Stroke is one of the leading causes of death and disability among adults worldwide [
1], resulting in up to 50% of survivors having post-stroke disabilities [
2]. The impairment or loss of upper limb functionality is one of the most common consequences of stroke. Upper limb impairment is a prominent issue that often prevents individuals from independently completing activities of daily living (ADLs). ADLs describe the fundamental daily activities that individuals can perform to maintain their quality of life. Rehabilitation is vital in treating post-stroke disabilities [
3]. It involves a multidisciplinary team that can include doctors, physiotherapists, and healthcare assistants who play a vital role in the post-stroke rehabilitation process. Therefore, more demand for healthcare professionals and intervention therapy leads to higher healthcare costs.
New technical devices, such as rehabilitation robotics, have the potential to provide assistance with rehabilitating injured upper limbs through repetitive and task-specific therapy. The literature reported several reviews [
4,
5,
6] showed positive upper limb motor function recovery results after using robotic rehabilitation devices for their therapy. Limited medical resources have created the need for rehabilitation robotics and other alternative therapy delivery methods. This situation has led to economic pressures to shorten the treatment periods of patients in hospitals [
7,
8]. Therefore, the role of robotic devices in rehabilitation research is becoming essential for providing rehabilitation treatment to individuals with stroke. The global aging of the population and an increasing number of stroke incidents point to such devices becoming necessary [
9,
10], with a special demand for therapy to regain lost arm and hand motor abilities. Robotic systems with precise and repeatable motions can fill some of these needs by providing intervention therapy that meets the established specifications of physiotherapists [
11].
Using techniques similar to those of physiotherapists, robots can deliver hand rehabilitation in both passive and semi-active modes. For passive treatments, the robot’s motion is the sole driving force that moves an individual’s fingers. Conversely, semi-active treatment involves using the volitional power of the individual’s own fingers and hand to drive the motion with some assistance from the robot. Therefore, treatments can mimic those of a physiotherapist, either by directly manipulating the motion of the fingers and hand or acting as a guide.
The development of robotic systems for rehabilitation settings has followed several paths. Initial clinical studies in rehabilitation robotics were performed using the MIT-MANUS robotic arm developed by MIT to investigate the effectiveness of target-based robotic-assisted movements [
7]. However, many studies related to the post-stroke robotic rehabilitation of the upper limb, such as those involving MIT-MANUS [
15] and GENTLE/S [
16], focused primarily on arm joints, including the shoulder and elbow, rather than the hand and the finger joints [
3]. As a result, the therapeutic exercises for some of these devices did not include the finger movements necessary to achieve pinching and grasping. These movements are required to obtain functional improvement, which is necessary for performing ADLs independently [
17,
18,
19].
Robotic Rehabilitation for Hands
Several studies on the upper limb joints found that improvements occurred primarily in the shoulder and elbow [
20,
21]. Because the joints exercised were the only joints to show improvement, the development of hand devices became necessary. For hands, the greatest need was to develop support for performing functional tasks, specifically the pinch and grasp motions. These motions allow for the manipulation of objects, which is necessary to achieve activities of daily living. As a result, several new forms of hand rehabilitation devices using robot-aided therapy have been researched in recent years. These devices can be categorized into two main types of robotic systems: exoskeletons and end effectors [
22].
These systems are mounted or worn by the patient on their hand. The patient controls its motion directly [
23,
24]. The Hand-Wrist Assisting Robotic Device (HWARD), developed at the University of California, is a standalone device for treating stroke patients. The HWARD system is considered one of the first exoskeleton devices that provides actuation on the thumb plus the fingers grouped as a whole. It allows for manipulating household objects during training in a virtual world [
25]. Another example of an exoskeleton device is the PMHand, developed by McConnell et al. at Heriot-Watt University [
26]. It uses a 3D-printed exoskeleton frame that utilizes wires linked to a motor to generate the hand’s and its fingers’ movement. An alternative to this is found in the emergence of a new version of wearable devices known as soft robots. These devices are fabricated from easily deformable materials, such as gel or soft polymers [
27], and tend to be less complex, safer, more portable, and flexible.
These systems interact with the patient through a single point and are either attached to the hand of the patient or gripped by the hand [
23,
24]. One first end-effector device developed for hand and wrist rehabilitation was the Space Interface Device for Artificial Reality (SPIDAR). This device was invented in 1989 at the Tokyo Institute of Technology [
28]. Another approach to hand and wrist rehabilitation consists of a knob-like two degrees of freedom interface for the user to grasp and use to exercise forearm movement. Another example is the haptic knob, which was developed by the National University of Singapore [
29].
Moggio et al. published a meta-analysis comparing exoskeleton versus end-effector robot-assisted therapy for finger-hand motor recovery in stroke survivors [
30]. Aggogeri et al. [
31] and Kabir et al. [
32] reviewed the research status in hand rehabilitation robotic technology and evaluated several devices to describe the current state of the art [
31]. Another overview of hand rehabilitation robotics, which addressed everything from the hardware systems to the training paradigms, was published in 2017 [
33].
Exoskeleton devices provide patients with active and passive rehabilitation using different sources of actuation and mechanisms. They both describe the means used in devices to generate and translate power from actuators to move the fingers. Actuation can be classified as elector motor, hydraulic, and pneumatic [
33]. Electromotors are widely used for hand rehabilitation design because they are reliable and easy to install and control with high precision [
34]. In contrast, hydraulic and pneumatic actuators are less used due to the issues related to noise, leakage, control, and storage of the compressed air and fluid. They also depend on multiple components, including the compressor, different values and regulators, pump, and control elements, which add complexity and cost to the control and design of the rehabilitation device [
35].
The actuation mechanism’s main function is to transform the motion of the actuator to the fingers to achieve the hand therapy exercises. They depend on the nature of the actuator and can be categorized into main classifications: pneumatic actuation [
27,
36], linkage-based actuation [
37,
38,
39,
40,
41], cable-driven systems [
33,
42,
43,
44,
45,
46,
47,
48], and gear-motor actuation [
32]. Linkage and gear-motor actuation are commonly used for hand rehabilitation devices. However, they had several rigid linkages, pullies, gears, and mechanisms to control the finger motions and their joint. As a result, bulky and complicated exoskeleton hand devices were designed and developed that are costly, heavy, and caused uncomfortable experiences for the users and had limited workspace area. All of these affected the device’s biomimetic qualities, and some devices resulted in misalignment with the finger’s anatomic axis during motion [
27,
33]. On the other hand, soft exoskeleton hand rehabilitation devices are simple, not rigid, and lightweight. ExHand Exoskeleton is a fabric-based soft hand exoskeleton for assistance in ADLs developed and evaluated by ten healthy users using a glove weighing 137 g [
49]. However, they were mainly actuated by the pneumatic system, which requires complex components and control systems and has low accuracy [
27,
50,
51,
52,
53].
The cable was also frequently used as the actuation mechanism in hand rehabilitation robots [
42,
43]. Some devices used a soft glove for hand rehabilitation with a cable-driven mechanism [
54,
55]. Some popular devices are the Exo-Glove [
56], HandCARE [
57], and Gloreha [
58]. A cable-driven mechanism attached to a soft glove instead of the rigid links can be an alternative to rigid linkage devices [
46,
54,
55]. Soft devices driven using cable mechanisms tend to be lighter, more comfortable, low-cost, and portable as the motors and sensors can be placed away from the hand device attachment or glove. However, using gloves can have limitations due to the different hand sizes and could induce significant joint reaction forces [
34,
43].
The common theme observed in the hand device studies presented in the literature is the inclusion of sensors to measure the position of the patient’s fingers and thumb, the force produced by them, or both. In addition, accomplishing the reduced power of actuation is an important goal to reduce the cost and complexity of the design. When looking at how each system actuates the fingers, it becomes apparent that the 21 DOFs available in the fingers of a human hand are simply too many to actuate and exercise individually. It would affect the system’s complexity and weight, making some of these devices relatively expensive to acquire and maintain. Another theme was hand rehabilitation focused on ADL tasks in 3D space, which is considered the most practical and beneficial to patients [
29,
59,
60,
61]. These tasks involve training the patient on repetitive motion patterns, such as gripping and releasing an apple.
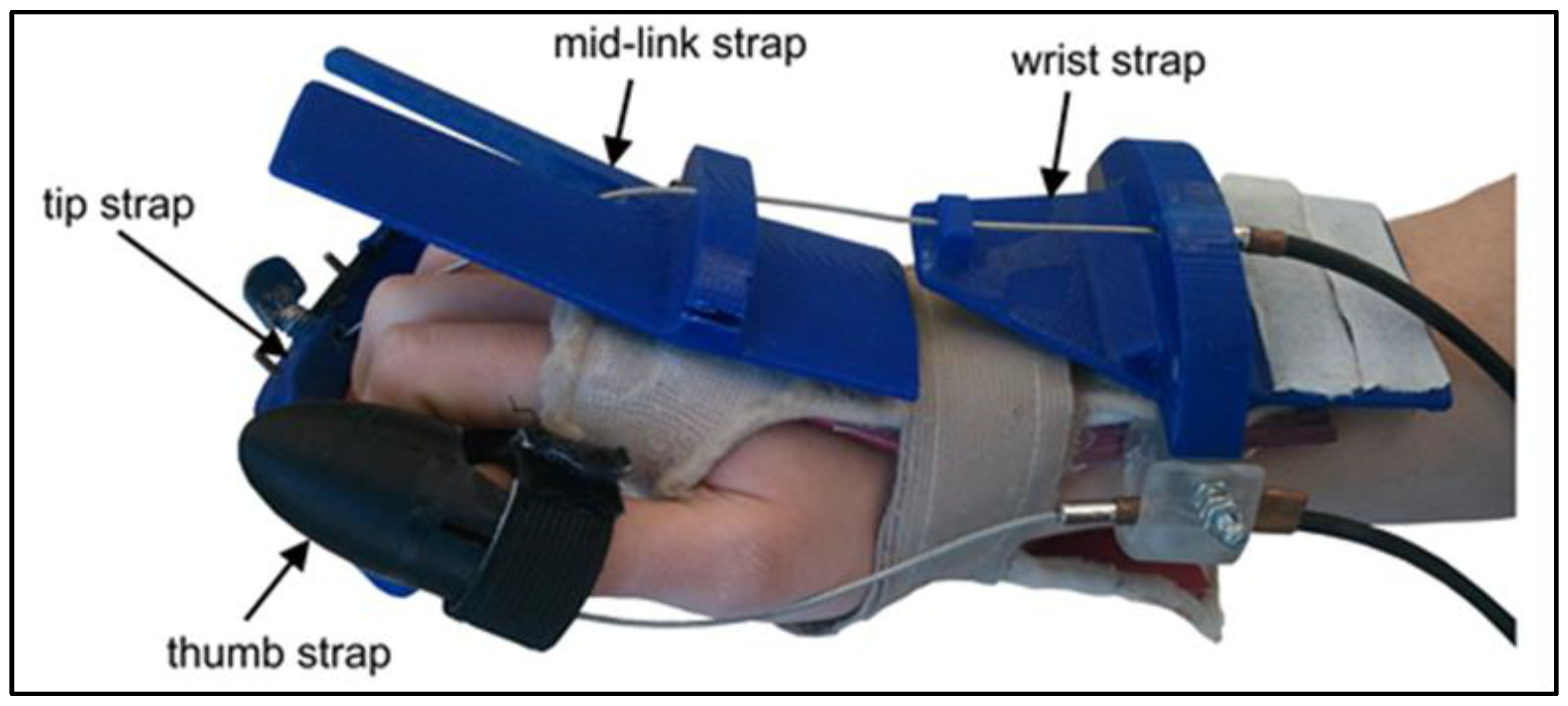
The proposed design approach addressed some of the challenges reported in the literature. It focused on developing a hand rehabilitation device with a flexible structure supporting different hand, finger, and thumb sizes. Provide a light and secure link to the auction and sensing system that was placed away from the palmar side of the hand so that the hand can be free to interact with real-world objects. This feature was selected based on our discussions with physiotherapists. The free hand helped users undergo training involving repetitive motion patterns with real objects. The proposed device features training for functional tasks with the four fingers grouped together in a reaching motion and the thumb actuated separately. Most other systems reported in the literature actuate each finger individually. The device used in the current approach simplifies the actuation, sensing, and control complexity because it uses only two motors rather than five. One motor activates the four fingers as a group, and the second activates the thumb. Moreover, the proposed hand device is light, with the total hand attachment at a weight of 246 g. It can be considered portable compared to other devices reported in the literature, making it comfortable for patients to wear and use. These features help to differentiate the proposed device from most of the systems reported in the literature, which actuate each finger individually. As a result, the specifications of the proposed device reduce its total complexity and cost.
This paper presents the results and analysis of a pilot study that examined the accessibility and reliability of the device, as well as how comfortable it was to use for healthy adults. These essential elements would affect stroke patients’ acceptance of the device and its efficacy. A pilot study that involved 52 healthy participants was conducted as a first step to obtaining regulatory permission for a clinical trial involving stroke patients. The study provided feedback regarding the comfort of the device. The quantifiable data collected for the system produced similar repeatable results for the repetitive motions being performed. Repeatability is the ability of the device to produce similar, quantifiable results as well as to operate predictably and reliably; this is critical when designing a rehabilitation device.