Nanocomposite Co3O4-ZnO Thin Films for Photoconductivity Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Co3O4-ZnO Thin Films
2.3. Materials’ Characterization
2.4. Photovoltaic Measurements
3. Results and Discussion
3.1. TGA and DSC
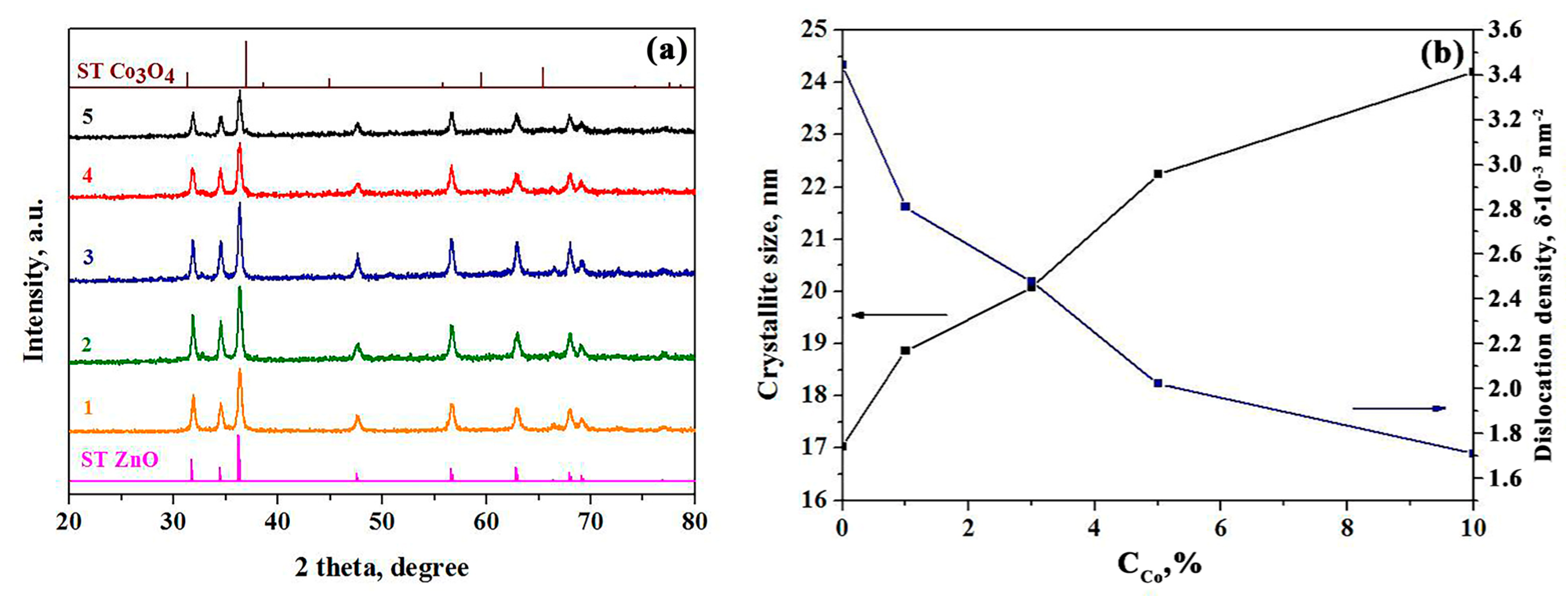
3.2. XRD
3.3. SEM
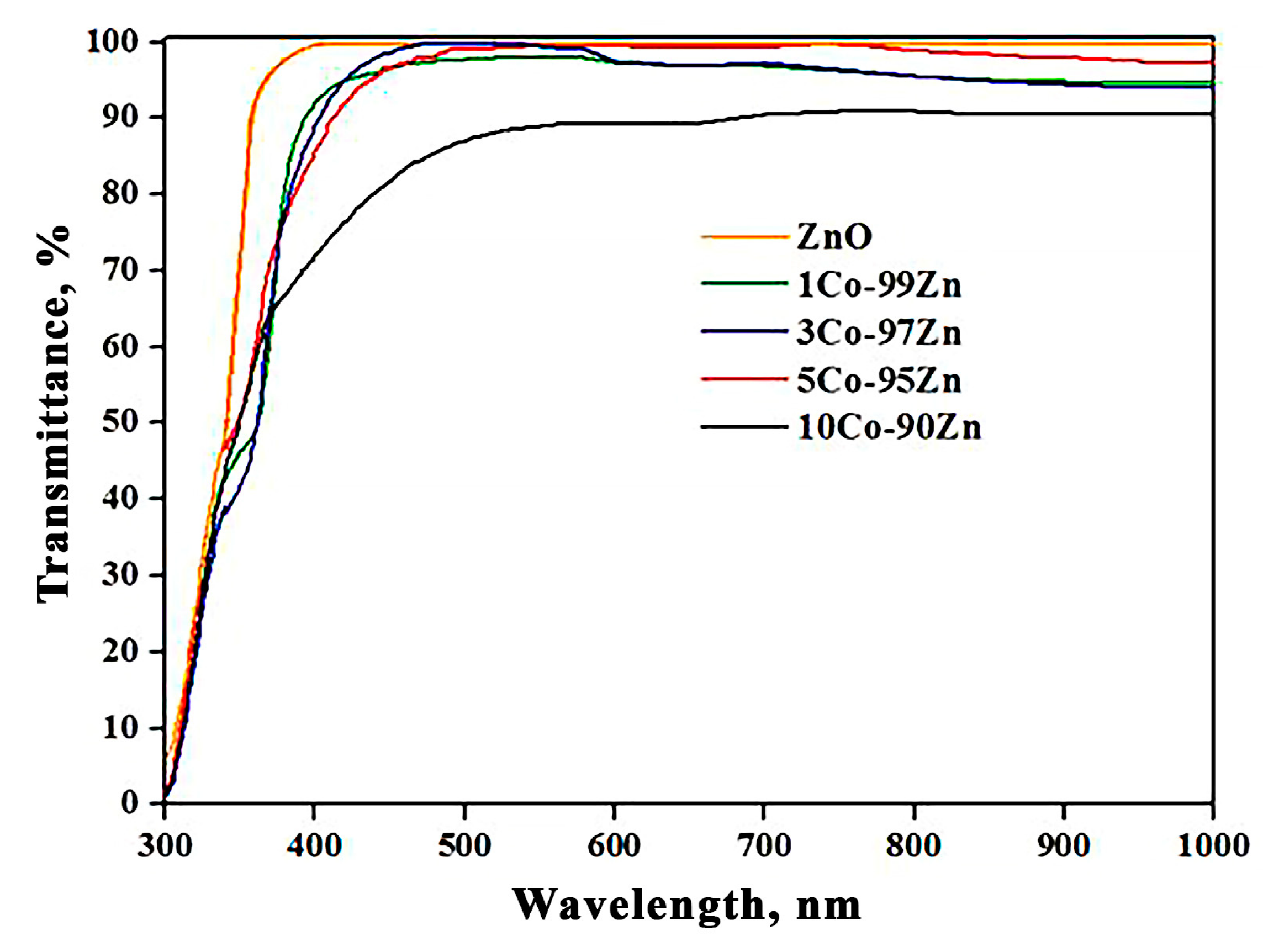
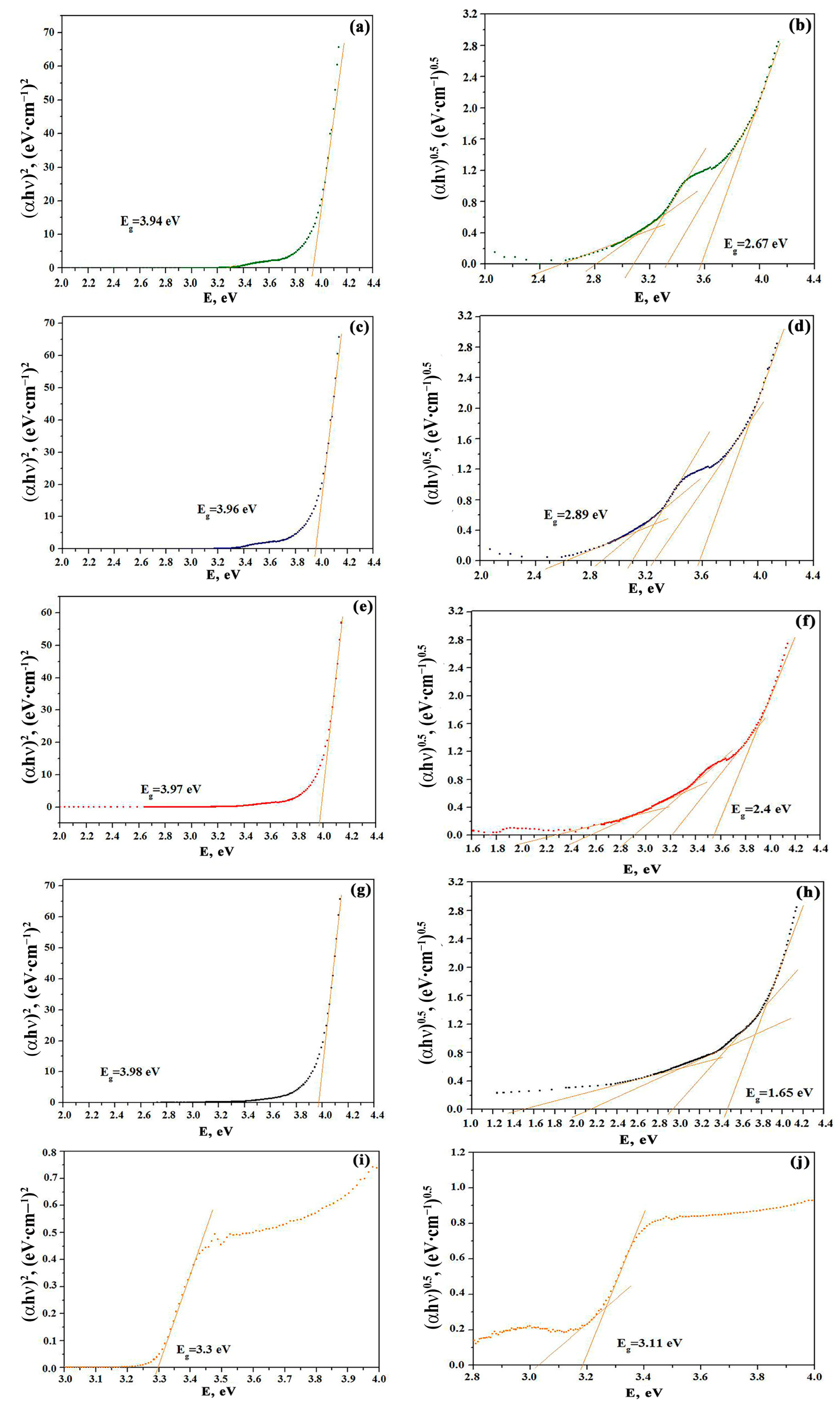
3.4. Optical Properties
3.5. Electrophysical Properties
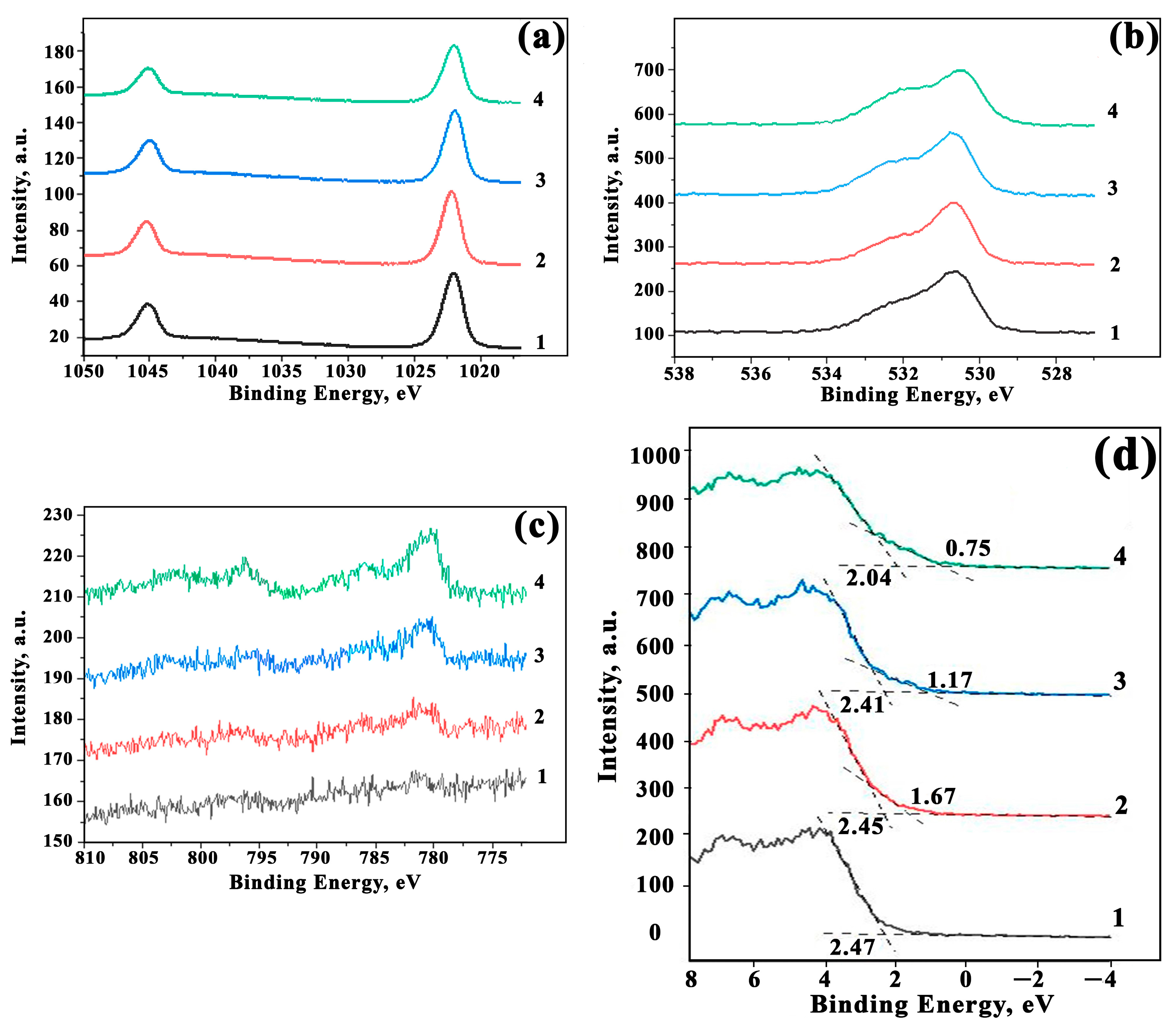
3.6. XPS Analysis
3.7. Surface Properties
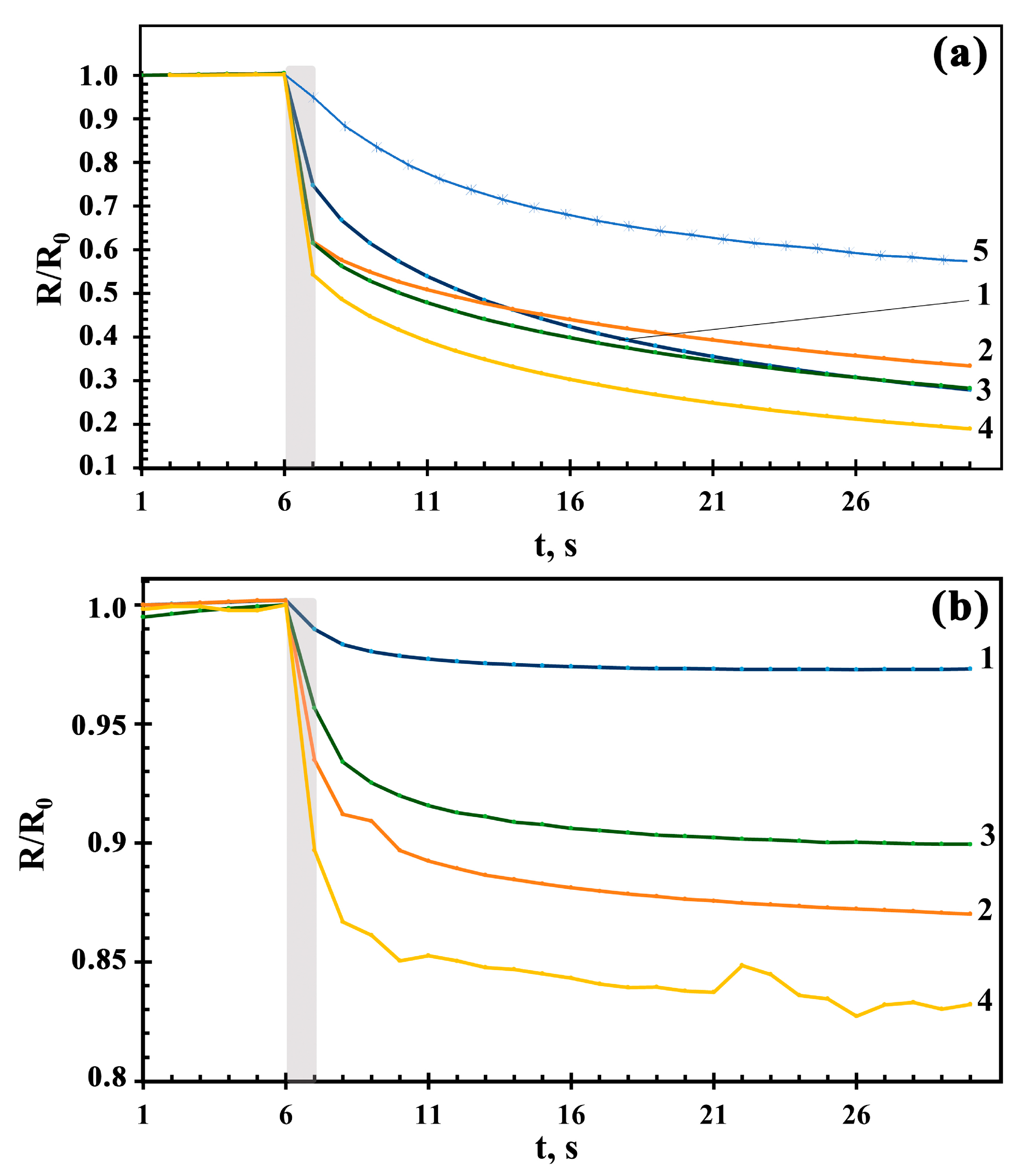
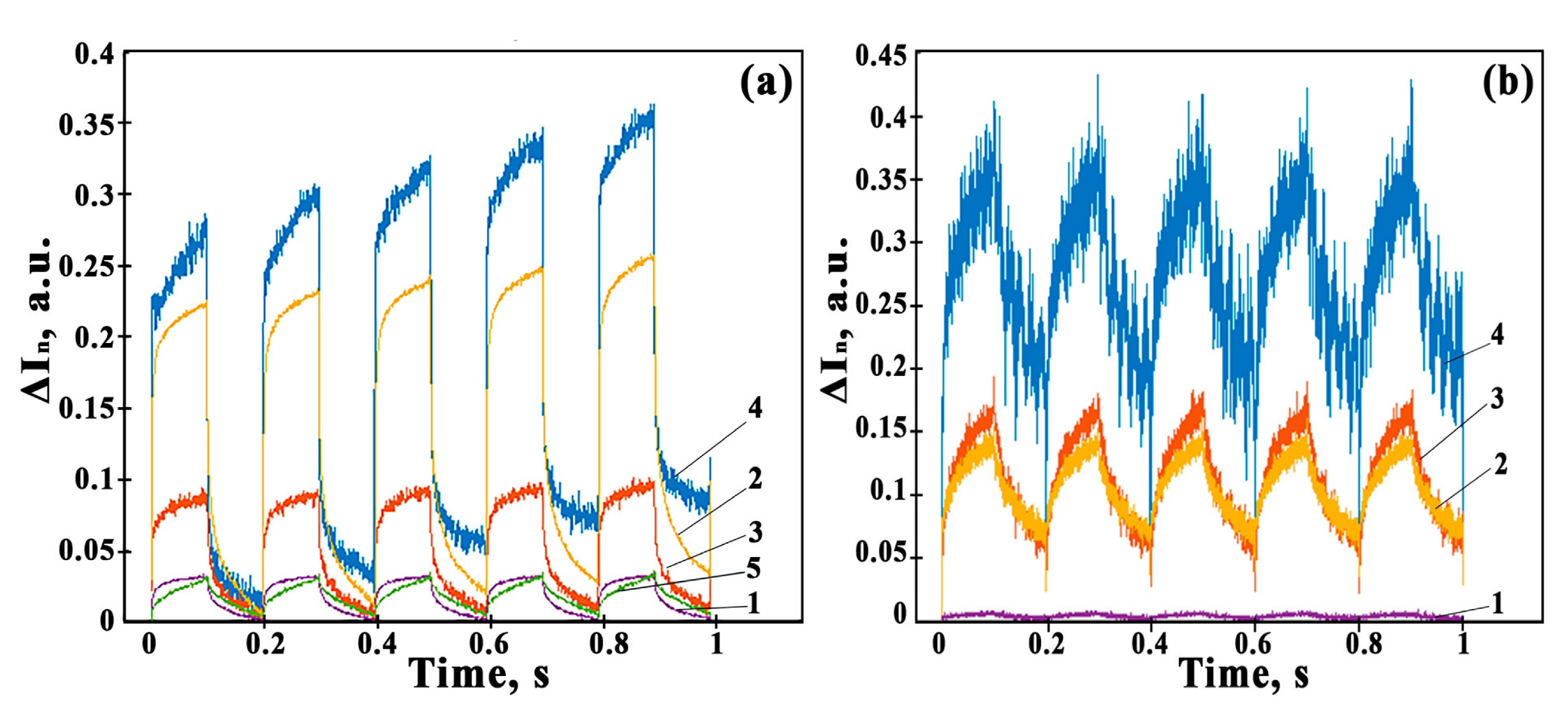
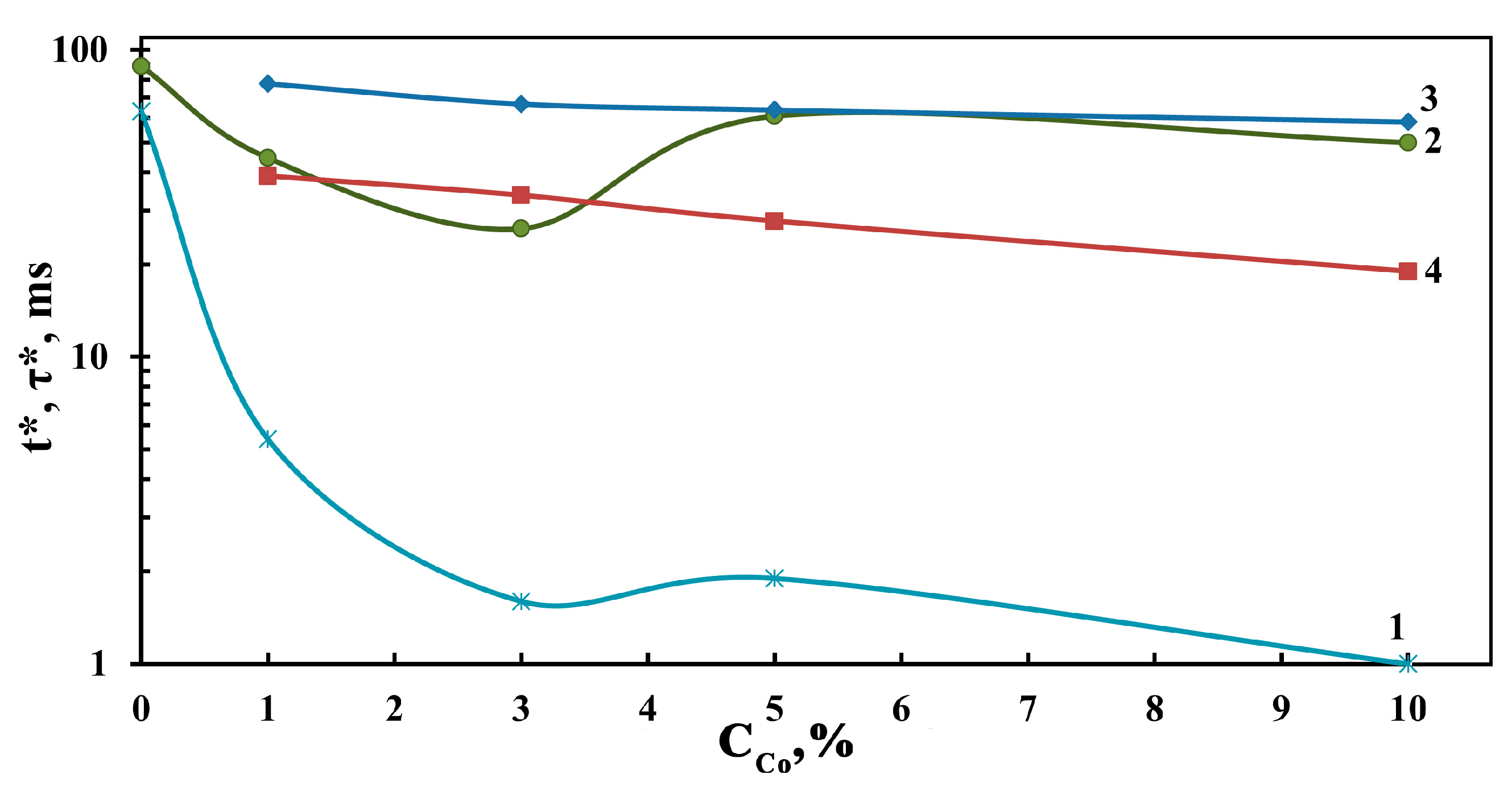
3.8. Photoconductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saadi, H.; Benzarti, Z.; Sanguino, P.; Pina, J.; Abdelmoula, N.; de Melo, J.S.S. Enhancing the electrical conductivity and the dielectric features of ZnO nanoparticles through Co doping effect for energy storage applications. J. Mater. Sci. Mater. Electron. 2023, 34, 116. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a functional material, a review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Sarma, B.; Barman, D.; Sarma, B.K. AZO (Al:ZnO) thin films with high figure of merit as stable indium free transparent conducting oxide. Appl. Surf. Sci. 2019, 479, 786–795. [Google Scholar] [CrossRef]
- Ignatieva, I.O.; Volkova, M.G.; Gulyaeva, I.A.; Starnikova, A.P.; Petrov, V.V.; Bayan, E.M. The optical and electrophysical properties of Al-ZnO thin films. Mater. Today Proc. 2022, 52, 191–194. [Google Scholar] [CrossRef]
- Rana, A.K.; Patel, M.; Nguyen, T.T.; Yun, J.H.; Kim, J. Transparent Co3O4/ZnO photovoltaic broadband photodetector. Mater. Sci. Semicond. Process. 2020, 117, 105192. [Google Scholar] [CrossRef]
- Badawi, A.; Althobaiti, M.G.; Ali, E.E.; Alharthi, S.S.; Alharbi, A.N. A comparative study of the structural and optical properties of transition metals (Mn = Fe, Co, Mn, Ni) doped ZnO films deposited by spray-pyrolysis technique for optoelectronic applications. Opt. Mater. 2022, 124, 112055. [Google Scholar] [CrossRef]
- Nayak, S.; Kumar, P. Review on structure, optical and magnetic properties of cobalt doped ZnO nanoparticles. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Zhumagulov, S.K.; Ismailova, G.A.; Kalkozova, Z.K.; Kudryashov, V.V.; Serikkanov, A.S. Synthesis of Heterogeneous ZnO/Co3O4 Nanostructures by Chemical Deposition from Solutions. Tech. Phys. 2020, 65, 1139–1143. [Google Scholar] [CrossRef]
- Kruefu, V.; Inpan, U.; Leangtanom, P.; Arkarvipath, C.; Kongpark, P.; Phokharatkul, D.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Enhanced gas-sensing performances of Ru-loaded p-Type Co3O4 nanoparticles. Phys. Status Solidi A 2018, 215, 1701015. [Google Scholar] [CrossRef]
- Vallejo, W.; Cantillo, A.; Salazar, B.; Diaz-Uribe, C.; Ramos, W.; Romero, E.; Hurtado, M. Comparative study of ZnO thin films doped with transition metals (Cu and Co) for methylene blue photodegradation under visible irradiation. Catalysts 2020, 11, 528. [Google Scholar] [CrossRef]
- Dhruvashi; Shishodia, P.K. Effect of cobalt doping on ZnO thin films deposited by sol-gel method. Thin Solid Films 2016, 612, 55–60. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, R.; Chauhan, M.S.; Kumar, G.; Chauhan, S. Spindle-like Co3O4-ZnO nanocomposites scaffold for hydrazine sensing and photocatalytic degradation of rhodamine B dye. Eng. Sci. 2021, 16, 288–300. [Google Scholar] [CrossRef]
- Li, T.; Ichimura, M. Drop-dry deposition of Co3O4 and fabrication of heterojunction solar cells with electrochemically deposited ZnO. Semicond. Sci. Technol. 2021, 36, 095030. [Google Scholar] [CrossRef]
- Obodo, R.M.; Chime, U.; Nkele, A.C.; Nwanya, A.C.; Bashir, A.; Madiba, I.; Asjad, M.; Ahmad, I.; Zhao, T.; Thovhogi, N.; et al. Effect of annealing on hydrothermally deposited Co3O4-ZnO thin films for supercapacitor applications. Mater. Today Proc. 2021, 36, 374–378. [Google Scholar] [CrossRef]
- Sutanto, H.; Wibowo, S.; Arifin, M.; Hidayanto, E. Photocatalytic activity of cobalt-doped zinc oxide thin film prepared using the spray coating technique. Mater. Res. Express 2017, 4, 076409. [Google Scholar] [CrossRef]
- Vempati, S.; Shetty, A.; Dawson, P.; Nanda, K.K.; Krupanidhi, S.B. Solution-based synthesis of cobalt-doped ZnO thin films. Thin Solid Films 2012, 524, 137–143. [Google Scholar] [CrossRef]
- Patel, M.; Park, S.H.; Kim, J. Rapid Thermal Treatment of Reactive Sputtering Grown Nanocrystalline Co3O4 for Enhanced All-Oxide Photovoltaics. Phys. Status Solidi A 2018, 215, 1800216. [Google Scholar] [CrossRef]
- Silva, A.L.C.; Rodrigues, A.D.G.; Jasinevicius, R.G.; de Godoy, M.P.F. Tuning the photoconductivity of Co3O4 thin films by defect engineering. Appl. Surf. Sci. 2022, 606, 154943. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Jing, C.; Zhang, H.; Shao, Q.; Ge, R. A visible-light active pn heterojunction ZnO/Co3O4 composites supported on Ni foam as photoanode for enhanced photoelectrocatalytic removal of methylene blue. Adv. Compos. Hybrid Mater. 2022, 5, 2406–2420. [Google Scholar] [CrossRef]
- Volkova, M.G.; Storozhenko, V.Y.; Petrov, V.V.; Bayan, E.M. Characterization of nanocrystalline ZnO thin films prepared by new pyrolysis method. J. Phys. Conf. Ser. 2020, 1695, 012023. [Google Scholar] [CrossRef]
- Petrov, V.V.; Ivanishcheva, A.P.; Volkova, M.G.; Storozhenko, V.Y.; Gulyaeva, I.A.; Pankov, I.V.; Volochaev, V.A.; Khubezhov, S.A.; Bayan, E.M. High Gas Sensitivity to Nitrogen Dioxide of Nanocomposite ZnO-SnO2 Films Activated by a Surface Electric Field. Nanomaterials 2022, 12, 2025. [Google Scholar] [CrossRef]
- Bayan, E.M.; Petrov, V.V.; Volkova, M.G.; Storozhenko, V.Y.; Chernyshev, A.V. SnO2–ZnO nanocomposite thin films: The influence of structure, composition and crystallinity on optical and electrophysical properties. J. Adv. Dielectr. 2021, 11, 2160008. [Google Scholar] [CrossRef]
- Gulyaeva, I.A.; Ignatieva, I.O.; Bayan, E.M.; Petrov, V.V. Study of structural properties and photoconductivity of Co3O4–ZnO thin films. ST PETER POLY U J-PH. 2022, 15, 271–275. [Google Scholar] [CrossRef]
- He, R.; Tang, B.; Ton-That, C.; Phillips, M.; Tsuzuki, T. Physical structure and optical properties of Co-doped ZnO nanoparticles prepared by co-precipitation. J. Nanopart. Res. 2013, 15, 2030. [Google Scholar] [CrossRef]
- Siegbahn, K. Electron spectroscopy for atoms, molecules, and condensed matter. Rev. Mod. Phys. 1982, 54, 709–728. [Google Scholar] [CrossRef]
- Gulyaeva, I.A.; Ivanisheva, A.P.; Volkova, M.G.; Storozhenko, V.Y.; Khubezhov, S.A.; Bayan, E.M.; Petrov, V.V. Investigation of electrophysical, photo- and gas-sensitive properties of ZnO–SnO2 sol–gel flms. J. Adv. Dielect. 2023, 13, 2245002. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Yalovega, G.E.; Shmatko, V.A.; Funik, A.O.; Petrov, V.V. SiO2CuOx films for nitrogen dioxide detection: Correlation between technological conditions and properties. Sens. Actuators B 2016, 230, 167–175. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database 20, Version 3.4 (Web Version); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Petrov, V.V.; Bayan, E.M.; Khubezhov, S.A.; Varzarev, Y.N.; Volkova, M.G. Investigation of Rapid Gas-Sensitive Properties Degradation of ZnO–SnO2 thin films grown on the glass substrate. Chemosensors 2020, 8, 40. [Google Scholar] [CrossRef]
- Shalimova, K.V. Physics Semiconductors, 2nd ed.; Revised and Expanded, Energy; Scientific Research: Moscow, Russia, 1976. [Google Scholar]
- Reis, R.Y.; Lima, A.E.; Costa, M.J.; Cruz-Filho, J.F.; Moura, J.P.; Santos, R.S.; Luz, G.E., Jr. Enhanced photoelectrocatalytic performance of ZnO films doped with N2 by a facile electrochemical method. Surf. Interfaces 2020, 21, 100675. [Google Scholar] [CrossRef]
- Al-Tuwirqi, R.; Al-Ghamdi, A.A.; Aal, N.A.; Umar, A.; Mahmoud, W.E. Facile synthesis and optical properties of Co3O4 nanostructures by the microwave route. Superlattices Microstruct. 2011, 49, 416–421. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Bashir, Z.; Riaz, S.; Naseem, S.; Saddiqe, Z. Transparent boron-doped zinc oxide films for antibacterial and magnetic applications. J. Mater. Sci. Mater. Electron. 2020, 31, 11911–11926. [Google Scholar] [CrossRef]
- Fathima, M.I.; Wilson, K.J.; Arulanantham, A.M.S. Cobalt doped ZnO films asantireflection coating application for solar cell. AIP Conf. Proc. 2020, 2265, 030262. [Google Scholar] [CrossRef]
- Kulkarni, S.D.; Choudhary, R.J.; Babu, P.D.; Rajendra, B.V. Influence of cobalt doping on structure, optical and magnetic properties of spray pyrolysed nano structured ZnO films. Phys. B Condens. Matter 2019, 572, 18–26. [Google Scholar] [CrossRef]
- Muchuweni, E.; Sathiaraj, T.S.; Nyakotyo, H. Synthesis and characterization of zinc oxide thin films for optoelectronic applications. Heliyon 2017, 3, e00285. [Google Scholar] [CrossRef]
- Petrov, V.V.; Sysoev, V.V.; Starnikova, A.P.; Volkova, M.G.; Kalazhokov, Z.K.; Storozhenko, V.Y.; Khubezhov, S.A.; Bayan, E.M. Synthesis, characterization and gas sensing study of ZnO-SnO2 nanocomposite thin films. Chemosensors 2021, 9, 124. [Google Scholar] [CrossRef]
- Rembeza, S.I.; Svistova, T.V.; Rembeza, E.S.; Borsyakova, O.I. The microstructure and physical properties of thin SnO2 films. Semiconductors 2001, 35, 762–765. [Google Scholar] [CrossRef]
- Hunsicker, R.A.; Klier, K.; Gaffney, T.S.; Kirner, J.G. Framework zinc-substituted zeolites: Synthesis, and core-level and valence-band XPS. Chem. Mater. 2002, 14, 4807–4811. [Google Scholar] [CrossRef]
- Kraut, E.A.; Grant, R.W.; Waldrop, J.R.; Kowalczyk, S.P. Precise determination of the valence-band edge in x-ray photoemission spectra: Application to measurement of semiconductor interface potentials. Phys. Rev. Lett. 1980, 44, 1620–1623. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Khmelevsky, N.; Grunin, A. Sensitization of ZnO Photoconductivity in the Visible Range by Colloidal Cesium Lead Halide Nanocrystals. Nanomaterials 2022, 12, 4316. [Google Scholar] [CrossRef]
- Ryvkin, S.M. Photoelectric Phenomena in Semiconductors; Fizmatgiz: Mosco, Russia, 1963; p. 496. [Google Scholar]
- Tahira, A.; Mazzaro, R.; Rigoni, F.; Nafady, A.; Shaikh, S.F.; Alothman, A.A.; Alshgari, R.A.; Ibupoto, Z.H. A simple and efficient visible light photodetector based on Co3O4/ZnO composite. Opt Quantum Electron 2021, 53, 534. [Google Scholar] [CrossRef]
- Ghamgosar, P.; Rigoni, F.; Kohan, M.G.; You, S.; Morales, E.A.; Mazzaro, R.; Morandi, V.; Almqvist, N.; Concina, I.; Vomiero, A. Self-powered photodetectors based on core–shell ZnO–Co3O4 nanowire heterojunctions. ACS Appl. Mater. Interfaces 2019, 11, 23454–23462. [Google Scholar] [CrossRef] [PubMed]




| Film Material | The Content of Element Atoms | ||
|---|---|---|---|
| O1s | Co2p | Zn2p | |
| 1Co-99Zn | 50 | 1 | 49 |
| 3Co-97Zn | 49 | 3 | 48 |
| 5Co-95Zn | 49 | 5 | 46 |
| 10Co-90Zn | 51 | 9 | 40 |
| Co3O4/ZnO-Films | tres (s), 400 nm | tres (s), 660 nm |
|---|---|---|
| ZnO | 15.5 | --- |
| 1Co-99Zn | 14.5 | 6.5 |
| 3Co-97Zn | 13.5 | 8.0 |
| 5Co-95Zn | 13.0 | 8.5 |
| 10Co-90Zn | 12.5 | 7.0 |
| Synthesis Method, Structure | The Optical Transmittance, % | Wavelength, nm | Response Time | Reference |
| Reactive sputtering method, Co3O4-ZnO p-n- heterojunction | ~76 | White light | 81.7 μs | [5] |
| Chemical growth method, Co3O4-ZnO composite structure | <8 | Solar simulator | 20 s | [44] |
| Spray pyrolysis, Co3O4 thin films | - | 442 nm −633 nm | 12 s 24 s | [18] |
| Sputtering method and hydrothermal synthesis, core–shell Co3O4-ZnO structures | <30 | Visible light | 6 s | [45] |
| Rapid liquid deposition, Co3O4-ZnO composite films (Co:Zn = 3:1) | - | 335 nm | 15 s | [19] |
| Solid-phase pyrolysis, 3Co-97Zn nanocomposite films, 10Co-90Zn nanocomposite films | >65 | 400 660 | 26.2 ms 58.3 ms | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, V.V.; Sysoev, V.V.; Ignatieva, I.O.; Gulyaeva, I.A.; Volkova, M.G.; Ivanishcheva, A.P.; Khubezhov, S.A.; Varzarev, Y.N.; Bayan, E.M. Nanocomposite Co3O4-ZnO Thin Films for Photoconductivity Sensors. Sensors 2023, 23, 5617. https://doi.org/10.3390/s23125617
Petrov VV, Sysoev VV, Ignatieva IO, Gulyaeva IA, Volkova MG, Ivanishcheva AP, Khubezhov SA, Varzarev YN, Bayan EM. Nanocomposite Co3O4-ZnO Thin Films for Photoconductivity Sensors. Sensors. 2023; 23(12):5617. https://doi.org/10.3390/s23125617
Chicago/Turabian StylePetrov, Victor V., Victor V. Sysoev, Irina O. Ignatieva, Irina A. Gulyaeva, Maria G. Volkova, Alexandra P. Ivanishcheva, Soslan A. Khubezhov, Yuri N. Varzarev, and Ekaterina M. Bayan. 2023. "Nanocomposite Co3O4-ZnO Thin Films for Photoconductivity Sensors" Sensors 23, no. 12: 5617. https://doi.org/10.3390/s23125617
APA StylePetrov, V. V., Sysoev, V. V., Ignatieva, I. O., Gulyaeva, I. A., Volkova, M. G., Ivanishcheva, A. P., Khubezhov, S. A., Varzarev, Y. N., & Bayan, E. M. (2023). Nanocomposite Co3O4-ZnO Thin Films for Photoconductivity Sensors. Sensors, 23(12), 5617. https://doi.org/10.3390/s23125617









