Methodological Considerations for Assessing Automatic Brightness Control in Endoscopy: Experimental Study
Abstract
:1. Introduction
1.1. Related Work
1.2. Proposed Work
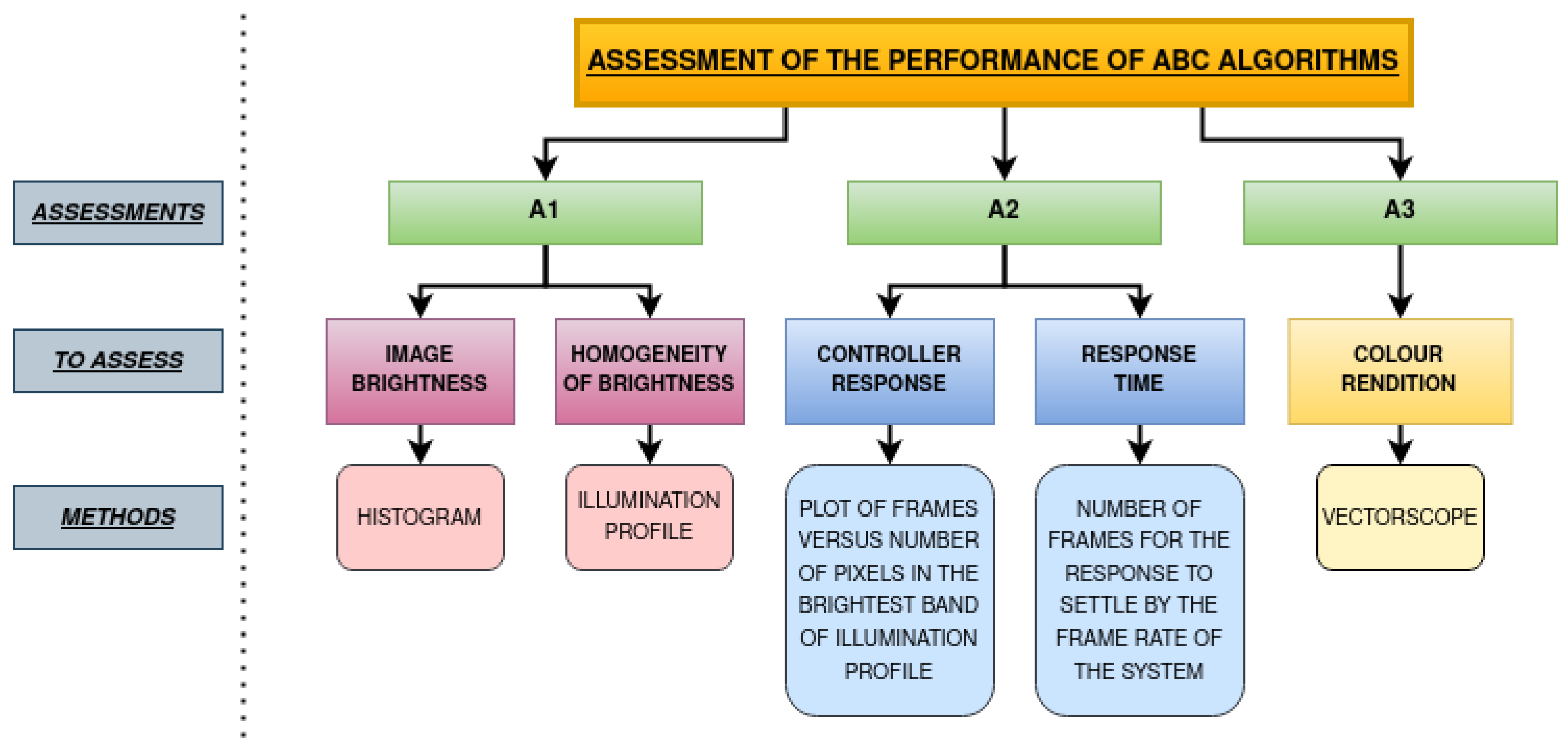
2. Methodology
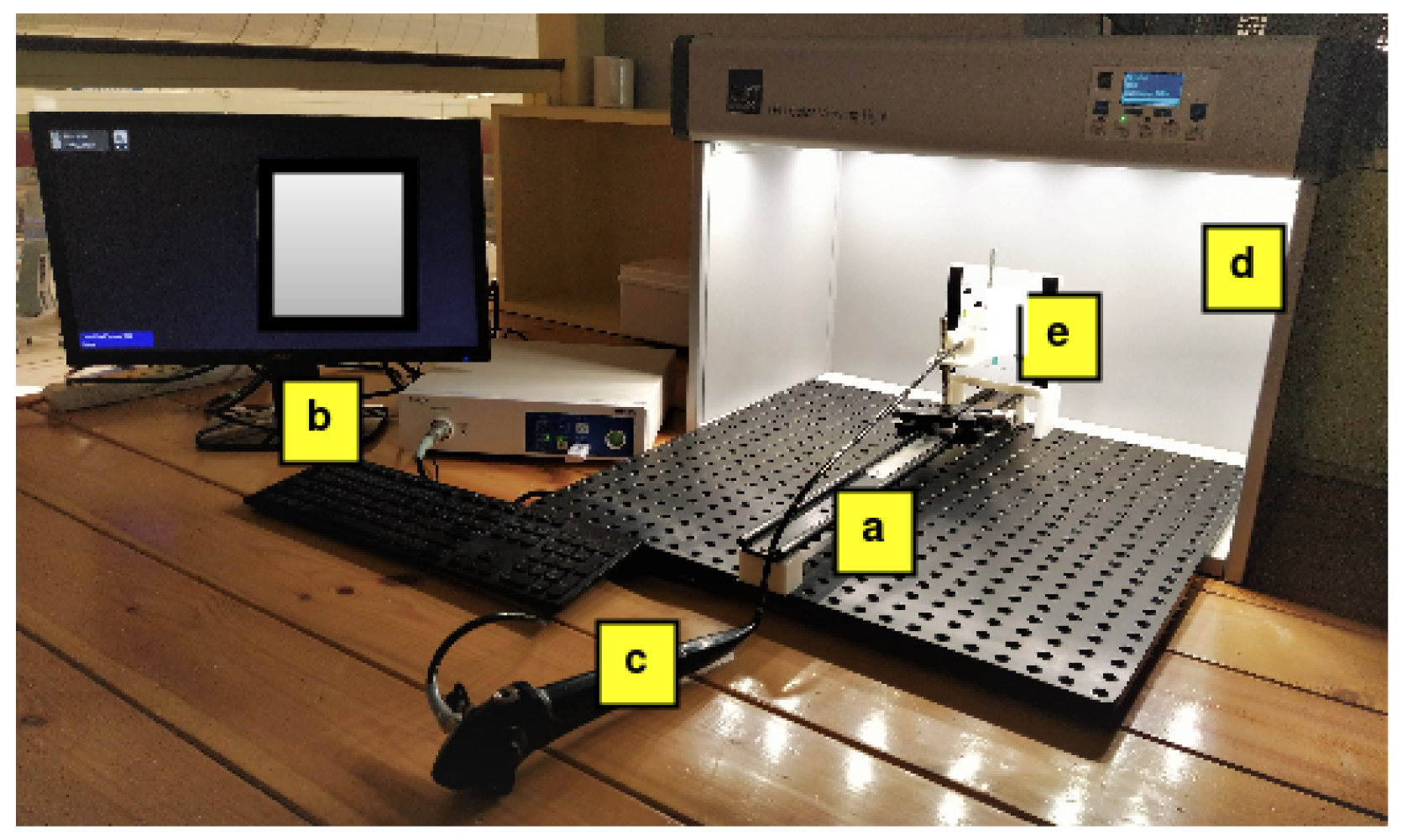
2.1. Experimental Setup
2.1.1. ImageLab
2.1.2. Test Charts
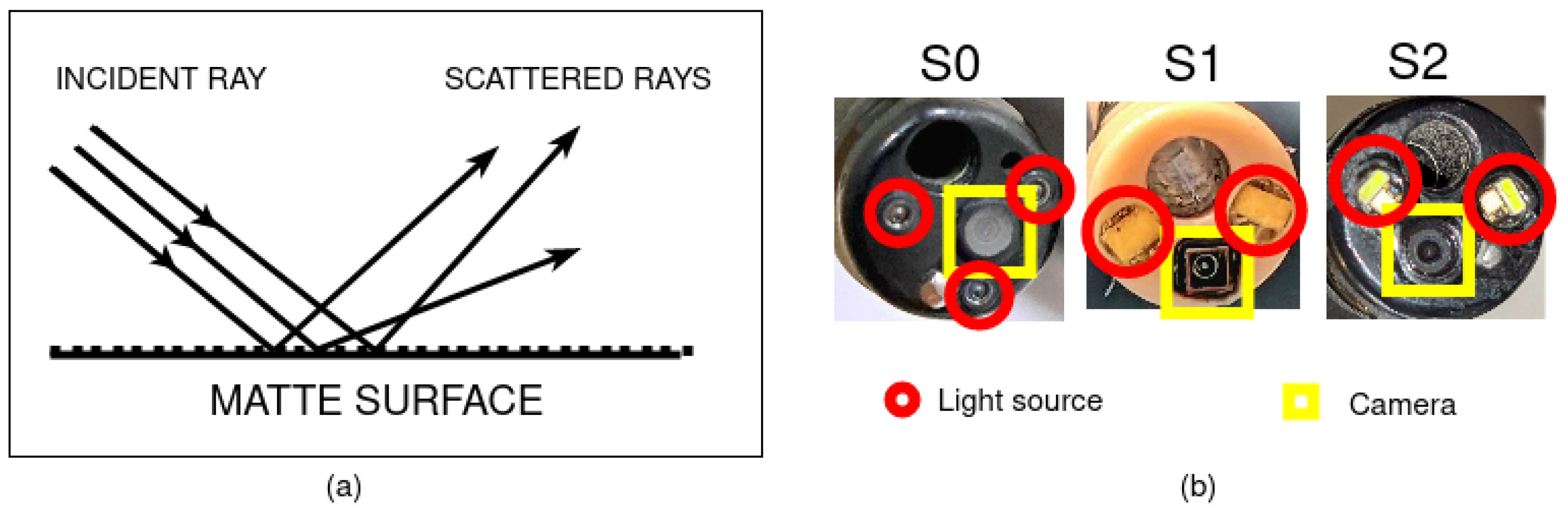
2.1.3. Endoscopy Systems
2.2. Experiments
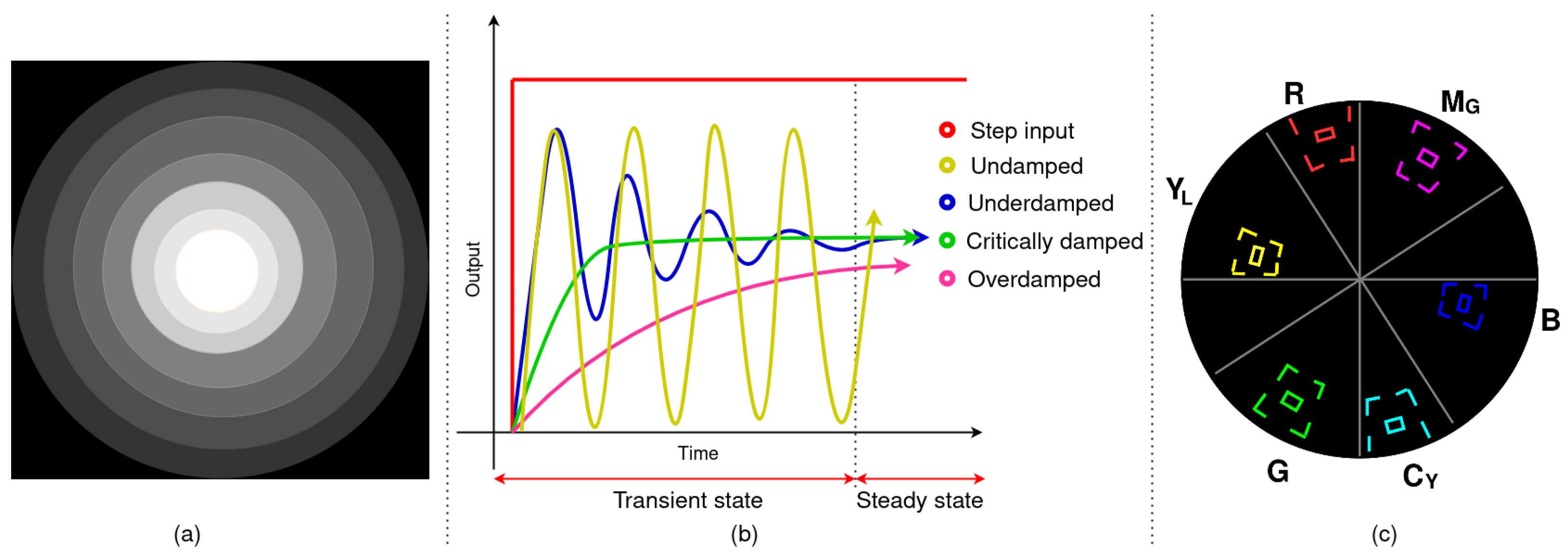
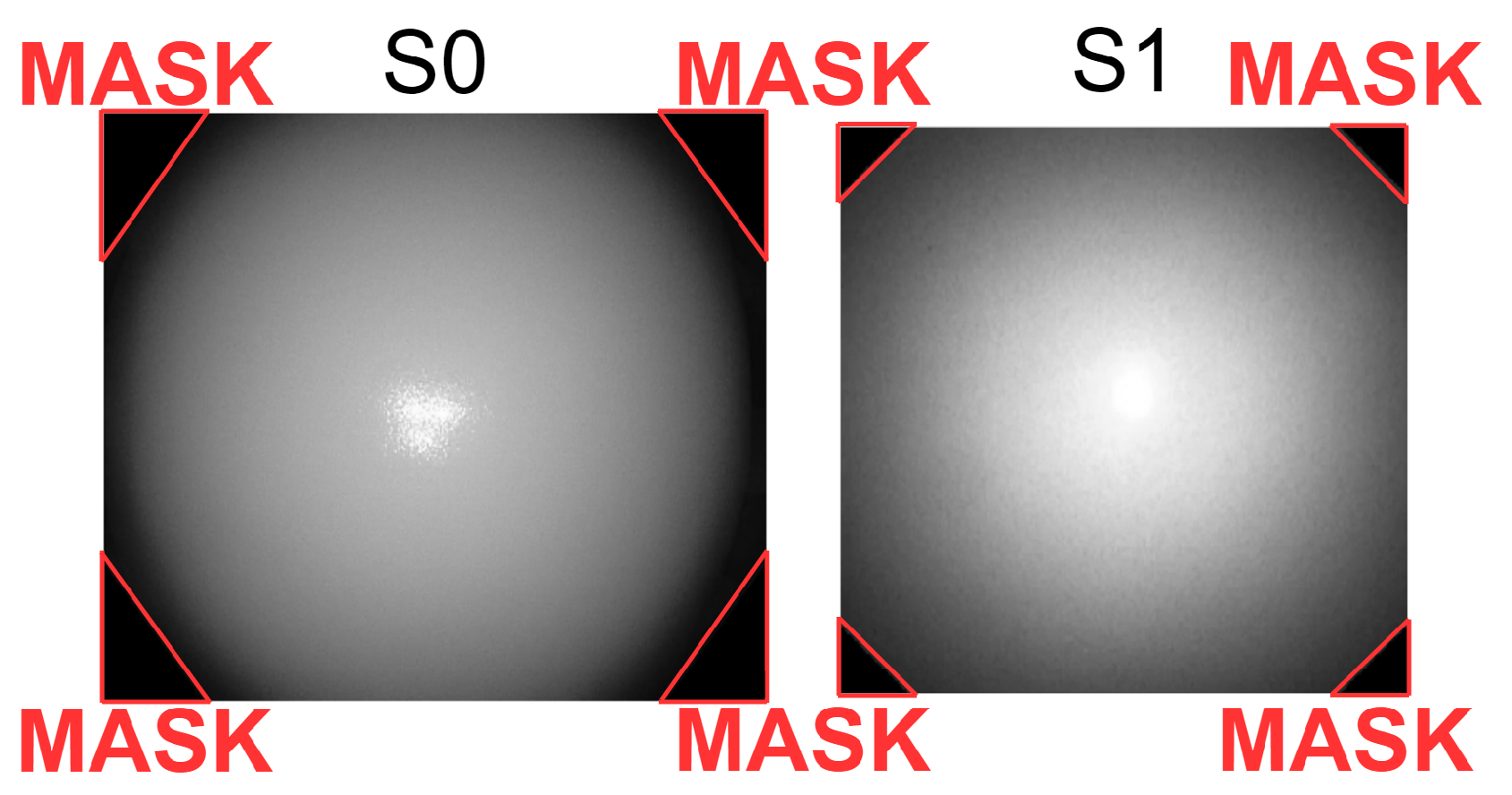
2.2.1. Assessment 1 (A1)—Image Brightness and Homogeneity of Brightness
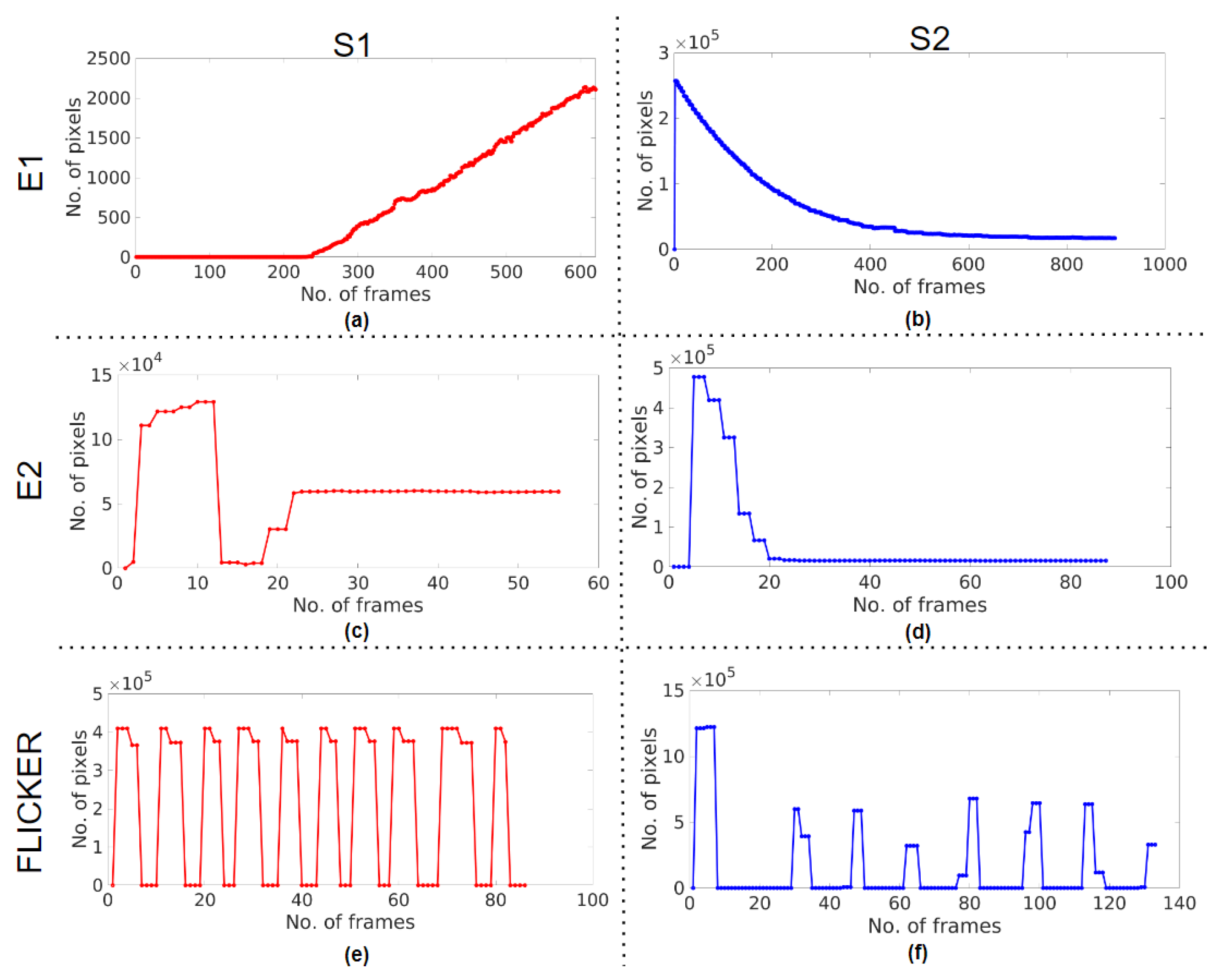
2.2.2. Assessment 2 (A2)—Controller Response and Response Time
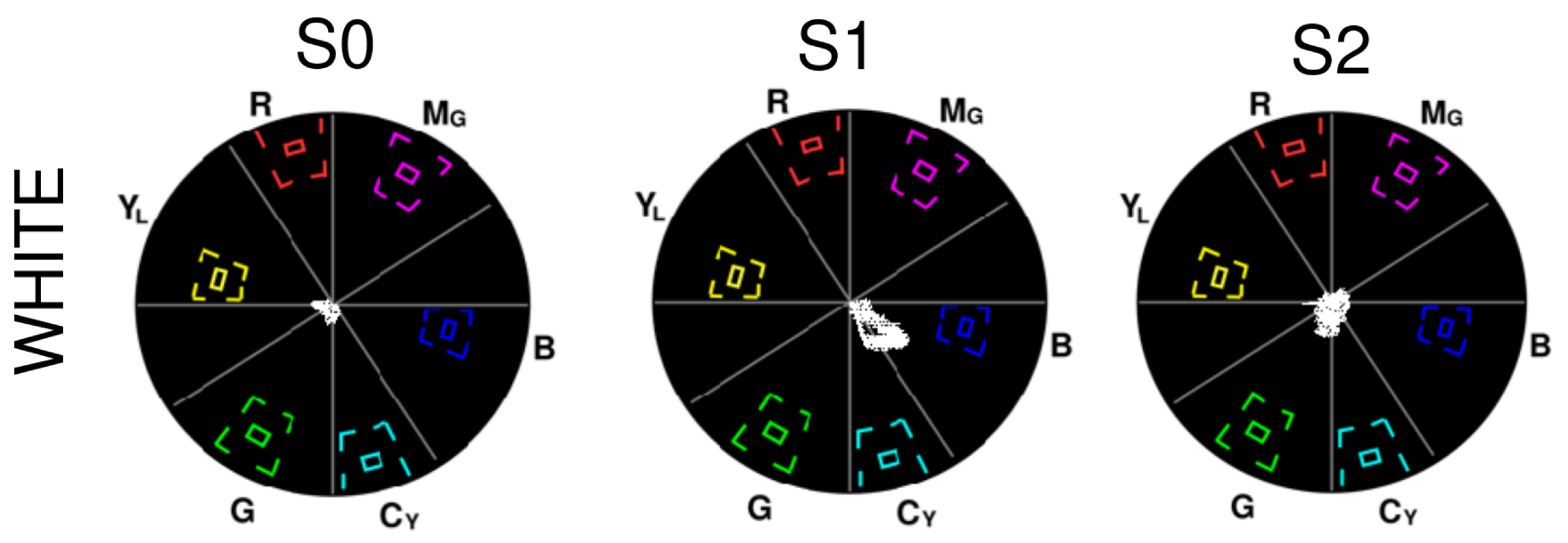
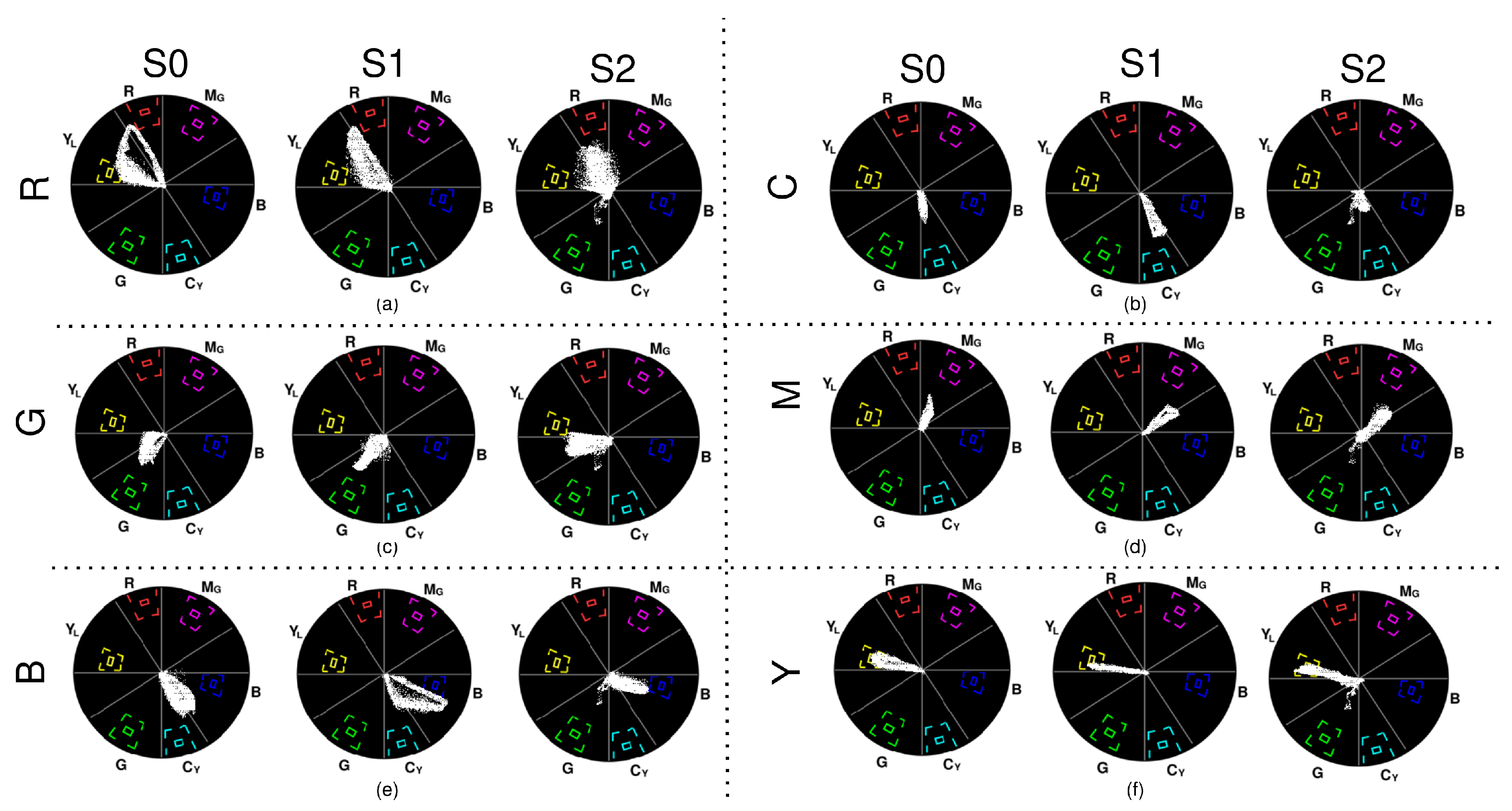
2.2.3. Assessment 3 (A3)—Colour Rendition
3. Results
3.1. A1—Image Brightness and Homogeneity of Brightness
3.2. A2—Controller Response and Response Time
3.3. A3—Colour Rendition
- White: S0 contains some amount of blue and green, S1 is skewed towards blue and cyan, and S2 contains some green.
- Red: S0 has most of the pixels within the yellow and orange phase and the remaining pixels in red. The maximum saturation obtained in the red phase is low. S1 renders red as orange, and the pixels in the red phase are very low in saturation. The red in S2 is spread across red, yellow, orange, and even magenta, with low saturation.
- Green: S0 renders green and deep green with some yellow at low saturation. S1 renders in deep green and yellow with better saturation than that observed in S0. Rendition in S2 is similar to S0 but with higher saturation. None of the outputs are close to the maximum chroma value of green.
- Blue: S0 has pixels in both blue and cyan, and the maximum blue saturation is close to the chroma gain of blue. Though S1 has some cyan too, its blue rendition is good, and many pixels fall within the bigger target for blue. S2 renders blue well but with less saturation.
- Cyan: S0 has pixels that fall between cyan and green with low saturation. S1 renders cyan well at better saturation than S0 but is still low compared to the cyan target. S2’s cyan is whiter due to very low saturation.
- Magenta: S0 renders magenta well with low saturation. S1 and S2 almost render magenta the same at similar saturation but have a few pixels spread in purple.
- Yellow: Yellow was rendered properly by all three systems, with many pixels achieving the required saturation by reaching the bigger targets. S2 has some pixels within the inner target too.
4. Discussion
- Objective Assessment: Though subjective assessment from experts could be useful as performed by McCallum et al. [11], it is unreliable unless there is a substantial amount of feedback, which makes it a time-consuming process. The proposed methodologies are objective assessment approaches that provide quantitative (A1 and A2) and qualitative (A3) assessments of the image data.
- Comprehensive Assessment: Shreshtha et al. [12] assess only image brightness and its homogeneity, and Cavallotti et al. [17] assess only the controller response. Sousa et al. [13] do not consider the assessment of colour rendition. To assess the overall performance of ABC, all of the assessment methodologies considered in the proposed work need to be considered to achieve homogeneous brightness rapidly and steadily without any colour shift.
- Blind Assessment: Cavallotti et al. [17] assess controller response based on the power consumption of the system which, would require access to the endoscopy hardware. Sousa et al. [13] also calculate response time from their endoscopy hardware. The results of the proposed methodologies could be carried out with only the endoscopy image data.
5. Conclusions
6. Limitations and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liedlgruber, M.; Uhl, A. Computer-aided decision support systems for endoscopy in the gastrointestinal tract: A review. IEEE Rev. Biomed. Eng. 2011, 4, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Dimas, G.; Bianchi, F.; Iakovidis, D.K.; Karargyris, A.; Ciuti, G.; Koulaouzidis, A. Endoscopic single-image size measurements. Meas. Sci. Technol. 2020, 31, 074010. [Google Scholar] [CrossRef]
- Rao, Z.; Xu, T.; Luo, J.; Guo, J.; Shi, G.; Wang, H. Non-uniform illumination endoscopic imaging enhancement via anti-degraded model and L 1 L 2-based variational retinex. EURASIP J. Wirel. Commun. Netw. 2017, 2017, 205. [Google Scholar] [CrossRef]
- Baumer. Types of Illumination for Industrial Image Processing—The Correct Light for Your Application. Available online: https://www.baumer.com/us/en/service-support/function-principle/illumination/a/illumination (accessed on 26 August 2022).
- Wang, L.; Li, Q.; Yang, H.; Huang, J.; Xu, K. A Sample-Based Color Correction Method for Laparoscopic Images. In Proceedings of the 2020 IEEE International Conference on Real-time Computing and Robotics (RCAR), Asahikawa, Japan, 28–29 September 2020; pp. 446–451. [Google Scholar]
- Khan, T.H.; Wahid, K.A. Subsample-based image compression for capsule endoscopy. J. Real-Time Image Process. 2013, 8, 5–19. [Google Scholar] [CrossRef]
- Abd-Alameer, S.A.; Daway, H.G.; Rashid, H.G. Quality of medical microscope Image at different lighting condition. In Proceedings of the IOP Conference Series: Materials Science and Engineering, The First International Conference of Pure and Engineering Sciences (ICPES2020), Karbala, Iraq, 26–27 February 2020. [Google Scholar]
- Festy, F.; Cook, R.; Watson, T. Automatic Exposure Control for Endoscopic Imaging. 2014. Available online: https://patents.google.com/patent/US10609291B2/en (accessed on 28 March 2023).
- Muehlebach, M. Camera Auto Exposure Control for VSLAM Applications; Autumn Term; Swiss Federal Institute of Technology Zurich: Zurich, Switzerland, 2010. [Google Scholar]
- Cline, R.W.; Fengler, J.J.P.; Figley, C.B.; Dawson, R.; Jaggi, B.W. Imaging System with Automatic Gain Control for Reflectance and Fluorescence Endoscopy. 2002. Available online: https://patents.google.com/patent/US6462770B1/en (accessed on 28 March 2023).
- McCallum, R.; McColl, J.; Iyer, A. The effect of light intensity on image quality in endoscopic ear surgery. Clin. Otolaryngol. 2018, 43, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Mohammed, S.K.; Hasan, M.M.; Zhang, X.; Wahid, K.A. Automated adaptive brightness in wireless capsule endoscopy using image segmentation and sigmoid function. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.M.; Wäny, M.; Santos, P.; Morgado-Dias, F.; Member, I. Automatic illumination control for an endoscopy sensor. Microprocess. Microsyst. 2020, 72, 102920. [Google Scholar] [CrossRef]
- Jiang, Q.; Peng, Z.; Yue, G.; Li, H.; Shao, F. No-reference image contrast evaluation by generating bidirectional pseudoreferences. IEEE Trans. Ind. Inform. 2020, 17, 6062–6072. [Google Scholar] [CrossRef]
- Wang, L.; Wu, B.; Wang, X.; Zhu, Q.; Xu, K. Endoscopic image luminance enhancement based on the inverse square law for illuminance and retinex. Int. J. Med. Robot. Comput. Assist. Surg. 2022, 18, e2396. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.; Cheng, D.; Zhou, T.; Hou, J.; Liu, W.; Xu, L.; Wang, T.; Cheng, J. Perceptual Quality Assessment of Enhanced Colonoscopy Images: A Benchmark Dataset and an Objective Method. IEEE Trans. Circuits Syst. Video Technol. 2023. [Google Scholar] [CrossRef]
- Cavallotti, C.; Merlino, P.; Vatteroni, M.; Valdastri, P.; Abramo, A.; Menciassi, A.; Dario, P. An FPGA-based versatile development system for endoscopic capsule design optimization. Sens. Actuators A Phys. 2011, 172, 301–307. [Google Scholar] [CrossRef]
- Nishitha, R.; Amalan, S.; Sharma, S.; Gurrala, A.K.; Preejith, S.; Joseph, J.; Sivaprakasam, M. Image Quality Assessment for Endoscopy Applications. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lausanne, Switzerland, 23–25 June 2021; pp. 1–6. [Google Scholar]
- Noordmans, H.J.; Van Mil, I.; Daoudi, S.; Kruit, S.; van den Brink, H.; Verdaasdonk, R. Optical quality assessment of rigid endoscopes during clinical lifetime. In Proceedings of the Endoscopic Microscopy, San Jose, CA, USA, 22 January 2006; International Society for Optics and Photonics: Bellingham, WA, USA, 2006; Volume 6082, p. 60820H. [Google Scholar]
- R, N.; S, A.; P, P.S.; Sivaprakasam, M. Identification of Structures to Perform Image Quality Assessment in Real-Time Endoscopy Imaging. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Nishitha, R.; Amalan, S.; Sharma, S.; Preejith, S.P.; Sivaprakasam, M. Image Quality Assessment for Interdependent Image Parameters Using a Score-Based Technique for Endoscopy Applications. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Imatest. Reflective Chart Quality Comparison: Inkjet vs. Photographic. 2019. Available online: https://www.imatest.com/2013/11/reflective-chart-quality-comparisoninkjet-vs-photographic/ (accessed on 30 August 2022).
- Auxomedical. Olympus CV-190 and CLV-190 Video Processor and Light Source. Available online: https://auxomedical.com/products/olympus-cv-190-and-clv-190-video-processor-and-light-source/ (accessed on 30 August 2022).
- Žukauskas, A.; Vaicekauskas, R.; Shur, M. Colour-rendition properties of solid-state lamps. J. Phys. D Appl. Phys. 2010, 43, 354006. [Google Scholar] [CrossRef]
- Hussain, K.; Rahman, S.; Rahman, M.; Khaled, S.M.; Wadud, A.A.; Hossain Khan, M.A.; Shoyaib, M. A histogram specification technique for dark image enhancement using a local transformation method. IPSJ Trans. Comput. Vis. Appl. 2018, 10, 3. [Google Scholar] [CrossRef]
- Fowler, K.R. Transient response for automatic gain control with multiple intensity thresholds for image-intensified camera. IEEE Trans. Instrum. Meas. 2005, 54, 1926–1933. [Google Scholar] [CrossRef]
- Alciatore, D.G.; Histand, M.B. Introduction to Mechatronics and Measurement Systems; McGraw-Hill: New York, NY, USA, 2007; Volume 3. [Google Scholar]
- Inman, D.J.; Singh, R.C. Engineering Vibration; Prentice Hall: Englewood Cliffs, NJ, USA, 1994; Volume 3. [Google Scholar]
- Mike Bedard, S.B. What Is a Vectorscope? How They Work and Why You Need One. 2021. Available online: https://www.studiobinder.com/blog/what-is-a-vectorscope-definition/ (accessed on 26 August 2022).
- Tektronix. NTSC Video Measurements. Available online: https://download.tek.com/document/25W_7247_1_rfs.pdf (accessed on 26 August 2022).
- Schreiber, P.; Kudaev, S.; Dannberg, P.; Zeitner, U.D. Homogeneous LED-illumination using microlens arrays. In Proceedings of the Nonimaging Optics and Efficient Illumination Systems II, San Diego, CA, USA, 31 July–1 August 2005; SPIE: Bellingham, WA, USA, 2005; Volume 5942, pp. 188–196. [Google Scholar]
- Design, V.S. An Illumination Profile. 2008. Available online: https://www.vision-systems.com/cameras-accessories/article/16739191/an-illuminating-profile (accessed on 26 August 2022).
- Mac Fhionnlaoich, N.; Taylor, A.; Guldin, S. Optimising Light Source Positioning for Even and Flux-Efficient Illumination. J. Open Source Softw. 2019, 4, 1392. [Google Scholar] [CrossRef]
- Munck, C.; Mordon, S.; Betrouni, N. Illumination profile characterization of a light device for the dosimetry of intra-pleural photodynamic therapy for mesothelioma. Photodiagn. Photodyn. Ther. 2016, 16, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.P. 5—Rotor Dynamics. In Gas Turbine Engineering Handbook, 4th ed.; Boyce, M.P., Ed.; Butterworth-Heinemann: Oxford, UK, 2012; pp. 215–250. [Google Scholar]
- Holmberg, U.; Myszkorowski, P.; Longchamp, R. Tracking control with a smooth transient response. IFAC Proc. Vol. 1996, 29, 1287–1291. [Google Scholar] [CrossRef]
- Passeri, D.; Ciampolini, P.; Bilei, G.; Casse, G.; Lemeilleur, F. Analysis of the transient response of LED-illuminated diodes under heavy radiation damage. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2000, 443, 148–155. [Google Scholar] [CrossRef]
- all2md. How to Solve the 5 Most Common Endoscopy Camera Problems by Yourself. 2019. Available online: https://all2md.com/how-to-solve-the-5-most-common-endoscopy-camera-problems-by-yourself/ (accessed on 12 September 2022).
- Jacobs, O. The damping ratio of an optimal control system. IEEE Trans. Autom. Control 1965, 10, 473–476. [Google Scholar] [CrossRef]




| Emulations | Systems | Imin | Imax | RANGE (Imax − Imin) | IP |
|---|---|---|---|---|---|
| S0 | 12 | 195 | 183 | 7 | |
| E0 | S1 | 46 | 231 | 185 | 7 |
| S2 | 48 | 231 | 184 | 8 | |
| E1 | S1 | 36 | 231 | 195 | 6 |
| S2 | 48 | 231 | 184 | 8 | |
| E2 | S1 | 46 | 250 | 204 | 7 |
| S2 | 48 | 231 | 184 | 8 |
| System/Emulations | Damping Ratio (No Unit) | Response Time (s) | ||||
|---|---|---|---|---|---|---|
| E0 | E1 | E2 | E0 | E1 | E2 | |
| S0 | 0.597 | X | X | 0.400 | X | X |
| S1 | - | - | 0.519 | >5 | >10 | 0.367 |
| S2 | 0.394 | 0.333 | 0.471 | 1.167 | >10 | 0.383 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravichandran, N.; Sreelatha Premkumar, P.; Sivaprakasam, M. Methodological Considerations for Assessing Automatic Brightness Control in Endoscopy: Experimental Study. Sensors 2023, 23, 4932. https://doi.org/10.3390/s23104932
Ravichandran N, Sreelatha Premkumar P, Sivaprakasam M. Methodological Considerations for Assessing Automatic Brightness Control in Endoscopy: Experimental Study. Sensors. 2023; 23(10):4932. https://doi.org/10.3390/s23104932
Chicago/Turabian StyleRavichandran, Nishitha, Preejith Sreelatha Premkumar, and Mohanasankar Sivaprakasam. 2023. "Methodological Considerations for Assessing Automatic Brightness Control in Endoscopy: Experimental Study" Sensors 23, no. 10: 4932. https://doi.org/10.3390/s23104932





