśkiewicz, M.; Augustynowicz, P. The effects of motor expertise on sensorimotor rhythm desynchronization during execution and imagery of sequential movements. Neuroscience 2018, 384, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, S.; Unenaka, S.; Shimada, S.; Ohki, Y. Distinct modulation of mu and beta rhythm desynchronization during observation of embodied fake hand rotation. Neuropsychologia 2021, 159, 107952. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: Exact, zero error localization. arXiv 2007, arXiv:0710.3341. [Google Scholar]
- Pascual-Marqui, R.D.; Pascual-Montano, A.D.; Lehmann, D.; Kochi, K.; Esslen, M.; Jancke, L.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; et al. Exact low resolution brain electromagnetic tomography (eLORETA). Neuroimage 2006, 31, S86. [Google Scholar]
- Pascual-Marqui, R.D. Theory of the EEG inverse problem. In Quantitative EEG Analysis: Methods and Clinical Applications; Artech House: Norwood, MA, USA, 2009; pp. 121–140. [Google Scholar] [CrossRef]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef]
- Baillet, S.; Mosher, J.C.; Leahy, R.M. Electromagnetic brain mapping. IEEE Signal Process. Mag. 2001, 18, 14–30. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Bastos, A.M.; Schoffelen, J.M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016, 9, 175. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Cruikshank, L.C.; Singhal, A.; Hueppelsheuser, M.; Caplan, J.B. Theta oscillations reflect a putative neural mechanism for human sensorimotor integration. J. Neurophysiol. 2012, 107, 65–77. [Google Scholar] [CrossRef]
- Tomassini, A.; Ambrogioni, L.; Medendorp, W.P.; Maris, E. Theta oscillations locked to intended actions rhythmically modulate perception. Elife 2017, 6, e25618. [Google Scholar] [CrossRef]
- Colgin, L.L. Mechanisms and functions of theta rhythms. Annu. Rev. Neurosci. 2013, 36, 295–312. [Google Scholar] [CrossRef]
- Başar, E. Theta Rhythms in Integrative Brain Function. In Brain Function and Oscillations; Springer: Berlin/Heidelberg, Germany, 1999; pp. 353–366. [Google Scholar]
- Kumar, J.; Iwabuchi, S.J.; Völlm, B.A.; Palaniyappan, L. Oxytocin modulates the effective connectivity between the precuneus and the dorsolateral prefrontal cortex. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 567–576. [Google Scholar] [CrossRef]
- Hervault, M.; Zanone, P.G.; Buisson, J.C.; Huys, R. Cortical sensorimotor activity in the execution and suppression of discrete and rhythmic movements. Sci. Rep. 2021, 11, 22364. [Google Scholar] [CrossRef]
- Jurkiewicz, M.T.; Gaetz, W.C.; Bostan, A.C.; Cheyne, D. Post-movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. Neuroimage 2006, 32, 1281–1289. [Google Scholar] [CrossRef]
- Panikratova, Y.R.; Vlasova, R.M.; Akhutina, T.V.; Korneev, A.A.; Sinitsyn, V.E.; Pechenkova, E.V. Functional connectivity of the dorsolateral prefrontal cortex contributes to different components of executive functions. Int. J. Psychophysiol. 2020, 151, 70–79. [Google Scholar] [CrossRef]
- van der Werf, Y.D.; Sanz-Arigita, E.J.; Menning, S.; van den Heuvel, O.A. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci. 2010, 11, 145. [Google Scholar] [CrossRef]
- Wu, X.; Ji, G.J.; Geng, Z.; Zhou, S.; Yan, Y.; Wei, L.; Qiu, B.; Tian, Y.; Wang, K. Strengthened theta-burst transcranial magnetic stimulation as an adjunctive treatment for Alzheimer’s disease: An open-label pilot study. Brain Stimul. 2020, 13, 484–486. [Google Scholar] [CrossRef]
- Northoff, G.; Heinzel, A.; de Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage 2006, 31, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering minds: The default network and stimulus-independent thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Mars, R.B.; Neubert, F.X.; Noonan, M.P.; Sallet, J.; Toni, I.; Rushworth, M.F.S. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 2012, 6, 189. [Google Scholar] [CrossRef]
- Xie, X.; Bratec, S.M.; Schmid, G.; Meng, C.; Doll, A.; Wohlschläger, A.; Finke, K.; Förstl, H.; Zimmer, C.; Pekrun, R.; et al. How do you make me feel better? Social cognitive emotion regulation and the default mode network. Neuroimage 2016, 134, 270–280. [Google Scholar] [CrossRef]
- Spreng, R.N.; Stevens, W.D.; Chamberlain, J.P.; Gilmore, A.W.; Schacter, D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 2010, 53, 303–317. [Google Scholar] [CrossRef]
- Beaty, R.E.; Benedek, M.; Kaufman, S.B.; Silvia, P.J. Default and Executive Network Coupling Supports Creative Idea Production. Sci. Rep. 2015, 5, 10964. [Google Scholar] [CrossRef]
- Glover, S.; Bibby, E.; Tuomi, E. Executive functions in motor imagery: Support for the motor-cognitive model over the functional equivalence model. Exp. Brain Res. 2020, 238, 931–944. [Google Scholar] [CrossRef]
- Scheeringa, R.; Bastiaansen, M.C.M.; Petersson, K.M.; Oostenveld, R.; Norris, D.G.; Hagoort, P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int. J. Psychophysiol. 2008, 67, 242–251. [Google Scholar] [CrossRef]
- White, T.P.; Jansen, M.; Doege, K.; Mullinger, K.J.; Park, S.B.; Liddle, E.B.; Gowland, P.A.; Francis, S.T.; Bowtell, R.; Liddle, P.F. Theta power during encoding predicts subsequent-memory performance and default mode network deactivation. Hum. Brain Mapp. 2013, 34, 2929–2943. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Schabus, M.; Doppelmayr, M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 2005, 57, 97–103. [Google Scholar] [CrossRef]
- Abellaneda-Pérez, K.; Vaqué-Alcázar, L.; Vidal-Piñeiro, D.; Jannati, A.; Solana, E.; Bargalló, N.; Santarnecchi, E.; Pascual-Leone, A.; Bartrés-Faz, D. Age-related differences in default-mode network connectivity in response to intermittent theta-burst stimulation and its relationships with maintained cognition and brain integrity in healthy aging. Neuroimage 2019, 188, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Bungert, A.; Bowtell, R.; Jackson, S.R. Modulating Brain Networks With Transcranial Magnetic Stimulation Over the Primary Motor Cortex: A Concurrent TMS/fMRI Study. Front. Hum. Neurosci. 2020, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Bilek, E.; Schäfer, A.; Ochs, E.; Esslinger, C.; Zangl, M.; Plichta, M.M.; Braun, U.; Kirsch, P.; Schulze, T.G.; Rietschel, M.; et al. Application of high-frequency repetitive transcranial magnetic stimulation to the DLPFC alters human prefrontal-hippocampal functional interaction. J. Neurosci. 2013, 33, 7050–7056. [Google Scholar] [CrossRef] [PubMed]
- Tik, M.; Hoffmann, A.; Sladky, R.; Tomova, L.; Hummer, A.; de Lara, L.N.; Bukowski, H.; Pripfl, J.; Biswal, B.; Lamm, C.; et al. Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage 2017, 162, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Oathes, D.J.; Chang, C.; Bradley, T.; Zhou, Z.W.; Williams, L.M.; Glover, G.H.; Deisseroth, K.; Etkin, A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 19944–19949. [Google Scholar] [CrossRef]
- Siebner, H.R.; Peller, M.; Willoch, F.; Minoshima, S.; Boecker, H.; Auer, C.; Drzezga, A.; Conrad, B.; Bartenstein, P. Lasting cortical activation after repetitive TMS of the motor cortex: A glucose metabolic study. Neurology 2000, 54, 956–963. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef]
- Thut, G.; Veniero, D.; Romei, V.; Miniussi, C.; Schyns, P.; Gross, J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr. Biol. 2011, 21, 1176–1185. [Google Scholar] [CrossRef]
- Daly, J.J.; Wolpaw, J.R. Brain–computer interfaces in neurological rehabilitation. Lancet Neurol. 2008, 7, 1032–1043. [Google Scholar] [CrossRef]
- Dimyan, M.A.; Cohen, L.G. Neuroplasticity in the context of motor rehabilitation after stroke. Nat. Rev. Neurol. 2011, 7, 76–85. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain–computer interface paradigms. J. Neural Eng. 2019, 16, 011001. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.A.; Mokienko, O.; Lyukmanov, R.; Biryukova, E.; Kotov, S.; Turbina, L.; Nadareyshvily, G.; Bushkova, Y. Post-stroke rehabilitation training with a motor-imagery-based brain-computer interface (BCI)-controlled hand exoskeleton: A randomized controlled multicenter trial. Front. Neurosci. 2017, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, M.A.; Nicolelis, M.A. Brain-machine interfaces: From basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 2017, 97, 767–837. [Google Scholar] [CrossRef] [PubMed]
- Liburkina, S.; Vasilyev, A.; Yakovlev, L.; Gordleeva, S.Y.; Kaplan, A.Y. A motor imagery-based brain–computer interface with vibrotactile stimuli. Neurosci. Behav. Physiol. 2018, 48, 1067–1077. [Google Scholar] [CrossRef]
- Lukoyanov, M.; Gordleeva, S.Y.; Pimashkin, A.; Grigor’ev, N.; Savosenkov, A.; Motailo, A.; Kazantsev, V.; Kaplan, A.Y. The efficiency of the brain-computer interfaces based on motor imagery with tactile and visual feedback. Hum. Physiol. 2018, 44, 280–288. [Google Scholar] [CrossRef]



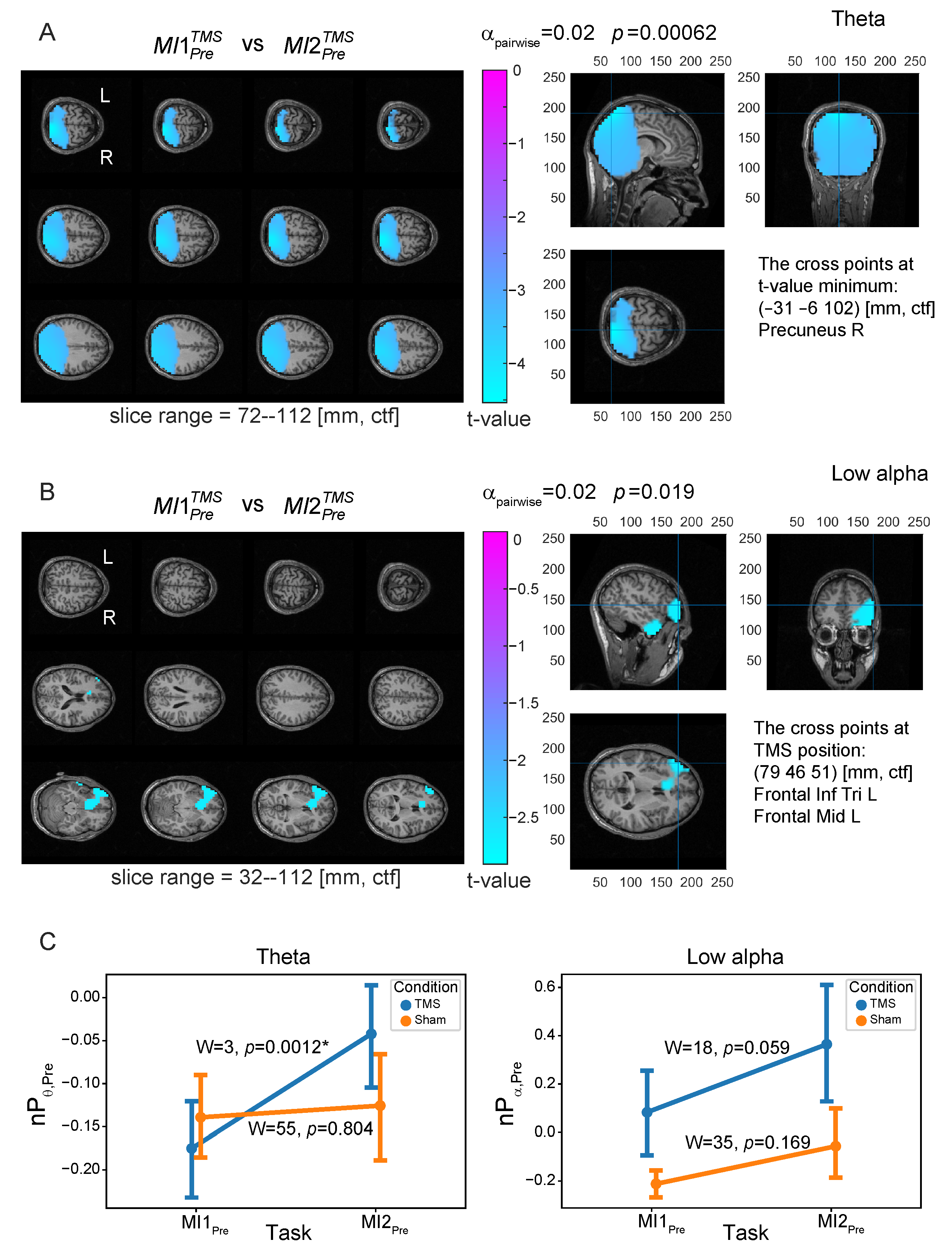
| Frequency Band | Condition 1 | Condition 2 | Significance | ROI, |
|---|---|---|---|---|
| CTF Coordinates (mm) | ||||
| theta | Rest2 | Rest3 | n.s. | – |
| , | PrecuneusR, | |||
| (−31, −6, 102) | ||||
| n.s. | – | |||
| n.s. | – | |||
| low alpha | Rest2 | Rest3 | n.s. | – |
| , | left DLPFC, | |||
| (79, 46, 51) | ||||
| n.s. | – | |||
| n.s. | – | |||
| high alpha | Rest2 | Rest3 | n.s. | – |
| n.s. | – | |||
| n.s. | – | |||
| n.s. | – | |||
| beta | Rest2 | Rest3 | n.s. | – |
| n.s. | – | |||
| n.s. | – | |||
| n.s. | – |
| Condition | Task 1/Group Mean | Task 2/Group Mean | W-Value | |
|---|---|---|---|---|
| MIBRT ± SE, s | MIBRT ± SE, s | |||
| Sham | MI1/1.56 ± 0.28 | MI2/1.69 ± 0.26 | 21 | 0.64 |
| TMS | MI1/1.36 ± 0.18 | MI2/1.18 ± 0.14 | 37 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurkin, S.; Gordleeva, S.; Savosenkov, A.; Grigorev, N.; Smirnov, N.; Grubov, V.V.; Udoratina, A.; Maksimenko, V.; Kazantsev, V.; Hramov, A.E. Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex Increases Posterior Theta Rhythm and Reduces Latency of Motor Imagery. Sensors 2023, 23, 4661. https://doi.org/10.3390/s23104661
Kurkin S, Gordleeva S, Savosenkov A, Grigorev N, Smirnov N, Grubov VV, Udoratina A, Maksimenko V, Kazantsev V, Hramov AE. Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex Increases Posterior Theta Rhythm and Reduces Latency of Motor Imagery. Sensors. 2023; 23(10):4661. https://doi.org/10.3390/s23104661
Chicago/Turabian StyleKurkin, Semen, Susanna Gordleeva, Andrey Savosenkov, Nikita Grigorev, Nikita Smirnov, Vadim V. Grubov, Anna Udoratina, Vladimir Maksimenko, Victor Kazantsev, and Alexander E. Hramov. 2023. "Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex Increases Posterior Theta Rhythm and Reduces Latency of Motor Imagery" Sensors 23, no. 10: 4661. https://doi.org/10.3390/s23104661
APA StyleKurkin, S., Gordleeva, S., Savosenkov, A., Grigorev, N., Smirnov, N., Grubov, V. V., Udoratina, A., Maksimenko, V., Kazantsev, V., & Hramov, A. E. (2023). Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex Increases Posterior Theta Rhythm and Reduces Latency of Motor Imagery. Sensors, 23(10), 4661. https://doi.org/10.3390/s23104661












