Curcuma longa-Based Optical Sensor for Hydrochloric Acid and Ammonia Vapor Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Testing of C. longa Pigment
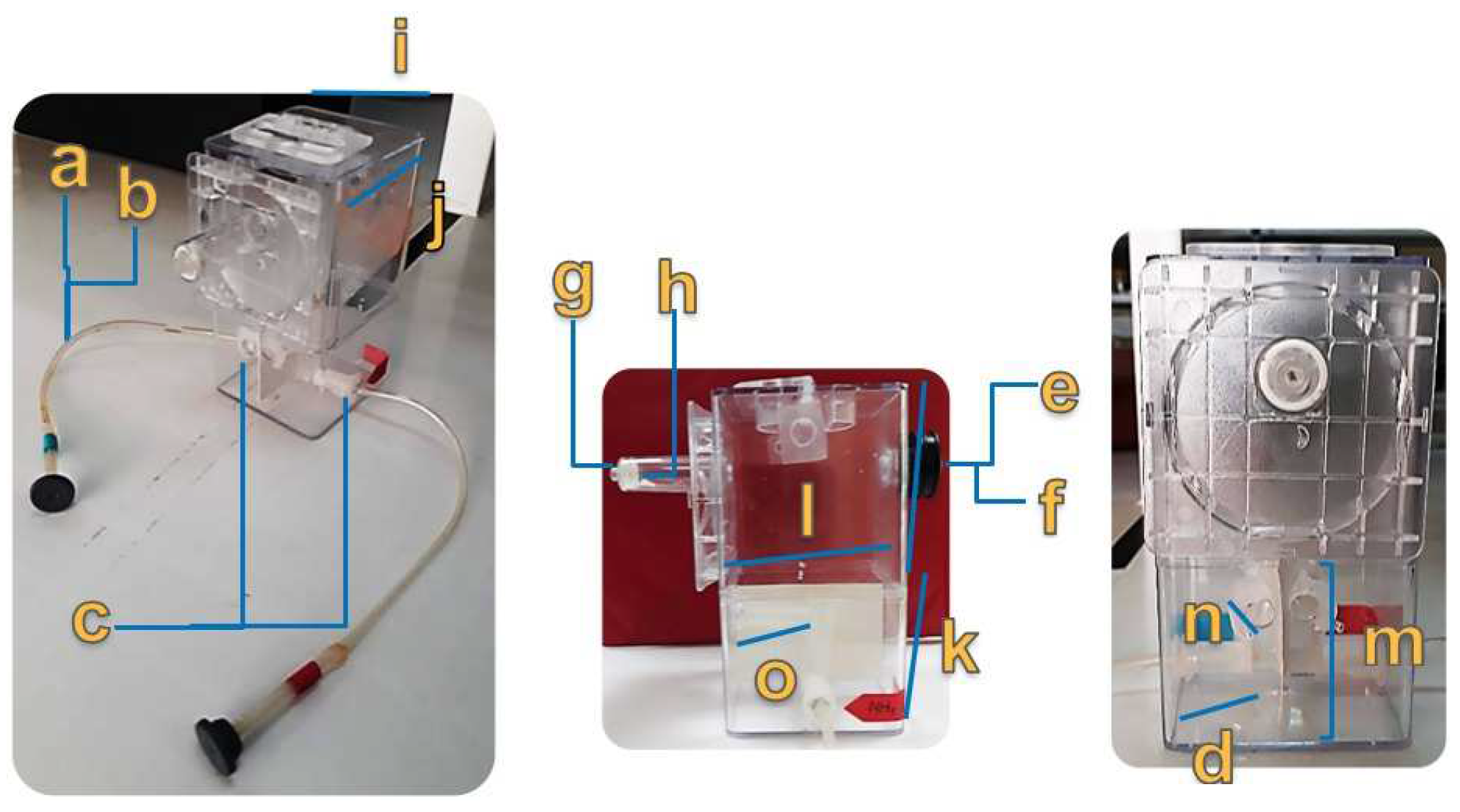
2.2. Sensor Cell Design and Construction
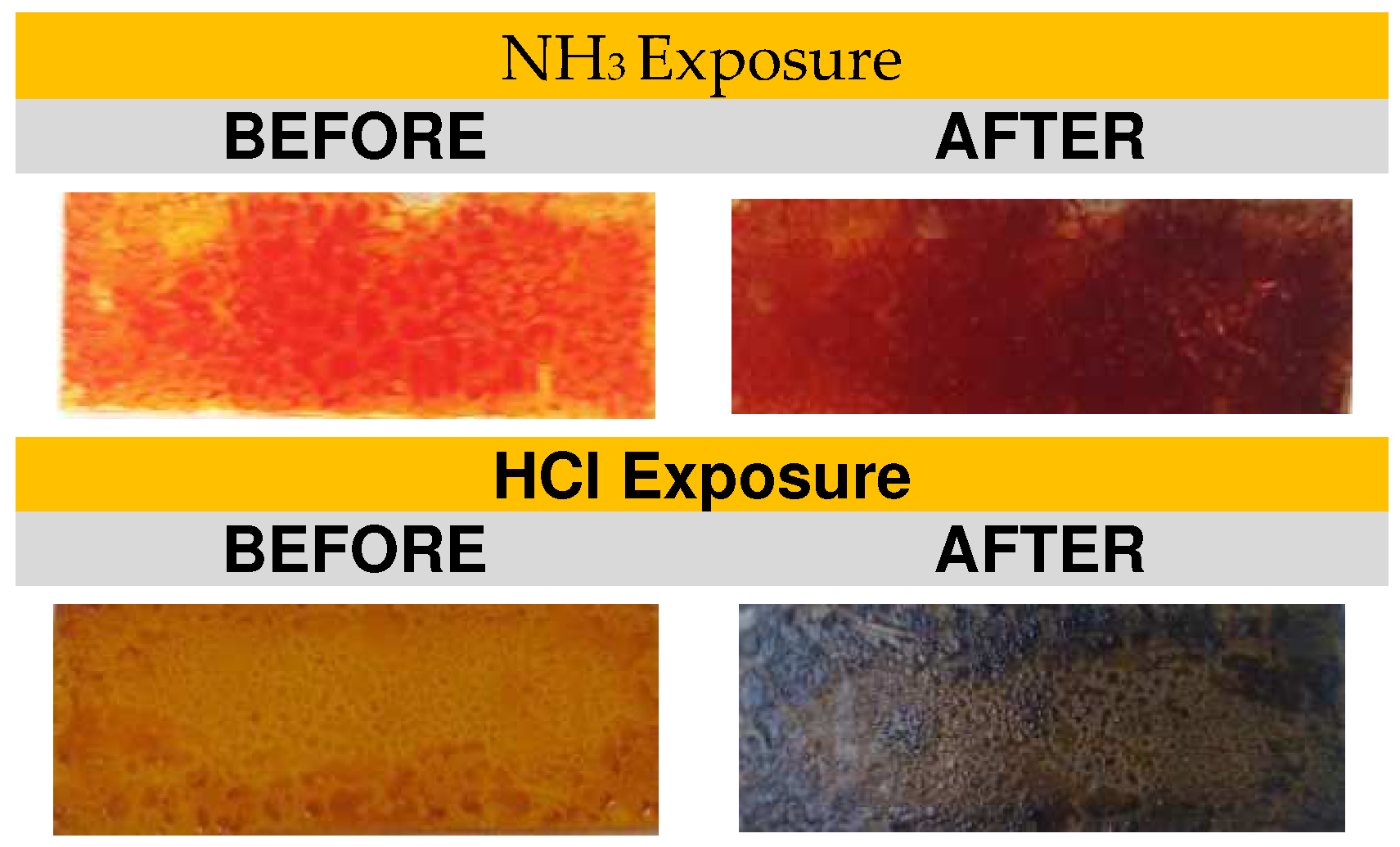
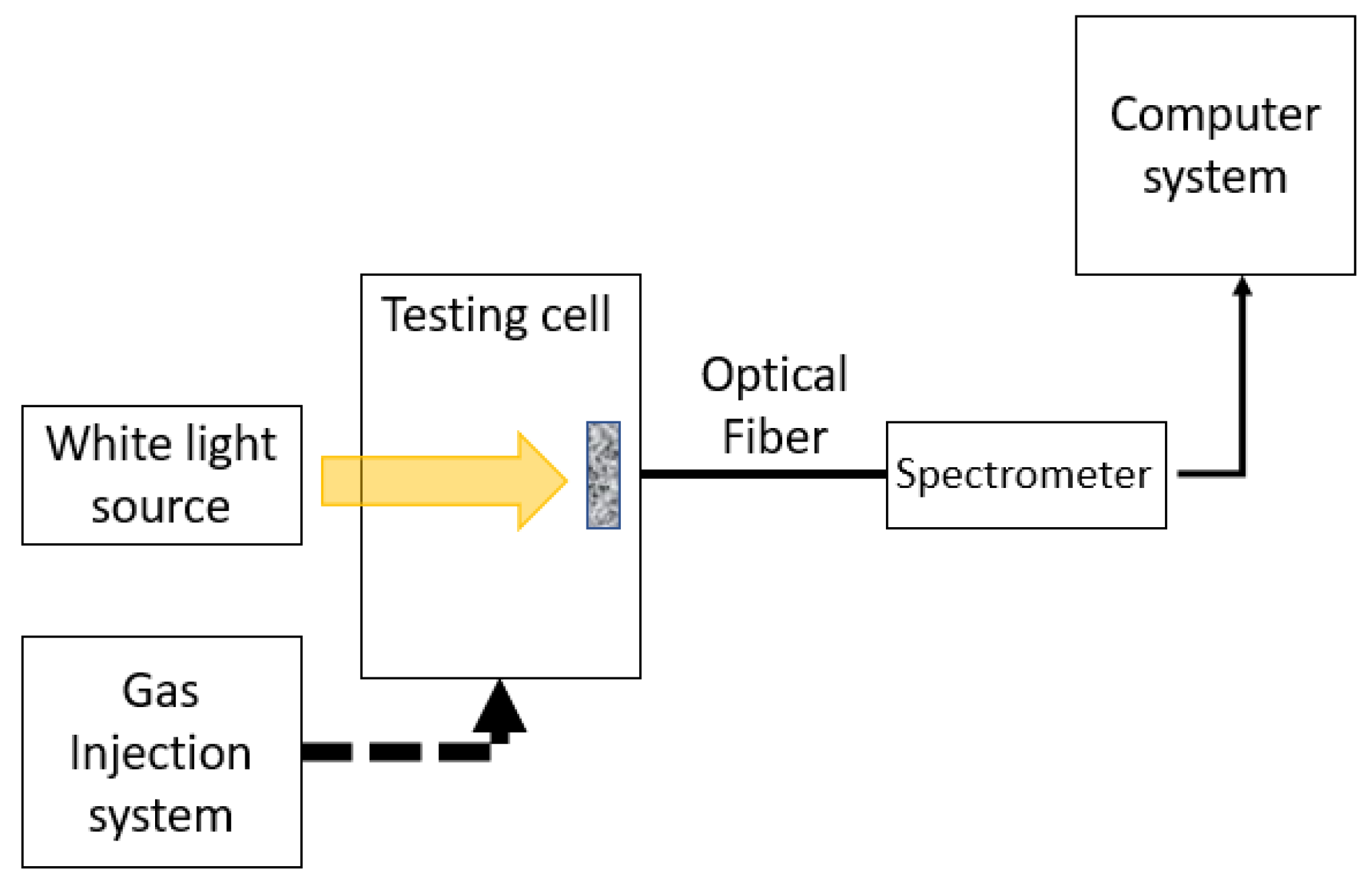
2.3. Main HCl y NH3 Exposure Experiments and Data Collection
2.4. Evaporation Rate
3. Results Discussion
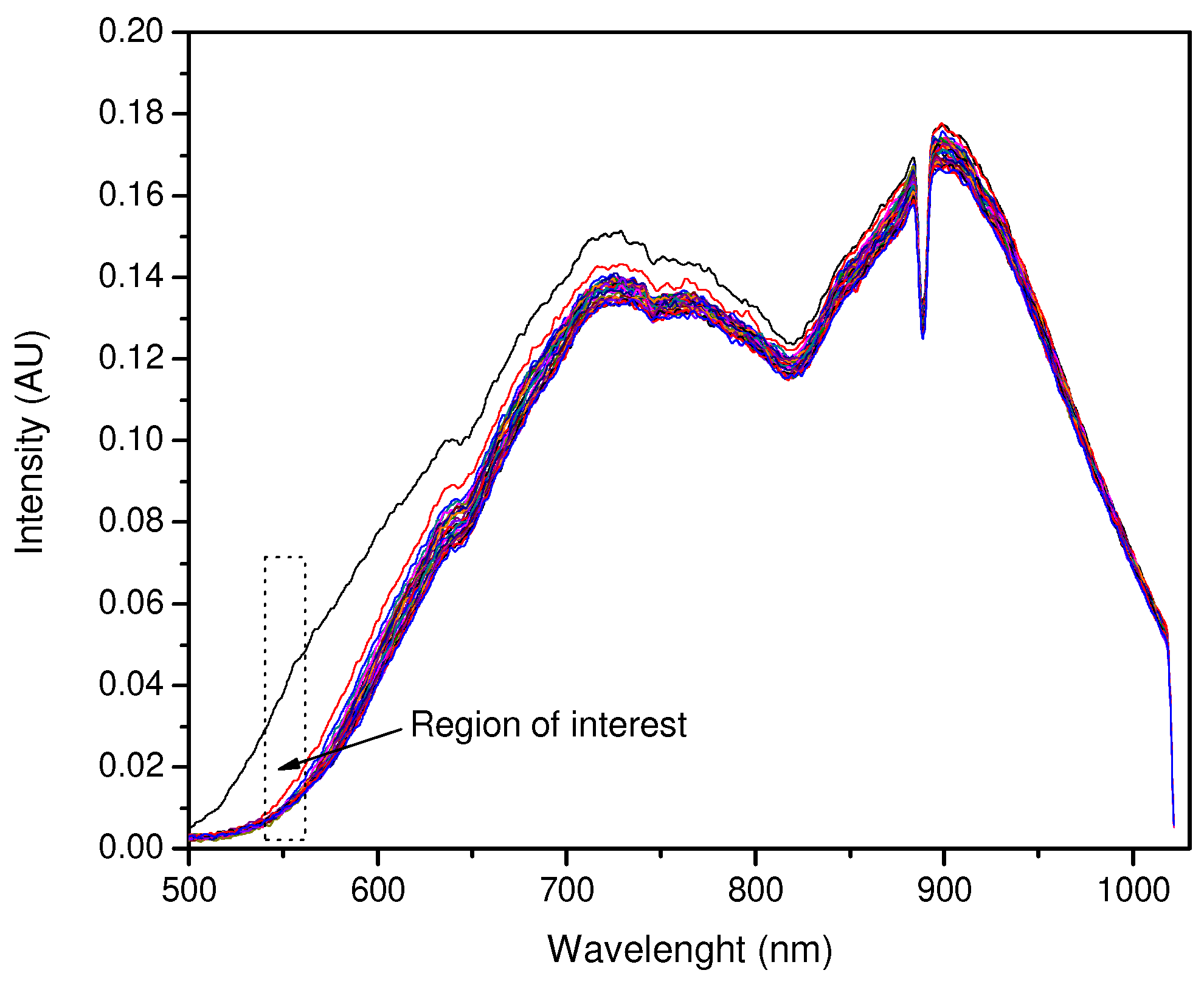
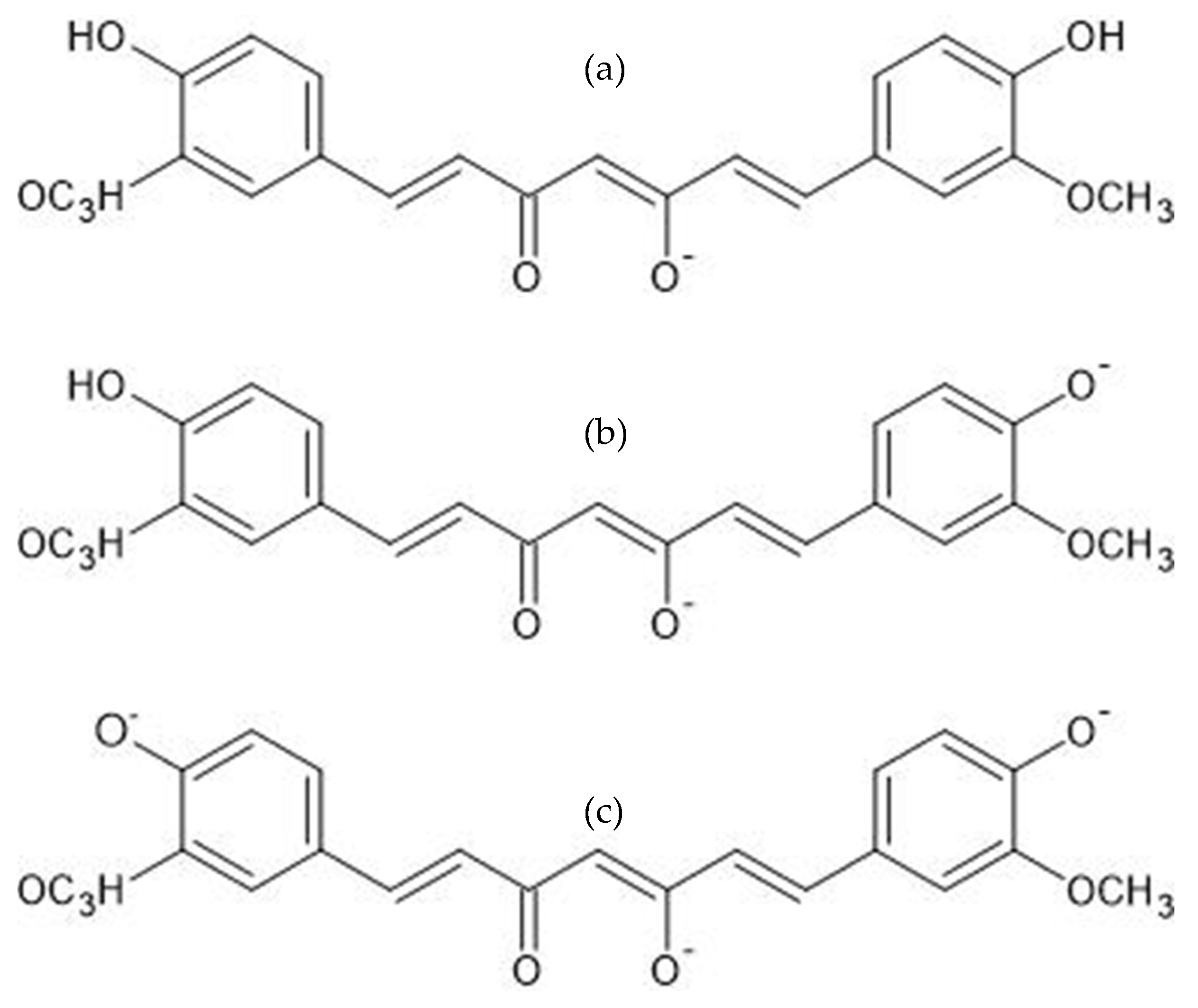
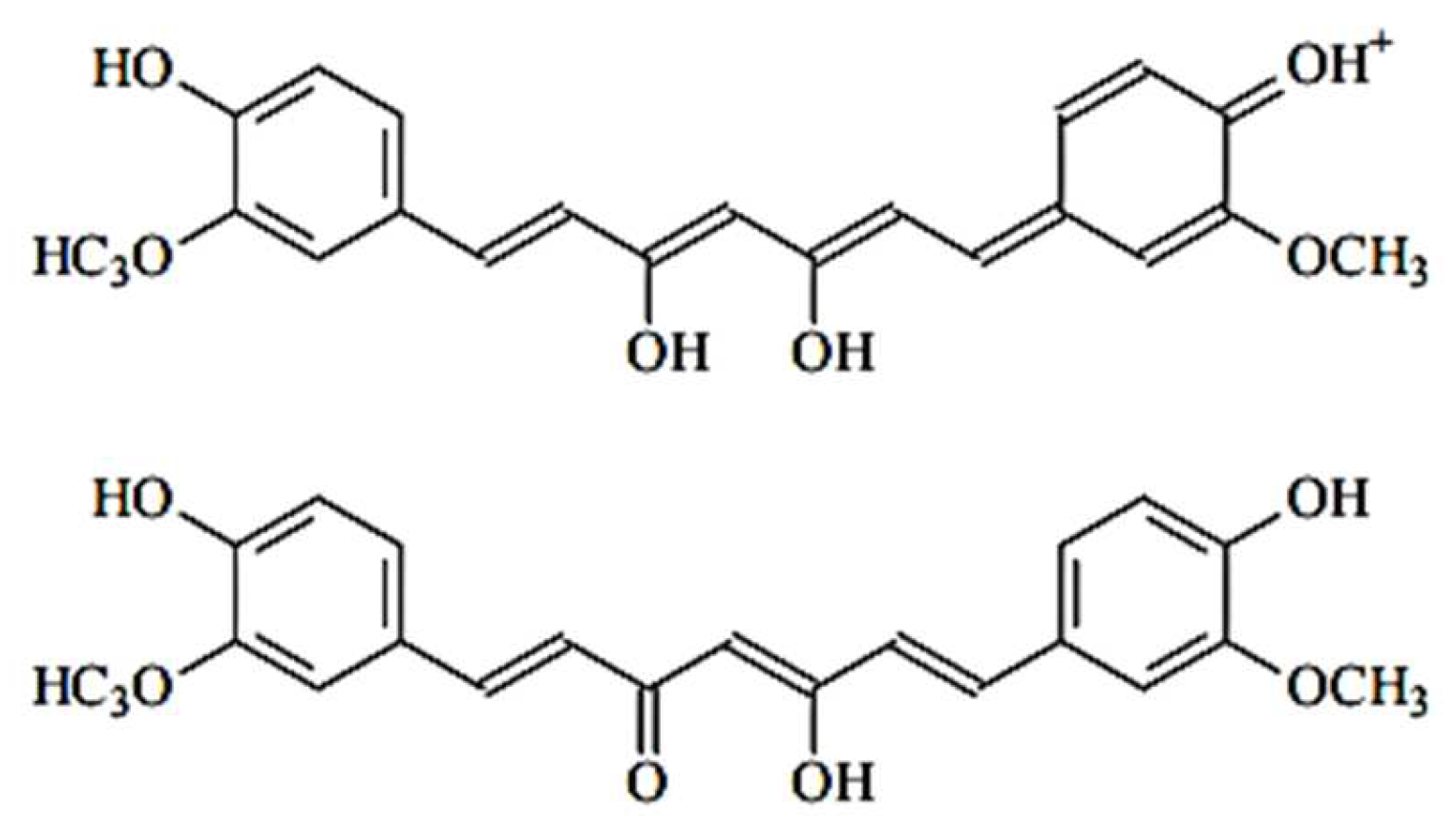
3.1. Curcumin Light Absorption and pH Interaction
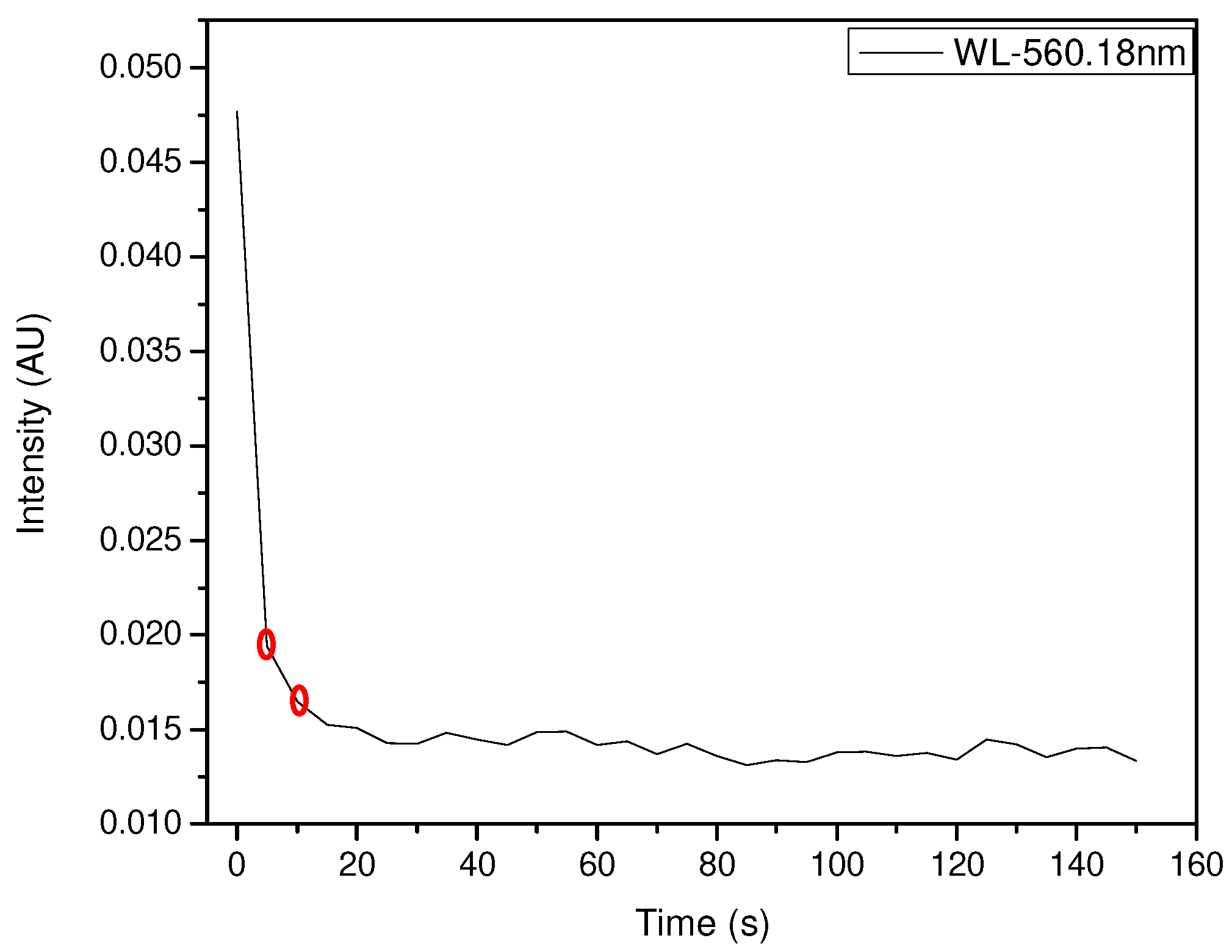
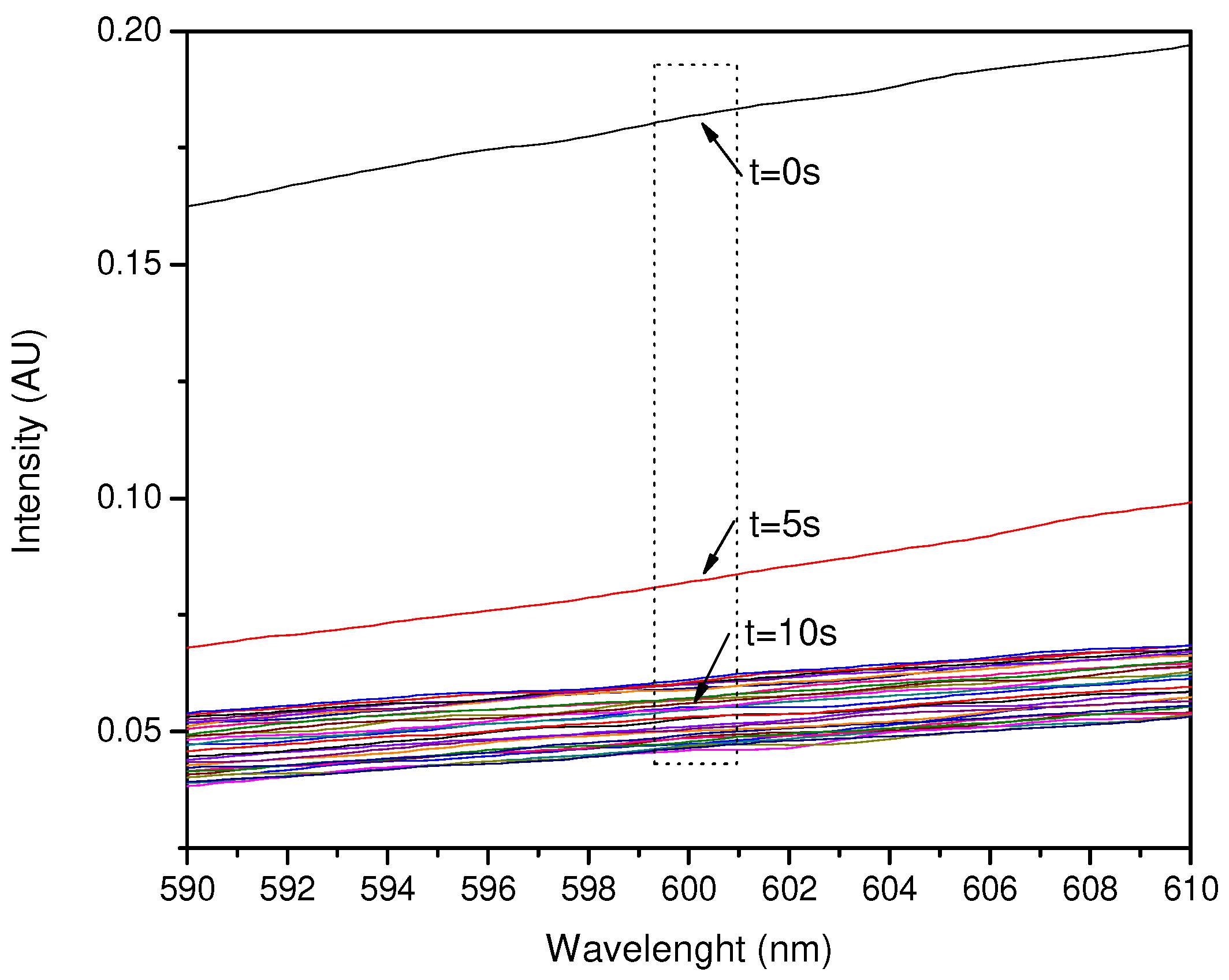
3.2. Main Sensor Test
3.3. Light Absorption of Curcumin and Interaction with pH Value
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krohn, D. Fiber Optic Sensors: Fundamentals and Applications; Spie Press: Bellingham, WA, USA, 2015. [Google Scholar]
- Wumaier, K.; Mamtmin, G.; Ma, Q.; Maimaiti, A.; Nizamidin, P.; Yimit, A. Zinc Phthalocyanine Thin Film-Based Optical Waveguide H2S Gas Sensor. Photonic Sens. 2022, 12, 74–83. [Google Scholar] [CrossRef]
- Tian, C.; Chang, J.; Wang, Q.; Wei, W.; Zhu, C. Realization of Rapid Debugging for Detection Circuit of Optical Fiber Gas Sensor: Using an Analog Signal Source. Photonic Sens. 2015, 5, 91–96. [Google Scholar] [CrossRef]
- Lesiak, P.; Wolí, T.; Jaroszewicz, L.; Zuchowska, M.; Marc, M.; Jakubowska, I.; Jaroszewicz, L.R. Threshold Volatile Organic Compounds Sensor Based on Polymer Microtip. Eng. Proc. 2022, 21, 44. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, L.; Bennion, I.; Koba, M.; Mikulic, P.; Bock, W.J. Towards Refractive Index Sensitivity of Long-Period Gratings at Level of Tens of μm per Refractive Index Unit: Fiber Cladding Etching and Nano-Coating Deposition. Opt. Express 2016, 24, 11897–11904. [Google Scholar] [CrossRef]
- Shu, X.; Gwandu, B.A.; Liu, Y.; Zhang, L.; Bennion, I. Sampled Fiber Bragg Grating for Simultaneous Refractive-Index and Temperature Measurement. Opt. Lett. 2001, 26, 774–776. [Google Scholar] [CrossRef]
- Ahsani, V.; Ahmed, F.; Jun, M.B.G.; Bradley, C. Tapered Fiber-Optic Mach-Zehnder Interferometer for Ultra-High Sensitivity Measurement of Refractive Index. Sensors 2019, 19, 1652. [Google Scholar] [CrossRef]
- Śmietana, M.; Janik, M.; Koba, M.; Bock, W.J. Transition between Bulk and Surface Refractive Index Sensitivity of Micro-Cavity in-Line Mach-Zehnder Interferometer Induced by Thin Film Deposition. Opt. Express 2017, 25, 26118–26123. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Coelho, L.C.C.; de Almeida, J.M.M.M. Measuring Water Vapor Sorption Hysteresis of Cement Paste through an Optical Fiber Sensor. Chemosensors 2023, 11, 123. [Google Scholar] [CrossRef]
- Bahos, F.A.; Vallejos, S.; Gràcia, I.; Cané, C.; Fernández, M.J.; Horrillo, M.C.; Matatagui, D. High-Performance Ammonia Sensor at Room Temperature Based on a Love-Wave Device with Fe2O3@WO3−x Nanoneedles. Proceedings 2017, 1, 484. [Google Scholar] [CrossRef]
- Mohd Noor, M.Y.; Khalili, N.; Skinner, I.; Peng, G.D. Optical Humidity Sensor Based on Air Guided Photonic Crystal Fiber. Photonic Sens. 2012, 2, 277–282. [Google Scholar] [CrossRef]
- Cordero, S.R.; Beshay, M.; Low, A.; Mukamal, H.; Ruiz, D.; Lieberman, R.A. A Distributed Fiber Optic Chemical Sensor for Hydrogen Cyanide Detection. Adv. Environ. Chem. Biol. Sens. Technol. III 2005, 5993, 599302. [Google Scholar] [CrossRef]
- Nitiss, E.; Bundulis, A.; Tokmakovs, A.; Busenbergs, J.; Rutkis, M. All-Organic Waveguide Sensor for Volatile Solvent Sensing. Photonic Sens. 2019, 9, 356–366. [Google Scholar] [CrossRef]
- Marć, P.; Żuchowska, M.; Jakubowska, I.; Jaroszewicz, L.R. Polymer Microtip on a Multimode Optical Fiber as a Threshold Volatile Organic Compounds Sensor. Sensors 2022, 22, 1246. [Google Scholar] [CrossRef]
- Renganathan, B.; Ganesan, A.R. Fiber Optic Gas Sensor with Nanocrystalline ZnO. Opt. Fiber Technol. 2014, 20, 48–52. [Google Scholar] [CrossRef]
- Hu, M.; Kang, W.; Cheng, B.; Li, Z.; Zhao, Y.; Li, L. Sensitive and Fast Optical HCl Gas Sensor Using a Nanoporous Fiber Membrane Consisting of Poly(Lactic Acid) Doped with Tetraphenylporphyrin. Microchim. Acta 2016, 183, 1713–1720. [Google Scholar] [CrossRef]
- Chu, J.; Shen, C.; Zhong, C.; Zou, X.; Li, K.; Dong, X. Optical Fiber Refractometer Based on a Long-Period Grating Inscribed in a Fiber Loop Mirror. In Proceedings of the 2012 Symposium on Photonics and Optoelectronics, SOPO 2012, Shanghai, China, 21–23 May 2012. [Google Scholar] [CrossRef]
- Stawska, H.I.; Popenda, M.A. Refractive Index Sensors Based on Long-Period Grating in a Negative Curvature Hollow-Core Fiber. Sensors 2021, 21, 1803. [Google Scholar] [CrossRef]
- Schroeder, K.; Ecke, W.; Mueller, R.; Willsch, R.; Andreev, A. A Fibre Bragg Grating Refractometer. Meas. Sci. Technol. 2001, 12, 757. [Google Scholar] [CrossRef]
- Yan, B.; Sun, L.; Luo, Y.; Yang, L.; Qi, H.; Chen, X.; Wang, K.; Yuan, J.; Sang, X.; Wang, C.; et al. Temperature Self-Compensated Refractive Index Sensor Based on Fiber Bragg Grating and the Ellipsoid Structure. Sensors 2019, 19, 5211. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.J.; Bartlett, R.; Wong, Y.M.; Scully, P.J.; Kadim, H.J.; Alexiou, V.; Bartlett, R.J. Automation and Dynamic Characterization of Light Intensity with Applications to Tapered Plastic Optical Fibre. J. Opt. A Pure Appl. Opt. 2003, 5, S51. [Google Scholar] [CrossRef]
- Zhang, L.; Lou, J.; Tong, L. Micro/Nanofiber Optical Sensors. Photonic Sens. 2011, 1, 31–42. [Google Scholar] [CrossRef]
- Luo, W.; Chen, Y.; Xu, F. Recent Progress in Microfiber-Optic Sensors. Photonic Sens. 2021, 11, 45–68. [Google Scholar] [CrossRef]
- Kocaadam, B.; Sanlier, N. Curcumin, an Active Component of Turmeric (Curcuma Longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Stankovik, I. Curcumin: Chemical and Technical Assessment. Available online: https://www.fao.org/fileadmin/templates/agns/pdf/jecfa/cta/61/Curcumin.pdf (accessed on 9 May 2023).
- Li, Z.; Hou, L.; Ran, L.; Kang, J.; Yang, J. Ultra-Sensitive Fiber Refractive Index Sensor with Intensity Modulation and Self-Temperature Compensation. Sensors 2019, 19, 3820. [Google Scholar] [CrossRef] [PubMed]
- Jauregui-Vazquez, D.; Alvarez-Chavez, J.A.; Lozano-Hernandez, T.; Estudillo-Ayala, J.M.; Sierra-Hernandez, J.M.; Offerhaus, H.L. Fiber Laser Sensor Configurations for Refractive Index, Temperature and Strain: A Review. Photonics 2023, 10, 495. [Google Scholar] [CrossRef]
- Oblov, K.Y.; Ivanova, A.V.; Soloviev, S.A.; Zhdanov, S.V.; Voronov, Y.A.; Florentsev, V.V. Carbon Dioxide Gas Sensor Based on Optical Control of Color in Liquid Indicator. IOP Conf. Ser. Mater. Sci. Eng. 2016, 151, 012031. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Kadowaki, Y. Poly(Acrylamide) Derivatives for QCM-Based HCl Gas Sensor Applications. Sens. Actuators B Chem. 2008, 130, 842–847. [Google Scholar] [CrossRef]
- Cano, M.; Castillero, P.; Roales, J.; Pedrosa, J.M.; Brittle, S.; Richardson, T.; González-Elipe, A.R.; Barranco, A. A Transparent TMPyP/TiO2 Composite Thin Film as an HCl Sensitive Optochemical Gas Sensor. Sens. Actuators B Chem. 2010, 150, 764–769. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, X.; Du, H.; Zhu, H.; Li, D.; Ao, D.; Guo, Y.; Fu, Y.Q.; Zu, X. Cellulose Nano-Crystals as a Sensitive and Selective Layer for High Performance Surface Acoustic Wave HCl Gas Sensors. Sens. Actuators A Phys. 2020, 301, 111792. [Google Scholar] [CrossRef]
- Vessally, E.; Behmagham, F.; Massoumi, B.; Hosseinian, A.; Edjlali, L. Carbon Nanocone as an Electronic Sensor for HCl Gas: Quantum Chemical Analysis. Vacuum 2016, 134, 40–47. [Google Scholar] [CrossRef]
- Yu, Y.L.; Kishikawa, H.; Oguchi, K.; Chiu, H.Y.; Liaw, S.K.; Liu, W.F. Graphene-assisted Synthesis NH3 Gas Sensor Based on Silicon Photonics Crystal Fiber and Surface Plasmon Resonance. Optik 2022, 267, 169654. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Zhang, H.; Yu, S.; Man, J. High Performance NH3 Sensor Based on CeO2/In2O3 Heterostructure for Mutton Preservation Detection. Mater. Sci. Semicond. Process. 2023, 161, 107475. [Google Scholar] [CrossRef]
- Wang, E.; Chow, K.F.; Wang, W.; Wong, C.; Yee, C.; Persad, A.; Mann, J.; Bocarsly, A. Optical Sensing of HCl with Phenol Red Doped Sol–Gels. Anal. Chim. Acta 2005, 534, 301–306. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Juárez, A.; Carrión, F.; Carrión, J.; Castillo, D.; Padilla-Martínez, J.P.; Cruz-Félix, Á. Curcuma longa-Based Optical Sensor for Hydrochloric Acid and Ammonia Vapor Detection. Sensors 2023, 23, 5602. https://doi.org/10.3390/s23125602
Sánchez Juárez A, Carrión F, Carrión J, Castillo D, Padilla-Martínez JP, Cruz-Félix Á. Curcuma longa-Based Optical Sensor for Hydrochloric Acid and Ammonia Vapor Detection. Sensors. 2023; 23(12):5602. https://doi.org/10.3390/s23125602
Chicago/Turabian StyleSánchez Juárez, A., Fabián Carrión, Javier Carrión, Darwin Castillo, J. P. Padilla-Martínez, and Ángel Cruz-Félix. 2023. "Curcuma longa-Based Optical Sensor for Hydrochloric Acid and Ammonia Vapor Detection" Sensors 23, no. 12: 5602. https://doi.org/10.3390/s23125602
APA StyleSánchez Juárez, A., Carrión, F., Carrión, J., Castillo, D., Padilla-Martínez, J. P., & Cruz-Félix, Á. (2023). Curcuma longa-Based Optical Sensor for Hydrochloric Acid and Ammonia Vapor Detection. Sensors, 23(12), 5602. https://doi.org/10.3390/s23125602





