Associating Functional Neural Connectivity and Specific Aspects of Sensorimotor Control in Chronic Stroke
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.2.1. Grip Control Data Acquisition
2.2.2. EEG Acquisition
2.2.3. MRI Acquisition
2.3. Analysis
2.3.1. Sensorimotor Grip Control
2.3.2. Brain Connectivity
2.3.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Relationship between Grip Measures and Functional Neural Connectivity
4. Discussion
4.1. Overall Findings
4.2. Connectivity and Sensorimotor Function
4.3. Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.S. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Lawrence, E.; Coshall, C.; Dundas, R.; Stewart, J.; Rudd, A.; Howard, R. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001, 32, 1279–1284. [Google Scholar] [CrossRef]
- Stewart, J.C.; Cramer, S.C. Patient-reported measures provide unique insights into motor function after stroke. Stroke 2013, 44, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.L.; Sturm, J.W.; Dewey, H.M.; Donnan, G.A.; Macdonell, R.A.; Thrift, A.G. Long-term outcome in the North East Melbourne Stroke Incidence Study: Predictors of quality of life at 5 years after stroke. Stroke 2005, 36, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Dellavalle, R.; Danaei, G.; Ezzati, M.; Fahimi, A.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Page, S.J.; Gauthier, L.V.; White, S. Size doesn’t matter: Cortical stroke lesion volume is not associated with upper extremity motor impairment and function in mild, chronic hemiparesis. Arch. Phys. Med. Rehabil. 2013, 94, 817–821. [Google Scholar] [CrossRef]
- Sterr, A.; Shen, S.; Szameitat, A.J.; Herron, K.A. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: A pilot study. Neurorehabil. Neural Repair. 2010, 24, 413–419. [Google Scholar] [CrossRef]
- Peters, D.M.; Fridriksson, J.; Richardson, J.D.; Stewart, J.C.; Rorden, C.; Bonilha, L.; Middleton, A.; Fritz, S.L. Upper and Lower Limb Motor Function Correlates with Ipsilesional Corticospinal Tract and Red Nucleus Structural Integrity in Chronic Stroke: A Cross-Sectional, ROI-Based MRI Study. Behav. Neurol. 2021, 2021, 3010555. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.A.; Kautz, S.A.; Schlaug, G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef]
- Zhu, L.L.; Lindenberg, R.; Alexander, M.P.; Schlaug, G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010, 41, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Schweighofer, N.; Haldar, J.P.; Leahy, R.M.; Winstein, C.J. Corticospinal Tract Microstructure Predicts Distal Arm Motor Improvements in Chronic Stroke. J. Neurol. Phys. Ther. 2021, 45, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Byblow, W.D.; Ackerley, S.J.; Smith, M.C.; Borges, V.M.; Barber, P.A. PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann. Clin. Transl. Neurol. 2017, 4, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Barber, P.A.; Smale, P.R.; Coxon, J.P.; Fleming, M.K.; Byblow, W.D. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007, 130 Pt 1, 170–180. [Google Scholar] [CrossRef]
- Basser, P.J.; Mettiello, J.; LeBihan, D. MR Diffusion Tensor Spectroscopy and Imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef]
- Schaechter, J.D.; Fricker, Z.P.; Perdue, K.L.; Helmer, K.G.; Vangel, M.G.; Greve, D.N.; Makris, N. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum. Brain Mapp. 2009, 30, 3461–3474. [Google Scholar] [CrossRef]
- Lin, D.J.; Cloutier, A.M.; Erler, K.S.; Cassidy, J.M.; Snider, S.B.; Ranford, J.; Parlman, K.; Giatsidis, F.; Burke, J.F.; Schwamm, L.H.; et al. Corticospinal Tract Injury Estimated From Acute Stroke Imaging Predicts Upper Extremity Motor Recovery after Stroke. Stroke 2019, 50, 3569–3577. [Google Scholar] [CrossRef]
- Gratton, C.; Nomura, E.M.; Perez, F.; D’Esposito, M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J. Cogn. Neurosci. 2012, 24, 1275–1285. [Google Scholar] [CrossRef]
- Alstott, J.; Breakspear, M.; Hagmann, P.; Cammoun, L.; Sporns, O. Modeling the impact of lesions in the human brain. PLoS Comput. Biol. 2009, 5, e1000408. [Google Scholar] [CrossRef]
- Kancheva, I.; Buma, F.; Kwakkel, G.; Kancheva, A.; Ramsey, N.; Raemaekers, M. Investigating secondary white matter degeneration following ischemic stroke by modelling affected fiber tracts. Neuroimage Clin. 2022, 33, 102945. [Google Scholar] [CrossRef]
- Sotelo, M.R.; Kalinosky, B.T.; Goodfriend, K.; Hyngstrom, A.S.; Schmit, B.D. Indirect Structural Connectivity Identifies Changes in Brain Networks After Stroke. Brain Connect. 2020, 10, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Honey, C.J.; Sporns, O. Dynamical consequences of lesions in cortical networks. Hum. Brain Mapp. 2008, 29, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.; Tononi, G. Diaschisis: Past, present, future. Brain 2014, 137 Pt 9, 2408–2422. [Google Scholar] [CrossRef] [PubMed]
- Schranz, C.; Vatinno, A.; Ramakrishnan, V.; Seo, N.J. Neuroplasticity after upper-extremity rehabilitation therapy with sensory stimulation in chronic stroke survivors. Brain Commun. 2022, 4, fcac191. [Google Scholar] [CrossRef]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing wolf motor function test as outcome measure for research in patients after stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Lecraw, D.E.; Barton, L.A.; Jann, B.B. Forced Use of Hemiplegic Upper Extremities to Reverse the Effect of Learned Nonuse among Chronic Stroke and Head-Injured Patients. Exper. Neurol. 1989, 104, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.M.; Uswatte, G.; Crago, J.E.; Cook, E.W., 3rd; Taub, E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 750–755. [Google Scholar] [CrossRef]
- Sanford, J.; Moreland, J.; Swanson, L.R.; Stratford, P.W.; Gowland, C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys. Ther. 1993, 73, 447–454. [Google Scholar] [CrossRef]
- Yozbatiran, N.; Der-Yeghiaian, L.; Cramer, S.C. A standardized approach to performing the action research arm test. Neurorehabil. Neural Repair. 2008, 22, 78–90. [Google Scholar] [CrossRef]
- Seo, N.J.; Rymer, W.Z.; Kamper, D.G. Delays in Grip Initiation and Termination in Persons With Stroke: Effects of Arm Support and Active Muscle Stretch Exercise. J. Neurophysiol. 2009, 101, 3108–3115. [Google Scholar] [CrossRef]
- Quaney, B.M.; Perera, S.; Maletsky, R.; Luchies, C.W.; Nudo, R.J. Impaired grip force modulation in the ipsilesional hand after unilateral middle cerebral artery stroke. Neurorehabil. Neural Repair. 2005, 19, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, N.; Russo, C.; Edwards, D.J. The sensory side of post-stroke motor rehabilitation. Restor. Neurol. Neurosci. 2016, 34, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.M.; Wodeyar, A.; Wu, J.; Kaur, K.; Masuda, A.K.; Srinivasan, R.; Cramer, S.C. Low-Frequency Oscillations Are a Biomarker of Injury and Recovery After Stroke. Stroke 2020, 51, 1442–1450. [Google Scholar] [CrossRef]
- Seo, N.J.; Lakshminarayanan, K.; Bonilha, L.; Lauer, A.W.; Schmit, B.D. Effect of imperceptible vibratory noise applied to wrist skin on fingertip touch evoked potentials—An EEG study. Physiol. Rep. 2015, 3, e12624. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.J.; Lakshminarayanan, K.; Lauer, A.W.; Ramakrishnan, V.; Schmit, B.D.; Hanlon, C.A.; George, M.S.; Bonilha, L.; Downey, R.J.; DeVries, W.; et al. Use of imperceptible wrist vibration to modulate sensorimotor cortical activity. Exp. Brain Res. 2019, 237, 805–816. [Google Scholar] [CrossRef]
- Wu, J.; Quinlan, E.B.; Dodakian, L.; McKenzie, A.; Kathuria, N.; Zhou, R.J.; Augsburger, R.; See, J.; Le, V.H.; Srinivasan, R.; et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain 2015, 138 Pt 8, 2359–2369. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The Post-Stroke Hemiplegic Patient. Scand. J. Rehab. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult Norms for the Box and Block Test of Manual Dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- Brant-Zawadzki, M.; Gillian, G.; Nitz, W. MP RAGE: A Three-dimensional, T1-weighted, Gradient-Echo Sequence-Initial Experience in the Brain. Radiology 1992, 182, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Rorden, C.; Brett, M. Stereotaxic display of brain lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Evans, A. An MRI-based stereotactic atlas from 250 young normal subjects. J. Soc. Neurosci. 1992, 18, 408. [Google Scholar]
- Hur, P.; Wan, Y.H.; Seo, N.J. Investigating the role of vibrotactile noise in early response to perturbation. IEEE Trans. Biomed. Eng. 2014, 61, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.J.; Enders, L.R.; Motawar, B.; Kosmopoulos, M.L.; Fathi-Firoozabad, M. The extent of altered digit force direction correlates with clinical upper extremity impairment in chronic stroke survivors. J. Biomech. 2015, 48, 383–387. [Google Scholar] [CrossRef]
- Seo, N.J.; Rymer, W.Z.; Kamper, D.G. Altered digit force direction during pinch grip following stroke. Exp. Brain Res. 2010, 202, 891–901. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Mognon, A.; Jovicich, J.; Bruzzone, L.; Buiatti, M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 2011, 48, 229–240. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Gramfort, A.; Papadopoulo, T.; Olivi, E.; Clerc, M. OpenMEEG: Opensource software for quasistatic bioelectromagnetics. Biomed. Eng. Online 2010, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Baillet, S.; Mosher, J.C.; Leahy, R.M. Electromagnetic Brain Mapping. IEEE Signal Process. Mag. 2001, 18, 14–30. [Google Scholar] [CrossRef]
- Nolte, G.; Bai, O.; Wheaton, L.; Mari, Z.; Vorbach, S.; Hallett, M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 2004, 115, 2292–2307. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K. Motor systems. Curr. Opin. Neurobiol. 2000, 10, 649–654. [Google Scholar] [CrossRef]
- Borich, M.R.; Brodie, S.M.; Gray, W.A.; Ionta, S.; Boyd, L.A. Understanding the role of the primary somatosensory cortex: Opportunities for rehabilitation. Neuropsychologia 2015, 79, 246–255. [Google Scholar] [CrossRef]
- Davare, M.; Andres, M.; Cosnard, G.; Thonnard, J.L.; Olivier, E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J. Neurosci. 2006, 26, 2260–2268. [Google Scholar] [CrossRef]
- Jensen, O.; Gelfand, J.; Kounios, J.; Lisman, J.E. Oscillations in the Alpha Band (9–12 Hz) Increase with Memory Load during Retention in a Short-term Memory Task. Cereb. Cortex 2002, 12, 877–882. [Google Scholar] [CrossRef]
- Chung, J.W.; Ofori, E.; Misra, G.; Hess, C.W.; Vaillancourt, D.E. Beta-band activity and connectivity in sensorimotor and parietal cortex are important for accurate motor performance. Neuroimage 2017, 144, 164–173. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Pierella, C.; Pirondini, E.; Kinany, N.; Coscia, M.; Giang, C.; Miehlbradt, J.; Magnin, C.; Nicolo, P.; Dalise, S.; Sgherri, G.; et al. A multimodal approach to capture post-stroke temporal dynamics of recovery. J. Neural Eng. 2020, 17, 045002. [Google Scholar] [CrossRef] [PubMed]
- Schranz, C.; Srivastava, S.; Seamon, B.A.; Marebwa, B.; Bonilha, L.; Ramakrishnan, V.; Wilmskoetter, J.; Neptune, R.R.; Kautz, S.A.; Seo, N.J. Different aspects of hand grip performance associated with structural connectivity of distinct sensorimotor networks in chronic stroke. Physiol. Rep. 2023, 11, e15659. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-Romagosa, M.; Udina, E.; Ortner, R.; Dinarès-Ferran, J.; Cho, W.; Murovec, N.; Matencio-Peralba, C.; Sieghartsleitner, S.; Allison, B.Z.; Guger, C. EEG Biomarkers Related with the Functional State of Stroke Patients. Front. Neurosci. 2020, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Carducci, F.; Cincotti, F.; Rossini, P.; Neuper, C.; Pfurtscheller, G.; Babiloni, F. Human Movement-Related Potentials vs Desynchronization of EEG Alpha Rhythm: A High-Resolution EEG Study. Neuroimage 1999, 10, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Platz, T.; Kim, I.H.; Pintschovius, H.; Winter, T.; Kieselbach, A.; Villringer, K.; Kurth, R.; Mauritz, K.H. Multimodal EEG analysis in man suggests impairment-specific changes in movement-related electric brain activity after stroke. Brain 2000, 123, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.R.; Croft, R.J.; Dominey, S.; Burgess, A.P.; Gruzelier, J.H. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int. J. Psychophysiol. 2003, 47, 65–74. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Central Beta Rhythm During Sensorimotor Activities in Man. Electroencephalogr. Clin. Neurophysiol. 1981, 51, 253–264. [Google Scholar] [CrossRef]
- Espenhahn, S.; Rossiter, H.E.; van Wijk, B.C.M.; Redman, N.; Rondina, J.M.; Diedrichsen, J.; Ward, N.S. Sensorimotor cortex beta oscillations reflect motor skill learning ability after stroke. Brain Commun. 2020, 7, fcaa161. [Google Scholar] [CrossRef]
- Struber, L.; Baumont, M.; Barraud, P.A.; Nougier, V.; Cignetti, F. Brain oscillatory correlates of visuomotor adaptive learning. Neuroimage 2021, 245, 118645. [Google Scholar] [CrossRef]
- Seo, N.J.; Fischer, H.W.; Bogey, R.A.; Rymer, W.Z.; Kamper, D.G. Effect of a serotonin antagonist on delay in grip muscle relaxation for persons with chronic hemiparetic stroke. Clin. Neurophysiol. 2011, 122, 796–802. [Google Scholar] [CrossRef]
- Kamper, D.; Barry, A.; Bansal, N.; Stoykov, M.E.; Triandafilou, K.; Vidakovic, L.; Seo, N.J.; Roth, E. Use of cyproheptadine hydrochloride (HCl) to reduce neuromuscular hypertonicity in stroke survivors: A Randomized Trial: Reducing Hypertonicity in Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106724. [Google Scholar] [CrossRef]
- Motawar, B.; Hur, P.; Stinear, J.; Seo, N.J. Contribution of intracortical inhibition in voluntary muscle relaxation. Exp. Brain Res. 2012, 221, 299–308. [Google Scholar] [CrossRef]
- Motawar, B.; Stinear, J.W.; Lauer, A.W.; Ramakrishnan, V.; Seo, N.J. Delayed grip relaxation and altered modulation of intracortical inhibition with aging. Exp. Brain Res. 2016, 234, 985–995. [Google Scholar] [CrossRef]
- Hermsdorfer, J.; Hagl, E.; Nowak, D.A.; Marquardt, C. Grip force control during object manipulation in cerebral stroke. Clin. Neurophysiol. 2003, 114, 915–929. [Google Scholar] [CrossRef]
- Lodha, N.; Misra, G.; Coombes, S.A.; Christou, E.A.; Cauraugh, J.H. Increased force variability in chronic stroke: Contributions of force modulation below 1 Hz. PLoS ONE 2013, 8, e83468. [Google Scholar] [CrossRef]
- Nowak, D.A.; Hermsdorfer, J.; Topka, H. Deficits of predictive grip force control during object manipulation in acute stroke. J. Neurol. 2003, 250, 850–860. [Google Scholar] [CrossRef]
- Meyer, S.; Kessner, S.S.; Cheng, B.; Bonstrup, M.; Schulz, R.; Hummel, F.C.; De Bruyn, N.; Peeters, A.; Van Pesch, V.; Duprez, T.; et al. Voxel-based lesion-symptom mapping of stroke lesions underlying somatosensory deficits. Neuroimage Clin. 2016, 10, 257–266. [Google Scholar] [CrossRef]
- Kessner, S.S.; Schlemm, E.; Cheng, B.; Bingel, U.; Fiehler, J.; Gerloff, C.; Thomalla, G. Somatosensory Deficits after Ischemic Stroke. Stroke 2019, 50, 1116–1123. [Google Scholar] [CrossRef]
- Lo, R.; Gitelman, D.; Levy, R.; Hulvershorn, J.; Parrish, T. Identification of critical areas for motor function recovery in chronic stroke subjects using voxel-based lesion symptom mapping. Neuroimage 2010, 49, 9–18. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Levin, M.F.; Berman, S.; Liebermann, D.G.; Baniña, M.C.; Solomon, J.M.; Ofir-Geva, S.; Soroker, N. Shared and distinct voxel-based lesion-symptom mappings for spasticity and impaired movement in the hemiparetic upper limb. Sci. Rep. 2022, 12, 10169. [Google Scholar] [CrossRef]
- Alexandre, A.M.; Colo, F.; Brunetti, V.; Valente, I.; Frisullo, G.; Pedicelli, A.; Scarcia, L.; Rollo, C.; Falcou, A.; Milonia, L.; et al. Mechanical thrombectomy in minor stroke due to isolated M2 occlusion: A multicenter retrospective matched analysis. J. Neurointerv. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Swayne, O.; Blankenburg, F.; Ruff, C.C.; Teo, J.; Weiskopf, N.; Driver, J.; Rothwell, J.C.; Ward, N.S. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J. Neurosci. 2010, 30, 11926–11937. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hutchinson, S.; Schlaug, G.; Pascual-Leone, A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. NeuroImage 2003, 20, 2259–2270. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.J. Involuntary contralateral upper extremity muscle activation pattern during unilateral pinch grip following stroke. J. Hand Ther. 2013, 26, 272–277. [Google Scholar] [CrossRef]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S. Neural correlates of outcome after stroke: A cross-sectional fMRI study. Brain 2003, 126 Pt 6, 1430–1448. [Google Scholar] [CrossRef]
- Vatinno, A.A.; Schranz, C.; Simpson, A.N.; Ramakrishnan, V.; Bonilha, L.; Seo, N.J. Predicting upper extremity motor improvement following therapy using EEG-based connectivity in chronic stroke. NeuroRehabilitation 2022, 50, 105–113. [Google Scholar] [CrossRef]
- Vatinno, A.A.; Simpson, A.; Ramakrishnan, V.; Bonilha, H.S.; Bonilha, L.; Seo, N.J. The Prognostic Utility of Electroencephalography in Stroke Recovery: A Systematic Review and Meta-Analysis. Neurorehabil. Neural Repair. 2022, 36, 255–268. [Google Scholar] [CrossRef]
- Srivastava, S.; Seamon, B.A.; Marebwa, B.K.; Wilmskoetter, J.; Bowden, M.G.; Gregory, C.M.; Seo, N.J.; Hanlon, C.A.; Bonilha, L.; Brown, T.R.; et al. The relationship between motor pathway damage and flexion-extension patterns of muscle co-excitation during walking. Front. Neurol. 2022, 13, 968385. [Google Scholar] [CrossRef]
- Seo, N.J.; Ramakrishnan, V.; Woodbury, M.L.; Bonilha, L.; Finetto, C.; Schranz, C.; Scronce, G.; Coupland, K.; Blaschke, J.; Baker, A.; et al. Concomitant sensory stimulation during therapy to enhance hand functional recovery post stroke. Trials 2022, 23, 262. [Google Scholar] [CrossRef]
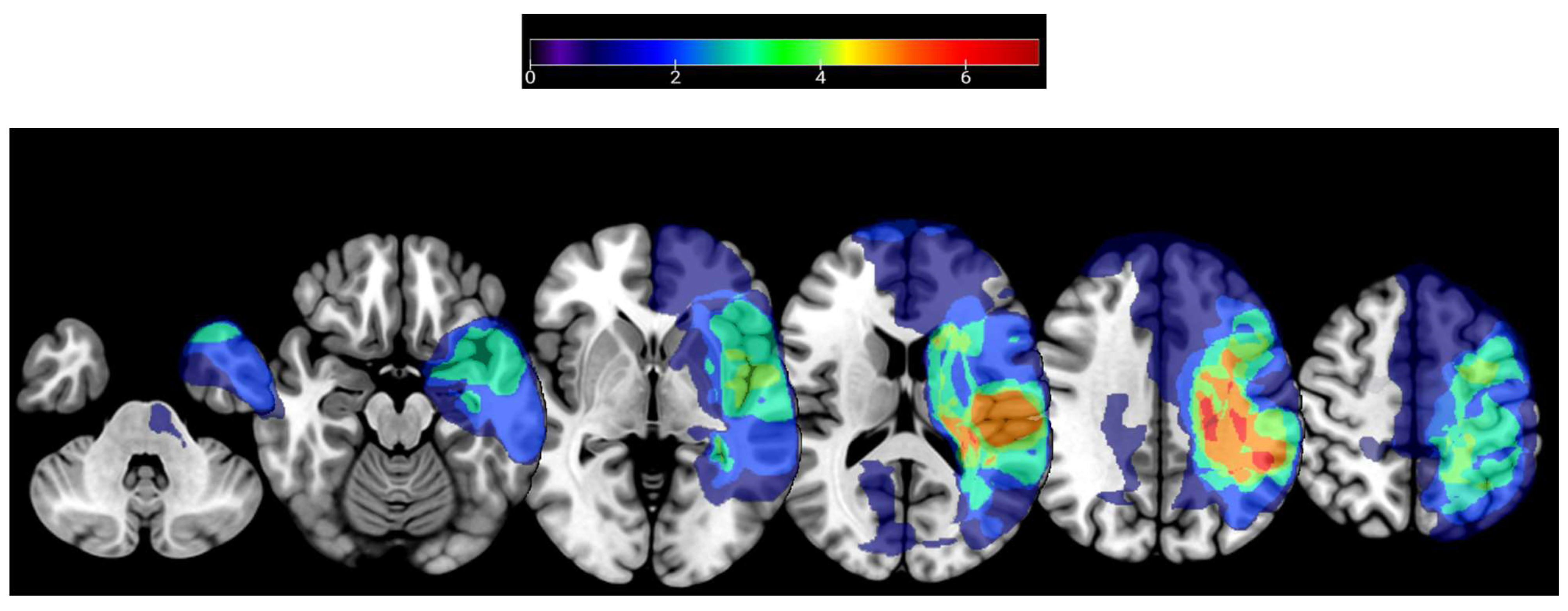

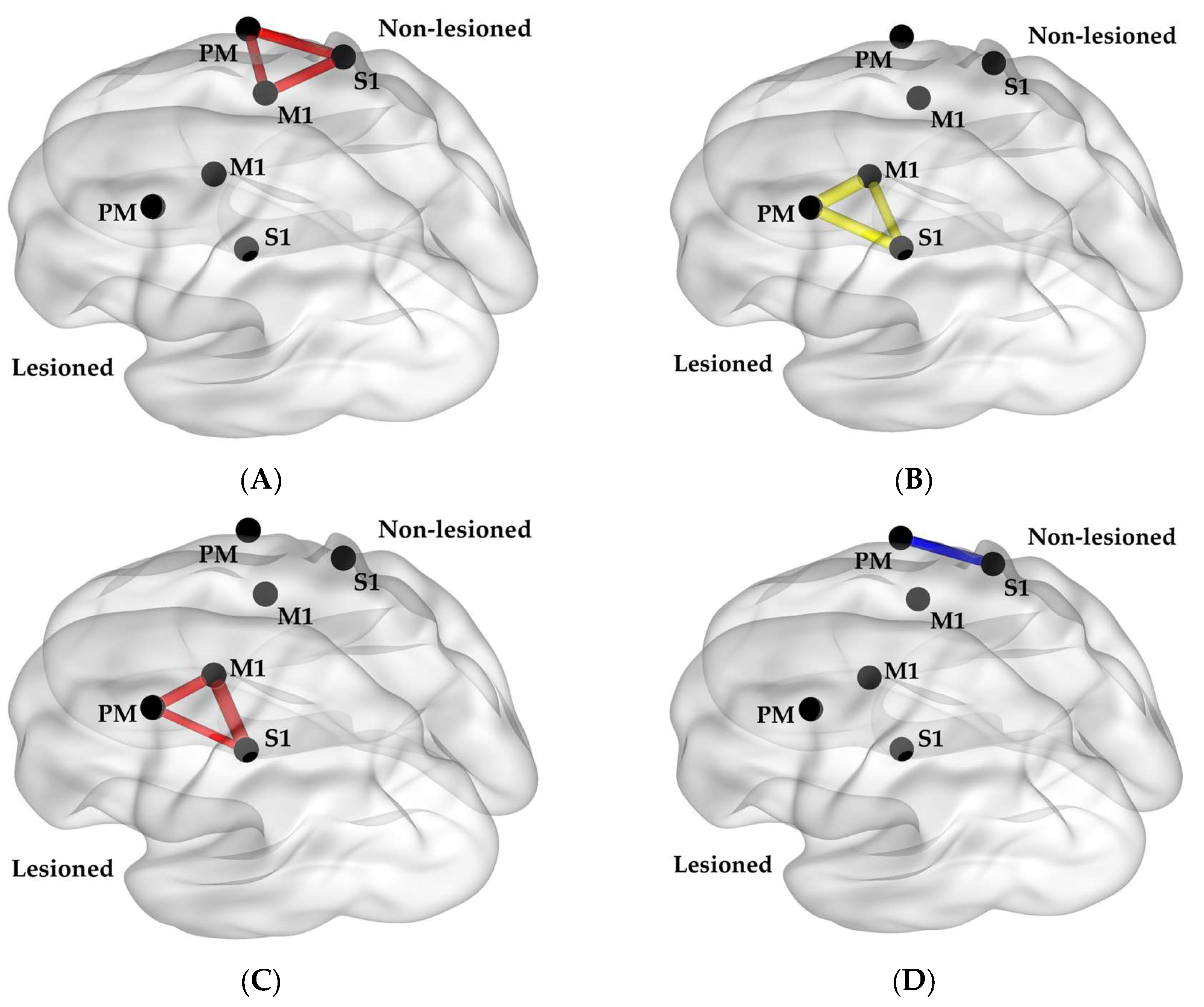

| Study ID | Age | Sex | Dominant Hand | Affected Upper Extremity | Time Since Stroke (Months) | Fugl-Meyer Upper Extremity Score (/66) | Wolf Motor Function Test Time (Seconds) | Box and Block Test Score |
|---|---|---|---|---|---|---|---|---|
| 1 | 58 | F | R | R | 75 | 57 | 3.5 | 46 |
| 2 | 61 | M | R | R | 23 | 41 | 29.8 | 12 |
| 3 | 63 | F | R | R | 65 | 58 | 5.0 | 31 |
| 4 | 46 | F | R | R | 17 | 35 | 33.2 | 12 |
| 5 | 64 | M | R | R | 39 | 59 | 2.4 | 50 |
| 6 | 59 | F | R | R | 27 | 51 | 9.7 | 18 |
| 7 | 58 | M | R | R | 34 | 43 | 14.9 | 17 |
| 8 | 68 | M | R | L | 193 | 53 | 4.5 | 35 |
| 9 | 73 | F | R | L | 15 | 40 | 4.6 | 34 |
| 10 | 66 | M | R | R | 47 | 43 | 6.3 | 32 |
| 11 | 53 | M | R | L | 25 | 39 | 16.0 | 24 |
| 12 | 79 | M | R | R | 178 | 53 | 6.6 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, A.; Schranz, C.; Seo, N.J. Associating Functional Neural Connectivity and Specific Aspects of Sensorimotor Control in Chronic Stroke. Sensors 2023, 23, 5398. https://doi.org/10.3390/s23125398
Baker A, Schranz C, Seo NJ. Associating Functional Neural Connectivity and Specific Aspects of Sensorimotor Control in Chronic Stroke. Sensors. 2023; 23(12):5398. https://doi.org/10.3390/s23125398
Chicago/Turabian StyleBaker, Adam, Christian Schranz, and Na Jin Seo. 2023. "Associating Functional Neural Connectivity and Specific Aspects of Sensorimotor Control in Chronic Stroke" Sensors 23, no. 12: 5398. https://doi.org/10.3390/s23125398
APA StyleBaker, A., Schranz, C., & Seo, N. J. (2023). Associating Functional Neural Connectivity and Specific Aspects of Sensorimotor Control in Chronic Stroke. Sensors, 23(12), 5398. https://doi.org/10.3390/s23125398








