Excluding Echo Shift Noise in Real-Time Pulse-Echo Speed-of-Sound Imaging
Abstract
:1. Introduction
2. Theory
2.1. Echo Shift Tracking
2.2. Reference Method
2.3. Proposed Method
3. Materials and Methods
3.1. Echo Shift Simulation
3.2. Synthetic Noise
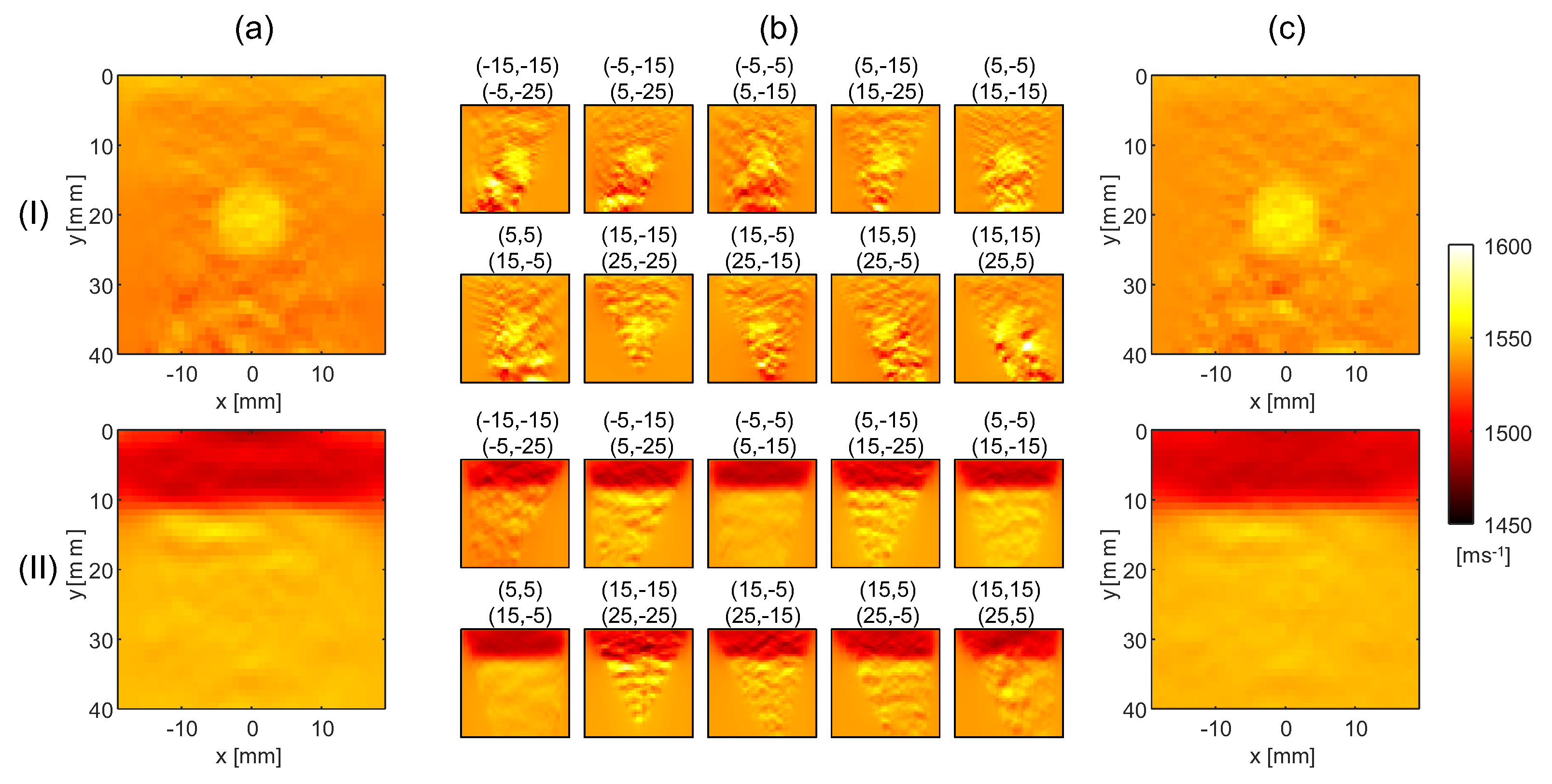
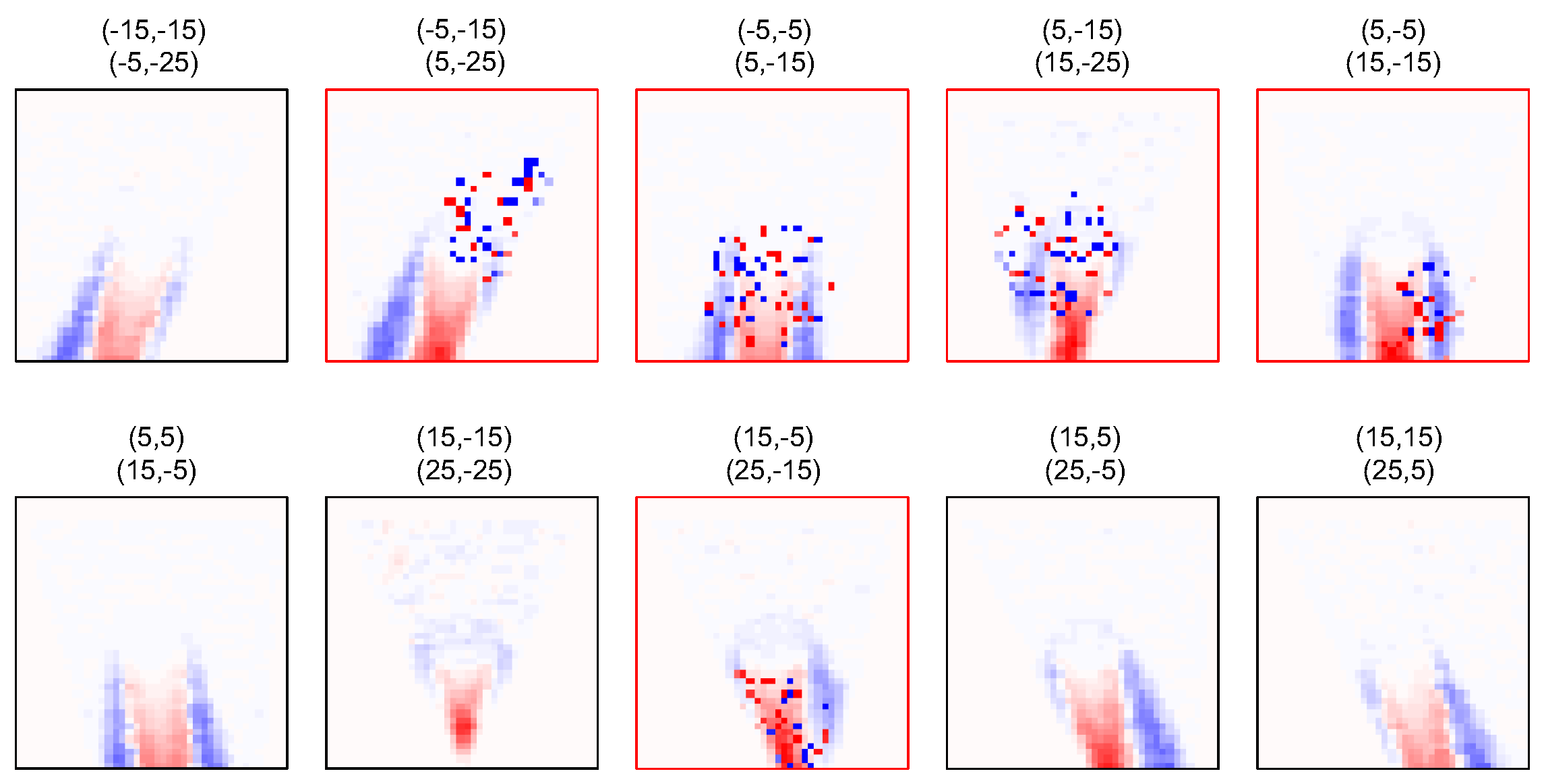
4. Results
4.1. Minimum Noise SoS Reconstructions
4.2. SoS Reconstruction from Noisy Echo Shift Maps
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgio, M.D.; Imbault, M.; Ronot, M.; Faccinetto, A.; Van Beers, B.E.; Rautou, P.E.; Castera, L.; Gennisson, J.L.; Tanter, M.; Vilgrain, V. Ultrasonic adaptive sound speed estimation for the diagnosis and quantification of hepatic steatosis: A pilot study. Ultraschall Med.-Eur. J. Ultrasound 2019, 40, 722–733. [Google Scholar] [CrossRef]
- Imbault, M.; Burgio, M.D.; Faccinetto, A.; Ronot, M.; Bendjador, H.; Deffieux, T.; Triquet, E.O.; Rautou, P.E.; Castera, L.; Gennisson, J.L.; et al. Ultrasonic fat fraction quantification using in vivo adaptive sound speed estimation. Phys. Med. Biol. 2018, 63, 215013. [Google Scholar] [CrossRef] [PubMed]
- Ruby, L.; Sanabria, S.J.; Martini, K.; Dedes, K.J.; Vorburger, D.; Oezkan, E.; Frauenfelder, T.; Goksel, O.; Rominger, M.B. Breast Cancer Assessment With Pulse-Echo Speed of Sound Ultrasound From Intrinsic Tissue Reflections: Proof-of-Concept. Investig. Radiol. 2019, 54, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duric, N.; Sak, M.; Fan, S.; Pfeiffer, R.M.; Littrup, P.J.; Simon, M.S.; Gorski, D.H.; Ali, H.; Purrington, K.S.; Brem, R.F.; et al. Using Whole Breast Ultrasound Tomography to Improve Breast Cancer Risk Assessment: A Novel Risk Factor Based on the Quantitative Tissue Property of Sound Speed. J. Clin. Med. 2020, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Natesan, R.; Wiskin, J.; Lee, S.; Malik, B.H. Quantitative assessment of breast density: Transmission ultrasound is comparable to mammography with tomosynthesis. Cancer Prev. Res. 2019, 12, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanabria, S.J.; Martini, K.; Freystätter, G.; Ruby, L.; Goksel, O.; Frauenfelder, T.; Rominger, M.B. Speed of sound ultrasound: A pilot study on a novel technique to identify sarcopenia in seniors. Eur. Radiol. 2019, 29, 3–12. [Google Scholar] [CrossRef]
- Ruby, L.; Kunut, A.; Nakhostin, D.N.; Huber, F.A.; Finkenstaedt, T.; Frauenfelder, T.; Sanabria, S.J.; Rominger, M.B. Speed of sound ultrasound: Comparison with proton density fat fraction assessed with Dixon MRI for fat content quantification of the lower extremity. Eur. Radiol. 2020, 30, 5272. [Google Scholar] [CrossRef]
- Korta Martiartu, N.; Nakhostin, D.; Ruby, L.; Frauenfelder, T.; Rominger, M.B.; Sanabria, S.J. Speed of sound and shear wave speed for calf soft tissue composition and nonlinearity assessment. Quant. Imaging Med. Surg. 2021, 11, 4149–4161. [Google Scholar] [CrossRef]
- Jaeger, M.; Robinson, E.; Akarçay, H.G.; Frenz, M. Full correction for spatially distributed speed-of-sound in echo ultrasound based on measuring aberration delays via transmit beam steering. Phys. Med. Biol. 2015, 60, 4497. [Google Scholar] [CrossRef] [Green Version]
- Rau, R.; Schweizer, D.; Vishnevskiy, V.; Goksel, O. Ultrasound Aberration Correction based on Local Speed-of-Sound Map Estimation. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 2003–2006. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.; Brevett, T.; Hyun, D.; Brickson, L.L.; Dahl, J.J. Distributed Aberration Correction Techniques Based on Tomographic Sound Speed Estimates. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 1714–1726. [Google Scholar] [CrossRef]
- Kondo, M.; Takamizawa, K.; Hirama, M.; Okazaki, K.; Iinuma, K.; Takehara, Y. An evaluation of an in vivo local sound speed estimation technique by the crossed beam method. Ultrasound Med. Biol. 1990, 16, 65–72. [Google Scholar] [CrossRef]
- Céspedes, I.; Ophir, J.; Huang, Y. On the feasibility of pulse-echo speed of sound estimation in small regions: Simulation studies. Ultrasound Med. Biol. 1992, 18, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, M.; Hsieh, S.; Ali, R.; Chau Loo Kung, G.; Hyun, D.; Dahl, J.J. Local speed of sound estimation in tissue using pulse-echo ultrasound: Model-based approach. J. Acoust. Soc. Am. 2018, 144, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Telichko, A.V.; Ali, R.; Brevett, T.; Wang, H.; Vilches-Moure, J.G.; Kumar, S.U.; Paulmurugan, R.; Dahl, J.J. Noninvasive estimation of local speed of sound by pulse-echo ultrasound in a rat model of nonalcoholic fatty liver. Phys. Med. Biol. 2022, 67, 015007. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, S.J.; Ozkan, E.; Rominger, M.; Goksel, O. Spatial domain reconstruction for imaging speed-of-sound with pulse-echo ultrasound: Simulation and in vivo study. Phys. Med. Biol. 2018, 63, 215015. [Google Scholar] [CrossRef] [Green Version]
- Feigin, M.; Anthony, D.F.B.W. A deep learning framework for single-sided sound speed inversion in medical ultrasound. IEEE Trans. Biomed. Eng. 2020, 67, 1142–1151. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, M.; Held, G.; Peeters, S.; Preisser, S.; Grünig, M.; Frenz, M. Computed ultrasound tomography in echo mode for imaging speed of sound using pulse-echo sonography: Proof of principle. Ultrasound Med. Biol. 2015, 41, 235–250. [Google Scholar] [CrossRef]
- Stähli, P.; Kuriakose, M.; Frenz, M.; Jaeger, M. Improved forward model for quantitative pulse-echo speed-of-sound imaging. Ultrasonics 2020, 108, 106168. [Google Scholar] [CrossRef]
- Stähli, P.; Frenz, M.; Jaeger, M. Bayesian Approach for a Robust Speed-of-Sound Reconstruction Using Pulse-Echo Ultrasound. IEEE Trans. Med. Imaging 2020, 40, 457–467. [Google Scholar] [CrossRef]
- Stähli, P.; Becchetti, C.; Korta Martiartu, N.; Berzigotti, A.; Frenz, M.; Jaeger, M. First-in-human diagnostic study of hepatic steatosis with computed ultrasound tomography in echo mode (CUTE). Res. Sq. 2022. [Google Scholar] [CrossRef]
- Jaeger, M.; Stähli, P.; Korta Martiartu, N.; Salemi Yolgunlu, P.; Frappart, T.; Fraschini, C.; Frenz, M. Pulse-echo speed-of-sound imaging using convex probes. Phys. Med. Biol. 2022, 67, 215016. [Google Scholar] [CrossRef] [PubMed]
- Beuret, S.; Hériard-Dubreuil, B.; Canales, S.; Thiran, J.P. Directional Cross-Correlation for Improved Aberration Phase Estimation in Pulse-Echo Speed-of-Sound Imaging. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Huang, L.; Quan, Y. Ultrasound pulse-echo imaging using the split-step Fourier propagator. In Proceedings of the 2007 SPIE Medical Imaging, San Diego, CA, USA, 17–22 February 2007; Volume 6513, p. 651305. [Google Scholar] [CrossRef]
- Huang, L.; Quan, Y.; Li, C.; Duric, N.; Hanson, K.M. Ultrasound Pulse-Echo Imaging with an Optimized Propagator. In Proceedings of the 2008 International Conference on BioMedical Engineering and Informatics, Sanya, China, 27–30 May 2008; pp. 281–285. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salemi Yolgunlu, P.; Korta Martiartu, N.; Gerber, U.R.; Frenz, M.; Jaeger, M. Excluding Echo Shift Noise in Real-Time Pulse-Echo Speed-of-Sound Imaging. Sensors 2023, 23, 5598. https://doi.org/10.3390/s23125598
Salemi Yolgunlu P, Korta Martiartu N, Gerber UR, Frenz M, Jaeger M. Excluding Echo Shift Noise in Real-Time Pulse-Echo Speed-of-Sound Imaging. Sensors. 2023; 23(12):5598. https://doi.org/10.3390/s23125598
Chicago/Turabian StyleSalemi Yolgunlu, Parisa, Naiara Korta Martiartu, Urs Richard Gerber, Martin Frenz, and Michael Jaeger. 2023. "Excluding Echo Shift Noise in Real-Time Pulse-Echo Speed-of-Sound Imaging" Sensors 23, no. 12: 5598. https://doi.org/10.3390/s23125598




