Validity of Estimated Results from a Wearable Device for the Tests Time Up and Go and Sit to Stand in Young Adults and in People with Chronic Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants Selection
2.2. Instrumentation
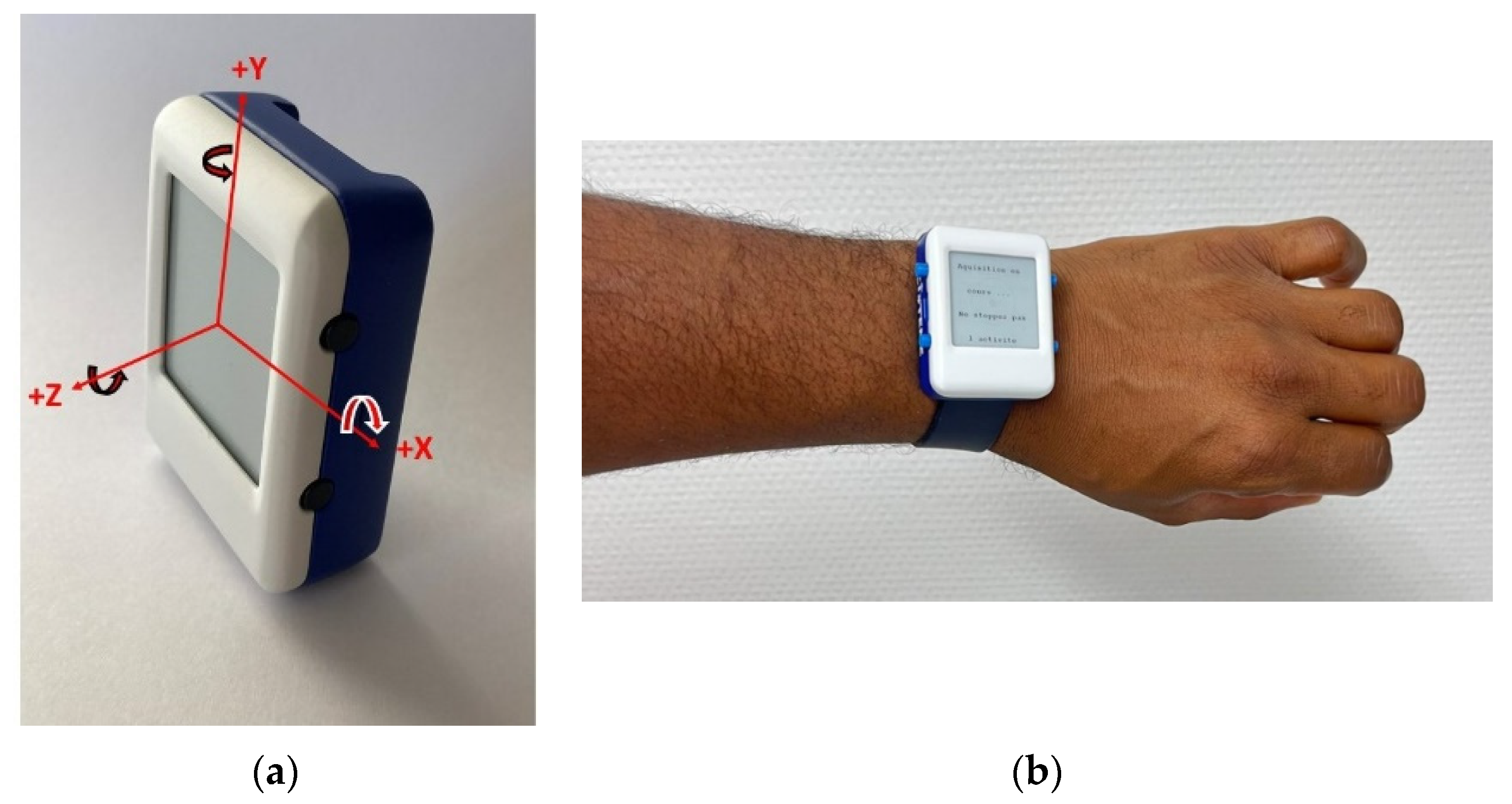
2.2.1. IMU-Based Custom Device Description
2.2.2. The Algorithms
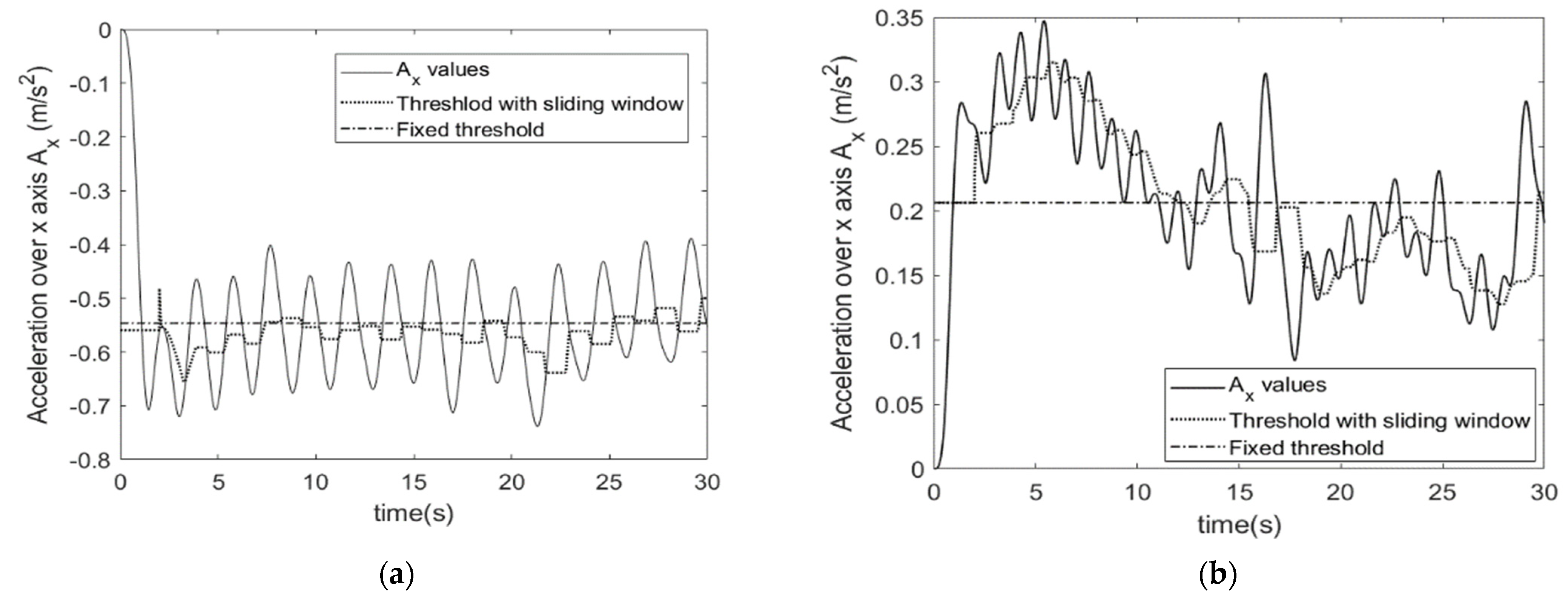
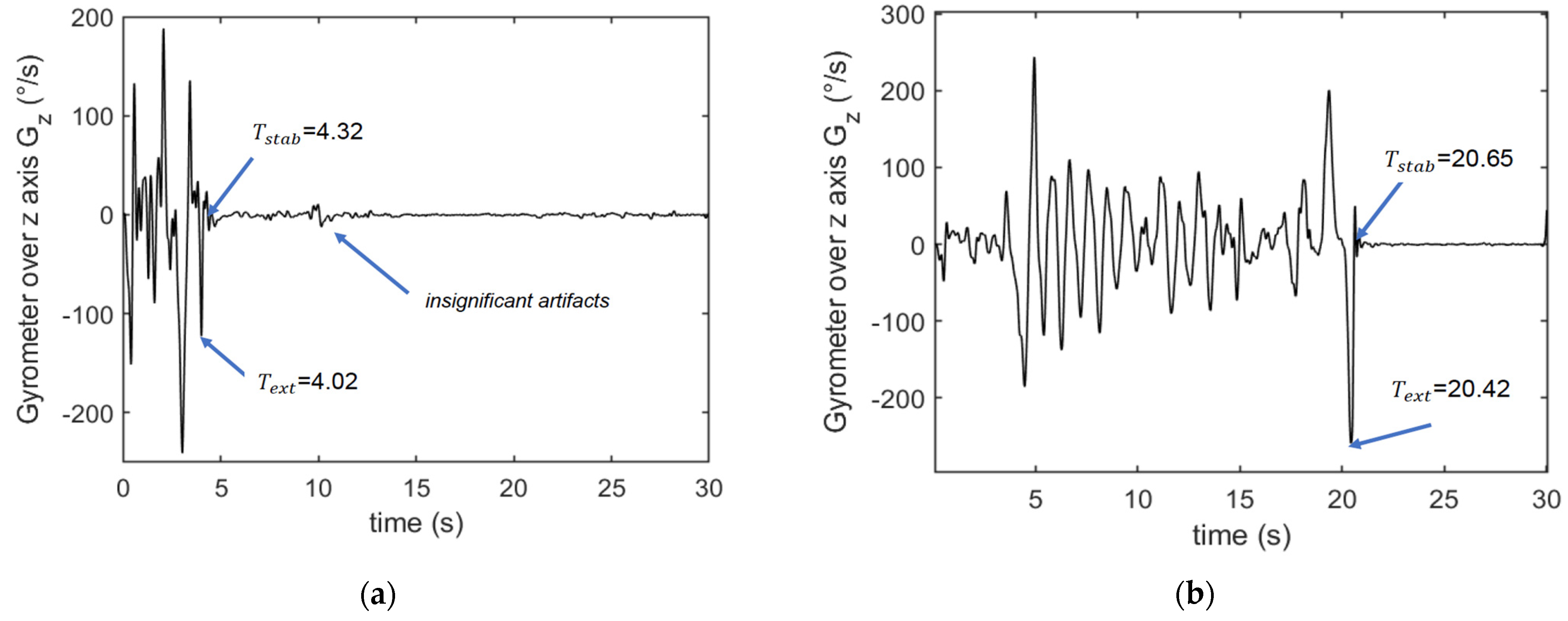
STS Algorithm
TUG Algorithm
2.3. Physical Fitness Tests
2.3.1. Sit to Stand Test
2.3.2. Timed “Up and Go” Test
2.4. Test Protocol and Data Processing
- A.
- Warm-up session with stretching, joint mobilization and muscle strengthening exercises.
- B.
- Installation of the custom device placed on the left wrist of the participant during all tests. The Installation of the sensor required a maximum of 3 min. Prior to use by each participant the device was automatically initialized via the supporting software (USB, prototype). To perform a test, the investigator had to choose the test from the menu, press the “start” button and 3 s later the device vibrated and the participant could start the test, see Figure 1, Figure 2 and Figure 3.
- C.
- Prior to each test, the participant performed a trial run to ensure that they understood the instructions (this step may take about 2 min). The participants performed STS and TUG tests, the results were also measured by the examiner using visual counts for STS and a stop-watch for TUG. The activity mode was automatically disabled at the end of each test. The data collection is carried out during all the tests which is supposed to take no more than 2 times 30 s, so less than 1 min. With the given acquisition frequency of 50 Hz, this corresponds to 3000 measurements. Each measurement corresponds to 9 values issued from the 9-axis accelerometer. With the 12 bits-ADC of the device, a 1-min recording corresponds to less than 40 kbytes.
- D.
- Download of the data from the device: Data from the sensor was downloaded via a universal serial bus (USB) and then processed. The members of the research team used a laptop to configure the device, start a test, and read the outcomes at the end of each test. The testing procedure is illustrated in Figure 4.
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
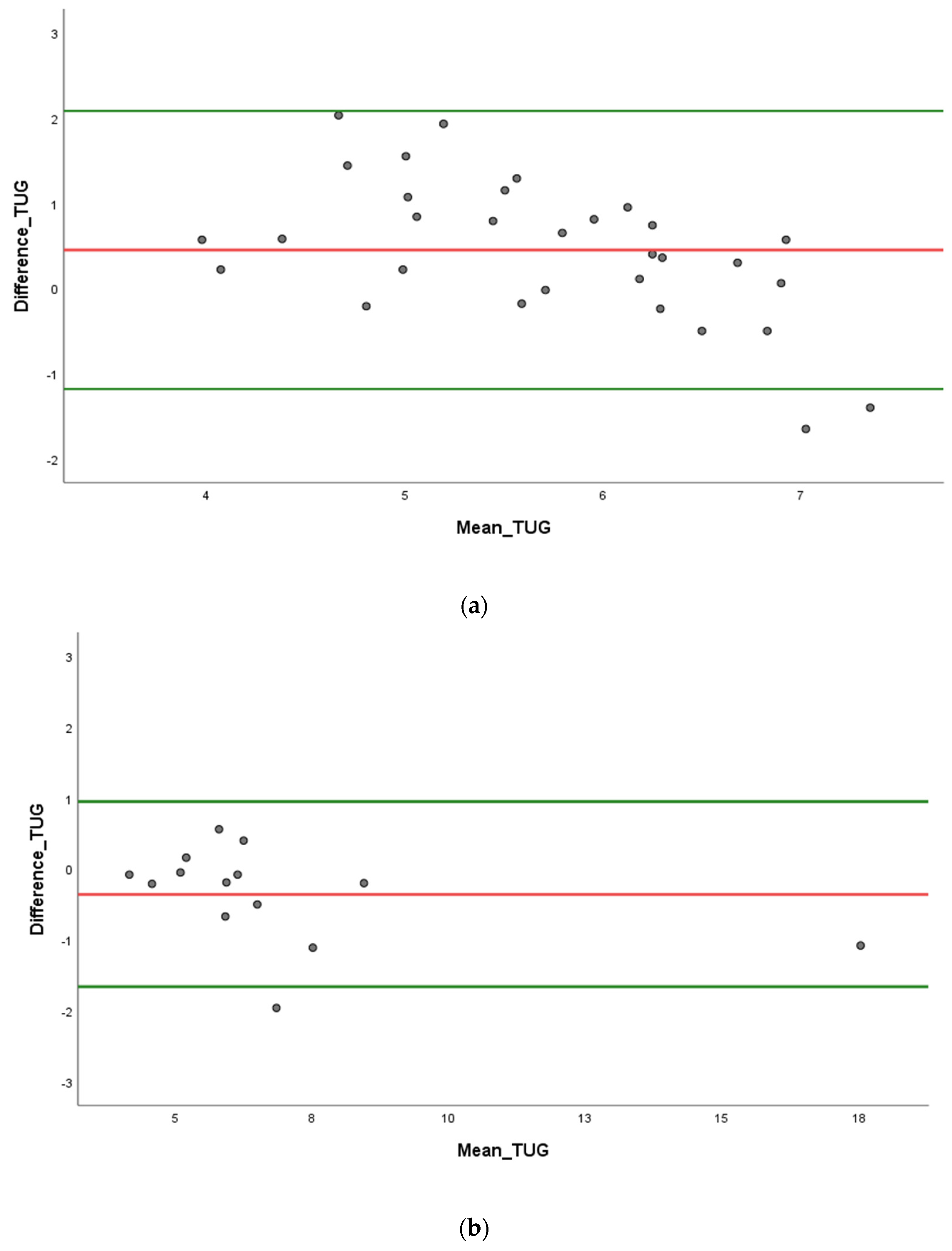
3.2. Validity Parameters
4. Discussion
4.1. Accuracy
4.2. Limitations
4.3. Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chassang, M.; Gautier, A. Les Maladies Chroniques. 2019. Available online: https://www.lecese.fr/actualites/maladies-chroniques-le-cese-adopte-son-avis (accessed on 11 June 2022).
- Chevreul, K.; Brunn, M.; Durand-Zaleski, I.; Farsi, F.; Knai, C.; Nolte, E. France [Internet]. Assessing Chronic Disease Management in European Health Systems: Country Reports [Internet]. European Observatory on Health Systems and Policies, 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK458739/ (accessed on 13 October 2022).
- Kunstler, B.E.; Cook, J.L.; Freene, N.; Finch, C.F.; Kemp, J.L.; O’Halloran, P.D.; Gaida, J.E. Physiotherapist-Led Physical Activity Interventions Are Efficacious at Increasing Physical Activity Levels: A Systematic Review and Meta-analysis. Clin. J. Sport Med. Off. J. Can Acad. Sport Med. 2018, 28, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M. Evidence on the effects of exercise therapy in the treatment of chronic disease. Br. J. Sports Med. 2009, 43, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Jerant, A.F.; von Friederichs-Fitzwater, M.M.; Moore, M. Patients’ perceived barriers to active self-management of chronic conditions. Patient Educ. Couns. 2005, 57, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Gomersall, S.; Maher, C.; English, C.; Rowlands, A.; Olds, T. Time regained: When people stop a physical activity program, how does their time use change? A randomised controlled trial. PLoS ONE 2015, 10, e0126665. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Lewis, S.F.; White, C.M.; Ashworth, M.; Rose, M.R. Validity of Fitbit activity monitoring for adults with progressive muscle diseases. Disabil. Rehabil. 2022, 44, 7543–7553. [Google Scholar] [CrossRef]
- Ndahimana, D.; Kim, E.K. Measurement Methods for Physical Activity and Energy Expenditure: A Review. Clin. Nutr. Res. 2017, 6, 68–80. [Google Scholar] [CrossRef]
- Jones, D.; Crossley, K.; Dascombe, B.; Hart, H.F.; Kemp, J. Validity and reliability of the fitbit flextm and actigraph gt3x+ at jogging and run-ning speeds. Int. J. Sports Phys. Ther. 2018, 13, 860–870. [Google Scholar] [CrossRef]
- Picerno, P.; Iosa, M.; D’Souza, C.; Benedetti, M.G.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis: A five-year update. Expert Rev. Med. Devices 2021, 18 (Suppl. S1), 79–94. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Bi, X. Wearable activity trackers for promoting physical activity: A systematic meta-analytic review. Int. J. Med. Inf. 2021, 152, 104487. [Google Scholar] [CrossRef]
- Colley, R.C.; Butler, G.; Garriguet, D.; Prince, S.A.; Roberts, K.C. Comparison of self-reported and accelerometer-measured physical activity in Canadian adults. Health Rep. 2018, 29, 3–15. [Google Scholar]
- Alcazar, J.; Kamper, R.S.; Aagaard, P.; Haddock, B.; Prescott, E.; Ara, I.; Suetta, C. Relation between leg extension power and 30-s sit-to-stand muscle power in older adults: Validation and translation to functional performance. Sci. Rep. 2020, 10, 16337. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed «Up & Go»: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Ng, S.S.; Hui-Chan, C.W. The Timed Up & Go Test: Its Reliability and Association With Lower-Limb Impairments and Locomotor Capacities in People With Chronic Stroke. Arch. Phys. Med. Rehabil. 2005, 6, 1641–1647. [Google Scholar]
- Gordt, K.; Gerhardy, T.; Najafi, B.; Schwenk, M. Effects of Wearable Sensor-Based Balance and Gait Training on Balance, Gait, and Functional Performance in Healthy and Patient Populations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gerontology 2018, 64, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Compagnat, M.; Batcho, C.S.; David, R.; Vuillerme, N.; Salle, J.Y.; Daviet, J.C.; Mandigout, S. Validity of the Walked Distance Estimated by Wearable Devices in Stroke Individuals. Sensors 2019, 19, 2497. [Google Scholar] [CrossRef]
- Millor, N.; Lecumberri, P.; Gomez, M.; Martinez-Ramirez, A.; Izquierdo, M. Kinematic parameters to evaluate functional performance of sit-to-stand and stand-to-sit transitions using motion sensor devices: A systematic review. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 926–936. [Google Scholar] [CrossRef]
- Salarian, A.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. iTUG, a sensitive and reliable measure of mobility. IEEE Trans. Neural. Syst. Rehabil. Eng. 2010, 18, 303–310. [Google Scholar] [CrossRef]
- Cobo, A.; Villalba-Mora, E.; Hayn, D.; Ferre, X.; Pérez-Rodríguez, R.; Sánchez-Sánchez, A.; Bernabe-Espiga, R.; Sanchez-Sanchez, J.L.; Lopez-Diez-Picazo, A.; Moral, C.; et al. Portable Ultrasound-Based Device for Detecting Older Adults’ Sit-to-Stand Transitions in Unsupervised 30-Second Chair-Stand Tests. Sensors 2020, 20, 1975. [Google Scholar] [CrossRef]
- Fudickar, S.; Pauls, A.; Lau, S.; Hellmers, S.; Gebel, K.; Diekmann, R.; Bauer, J.M.; Hein, A.; Koppelin, F. Measurement System for Unsupervised Standardized Assessments of Timed Up and Go Test and 5 Times Chair Rise Test in Community Settings—A Usability Study. Sensors 2022, 22, 731. [Google Scholar] [CrossRef]
- Hardy, R.; Cooper, R.; Shah, I.; Harridge, S.; Guralnik, J.; Kuh, D. Is chair rise performance a useful measure of leg power? Aging Clin. Exp. Res. 2010, 22, 412–418. [Google Scholar] [CrossRef]
- Cooper, R.; Kuh, D.; Hardy, R.; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. BMJ 2010, 341, c4467. [Google Scholar] [CrossRef]
- Stephens, M.A. Introduction to Kolmogorov (1933) On the Empirical Determination of a Distribution. In Breakthroughs in Statistics: Methodology and Distribution [Internet]; Springer Series in Statistics; Kotz, S., Johnson, N.L., Eds.; Springer: New York, NY, USA, 1992; pp. 93–105. [Google Scholar] [CrossRef]
- Smirnov, N. Table for Estimating the Goodness of Fit of Empirical Distributions. Ann. Math. Stat. 1948, 19, 279–281. [Google Scholar] [CrossRef]
- Walter, S.D.; Eliasziw, M.; Donner, A. Sample size and optimal designs for reliability studies. Stat. Med. 1998, 17, 101–210. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Terwee, C.B.; Bot, S.D.M.; de Boer, M.R.; van der Windt, D.A.W.M.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Beshara, P.; Chen, J.F.; Read, A.C.; Lagadec, P.; Wang, T.; Walsh, W.R. The Reliability and Validity of Wearable Inertial Sensors Coupled with the Microsoft Kinect to Measure Shoulder Range-of-Motion. Sensors 2020, 20, 7238. [Google Scholar] [CrossRef]
- Cavazzana, A.; Röhrborn, A.; Garthus-Niegel, S.; Larsson, M.; Hummel, T.; Croy, I. Sensory-specific impairment among older people. An investigation using both sensory thresholds and subjective measures across the five senses. PLoS ONE 2018, 13, e0202969. [Google Scholar] [CrossRef]
- Reuter, E.M.; Voelcker-Rehage, C.; Vieluf, S.; Godde, B. Touch perception throughout working life: Effects of age and expertise. Exp. Brain Res. 2012, 216, 287–297. [Google Scholar] [CrossRef]
- Hetzler, R.K.; Stickley, C.D.; Lundquist, K.M.; Kimura, I.F. Reliability and Accuracy of Handheld Stopwatches Compared With Electronic Timing in Measuring Sprint Performance. J. Strength Cond. Res. 2008, 22, 1969. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.N.; Decker, A.J.; Baarts, J.N.; Dupont, A.M.; Epema, J.S.; Reuther, M.C.; Houser, J.J.; Mayhew, J.L. Effect of competitiveness on forty-yard dash performance in college men and women. J. Strength Cond. Res. 2007, 21, 385–388. [Google Scholar] [PubMed]
- Falter, M.; Budts, W.; Goetschalckx, K.; Cornelissen, V.; Buys, R. Accuracy of Apple Watch Measurements for Heart Rate and Energy Expenditure in Patients With Cardiovascular Disease: Cross-Sectional Study. JMIR mHealth uHealth 2019, 7, e11889. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Value |
|---|---|
| IMU 9-axis sensor | ICM20948 |
| Microcontroller processing unit | STM32WB55RG |
| On chip memory (program + data) | 1 Mbyte |
| DAC resolution | 12 bits |
| Sampling frequency | 50 Hz |
| Connectivity | BLE and USB |
| Power | Lithium ion battery 560 mAh 3.7 V |
| Characteristic | Healthy Adults, n = 31 | Chronic Disease, n = 14 |
|---|---|---|
| Sex | ||
| Female | 15 (48%) | 9 (64%) |
| Male | 16 (52%) | 5 (36%) |
| Weight (Kg) | 70.0 [12] | 82.0 [10] |
| Height (m) | 1.7 ± 0.1 | 1.6 ± 0.1 |
| STS observed (n) | 18.9 ± 4.5 | 14.1 ± 4.0 |
| STS sensor (n) | 18.7 ± 3.8 | 14.2 ± 3.0 |
| TUG observed (s) | 5.9 ± 0.8 | 6.7 ± 3.1 |
| TUG Sensor (s) | 5.5 ± 1.2 | 7.0 ± 3.4 |
| Measures | r | p-Value | ICC | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| STS group A | 0.96 | <0.001 | 0.95 | 0.9, 0.97 | <0.001 | |
| STS group B | 0.94 | <0.001 | 0.90 | 0.74, 0.97 | <0.001 | |
| TUG group A | 0.80 | <0.001 | 0.75 | 0.43, 0.79 | <0.001 | |
| TUG group B | 0.87 | <0.001 | 0.98 | 0.91, 0.99 | <0.001 |
| Measures | Mean Bias | Percentage Difference (%) | 95% LoA Down | 95% LoA Up | RMSE | Percentage RMSE (%) |
|---|---|---|---|---|---|---|
| STS group A | 0.19 | 0.08 | −2.5 | 2.89 | 1.37 | 7.22 |
| STS group B | −0.14 | −3.21 | −3.23 | 2.94 | 1.52 | 10.83 |
| TUG group A | 0.45 | 9.24 | −1.18 | 2.08 | 0.94 | 15.75 |
| TUG group B | −0.36 | −4.56 | −1.66 | 0.95 | 0.74 | 11.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbohessou, K.G.; Sahuguede, S.; Lacroix, J.; Hamdan, F.; Conchon, E.; Dumas, Y.; Julien-Vergonjanne, A.; Mandigout, S. Validity of Estimated Results from a Wearable Device for the Tests Time Up and Go and Sit to Stand in Young Adults and in People with Chronic Diseases. Sensors 2023, 23, 5742. https://doi.org/10.3390/s23125742
Agbohessou KG, Sahuguede S, Lacroix J, Hamdan F, Conchon E, Dumas Y, Julien-Vergonjanne A, Mandigout S. Validity of Estimated Results from a Wearable Device for the Tests Time Up and Go and Sit to Stand in Young Adults and in People with Chronic Diseases. Sensors. 2023; 23(12):5742. https://doi.org/10.3390/s23125742
Chicago/Turabian StyleAgbohessou, Kokouvi Geovani, Stephanie Sahuguede, Justine Lacroix, Fadel Hamdan, Emmanuel Conchon, Yannick Dumas, Anne Julien-Vergonjanne, and Stephane Mandigout. 2023. "Validity of Estimated Results from a Wearable Device for the Tests Time Up and Go and Sit to Stand in Young Adults and in People with Chronic Diseases" Sensors 23, no. 12: 5742. https://doi.org/10.3390/s23125742
APA StyleAgbohessou, K. G., Sahuguede, S., Lacroix, J., Hamdan, F., Conchon, E., Dumas, Y., Julien-Vergonjanne, A., & Mandigout, S. (2023). Validity of Estimated Results from a Wearable Device for the Tests Time Up and Go and Sit to Stand in Young Adults and in People with Chronic Diseases. Sensors, 23(12), 5742. https://doi.org/10.3390/s23125742








